Optimizing Microwave-Assisted Extraction from Levisticum officinale WDJ Koch Roots Using Pareto Optimal Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Microwave-Assisted Extraction (MAE)
2.3. Bioactive Characterization of Obtained Extracts
2.4. Mathematical Model
- —degrees of freedom
- —sum of squares due to error
- —the sum of squares about the mean
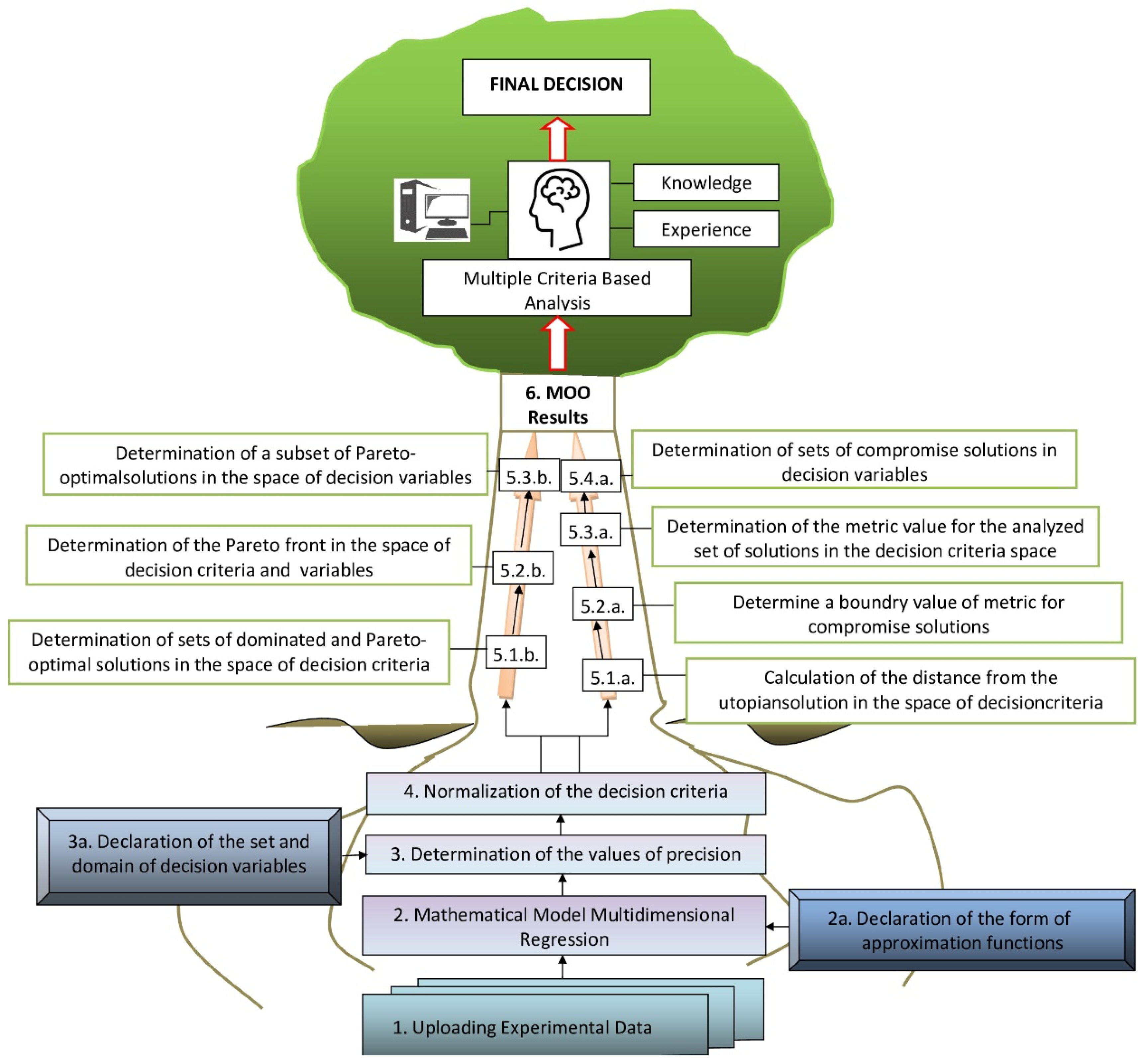
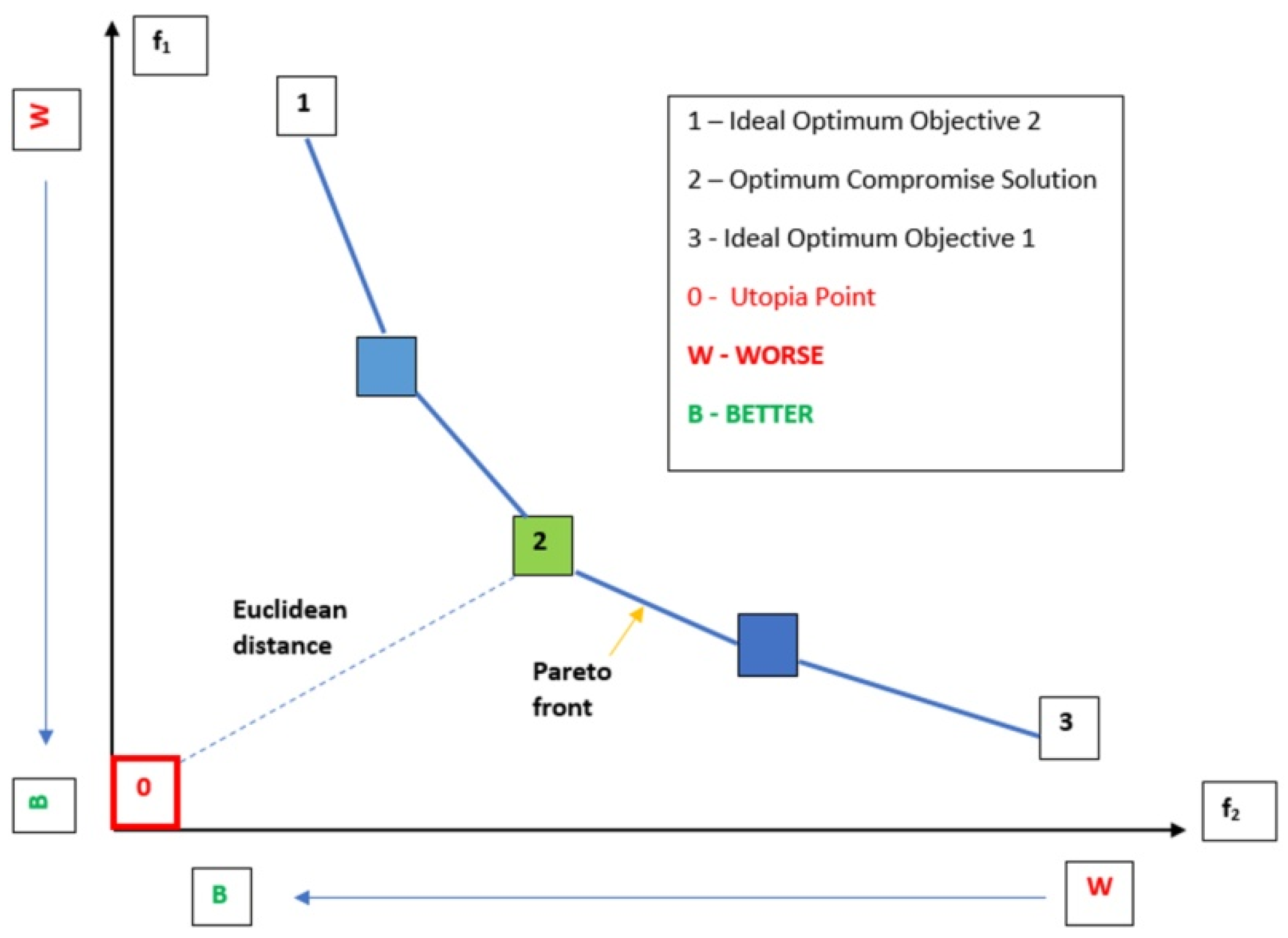
2.5. Multi-Objective Optimization
2.6. Smart Pareto Filter—Pareto Frontier Exploration Using Weighting of Decision Criteria

2.7. Reducing the Set of Pareto Optimal Solutions—The Compromise Solutions
2.8. Statistical Analysis
3. Results
3.1. The Mathematical Models—Multivariate Regression
3.2. Multi-Criteria Optimization of Microwave-Assisted Aqueous Extraction Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, G.; Chan, S.S.K.; Chung, H.S.; Li, S.L. Chemistry and biological activities of naturally occurring phthalides. Stud. Nat. Prod. Chem. 2005, 32, 611–669. [Google Scholar] [CrossRef]
- Beck, J.J.; Chou, S.C. The structural diversity of phthalides from the Apiaceae. J. Nat. Prod. 2007, 70, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Li, J.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Wu, N.; Liu, X.L. Optimisation of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chem. 2010, 122, 373–380. [Google Scholar] [CrossRef]
- Pannek, J.; Gach, J.; Boratyński, F.; Olejniczak, T. Antimicrobial activity of extracts and phthalides occurring in Apiaceae plants. Phytother. Res. 2018, 32, 1459–1487. [Google Scholar] [CrossRef] [PubMed]
- Nalini, P.; Poonam, Y. Synthesis and Biological activities of some new Phthalides. Orient. J. Chem. 2012, 2, 57–61. [Google Scholar]
- Song, J.; Li, D.; Liu, C.; Zhang, Y. Optimized microwave-assisted extraction of total phenolics (TP) from Ipomoea batatas leaves and its antioxidant activity. Innovat. Food Sci. Emerg. Technol. 2011, 12, 282–287. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kapusta, I.; Zaguła, G. Exploring the agro-potential of extract from Levisticum officinale WDJ Koch in soybean cultivation. Ind. Crop. Prod. 2023, 203, 117235. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kapusta, I.; Zaguła, G. Solid–liquid extraction of bioactive compounds as a green alternative for developing novel biostimulant from Linum usitatissimum L. Chem. Biol. Technol. Agric. 2023, 10, 108. [Google Scholar] [CrossRef]
- Szparaga, A. Biostimulating Extracts from Arctium lappa L. As Ecological Additives in Soybean Seed Coating Applications. Agric. Eng. 2023, 27, 1–10. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Jerman, T.; Trebse, P.; Mozetic Vodopivec, B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Camel, V. Microwave-assisted solvent extraction of environmental samples. TrAC Trends Anal. Chem. 2000, 19, 229–248. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; De Castro, M.L. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Milutinović, M.; Miladinović, M.; Gašić, U.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Recovery of bioactive molecules from Hypericum perforatum L. dust using microwave-assisted extraction. Biomass Conv. Bioref. 2024, 14, 7111–7123. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules 2010, 15, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Šeremet, D.; Jokić, S.; Aladić, K.; Cebin, A.V.; Božac, N.; Mandura, A.; Komes, D. Optimization of heat-, microwave-assisted and subcritical water extraction of phenolic compounds from ground ivy (Glechoma hederacea L.) using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100346. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Popa, V.I.; Beleca, C. On the role played by some natural products, with aromatic structure, in processes of plant growing. Cell. Chem. Technol. 1994, 28, 613–620. [Google Scholar]
- Balas, A.; Popa, V. Bioactive compounds extracted from Picea abies bark. In Proceedings of the 10th European workshop on Lignocellulosics and Pulp, Stockholm, Sweden, 25–28 August 2008; pp. 345–356. [Google Scholar]
- Tanase, C.; Bujor, O.C.; Popa, V.I. Phenolic natural compounds and their influence on physiological processes in plants. In Polyphenols in Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–58. [Google Scholar] [CrossRef]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; Ramírez de Molina, A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009, 113, 160–165. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Barbosa, L.C.A.; Queiroz, J.H.; Knödler, M.; Schieber, A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 2008, 110, 620–626. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Sirajuddi; Chan, K.W.; Sarfraz, R.A.; Uddin, K. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef]
- Lee, L.S.; Lee, N.; Kim, Y.H.; Lee, C.H.; Hong, S.P.; Jeon, Y.W.; Kim, Y.E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef] [PubMed]
- Krivorotova, T.; Sereikaite, J. Determination of fructan exohydrolase activity in the crude extracts of plants. Electron. J. Biotechnol. 2014, 17, 329–333. [Google Scholar] [CrossRef]
- Curve Fitting Toolbox for Use with Matlab; The MathWorks Inc.: Natick, MA, USA; Available online: http://cda.psych.uiuc.edu/matlab_pdf/curvefit.pdf (accessed on 10 April 2024).
- Gómez-Salazar, J.A.; Patlán-González, J.; Sosa-Morales, M.E.; Segovia-Hernandez, J.G.; Sánchez-Ramírez, E.; Ramírez-Márquez, C. Multi-objective optimization of sustainable red prickly pear (Opuntia streptacantha) peel drying and biocompounds extraction using a hybrid stochastic algorithm. Food Bioprod. Process. 2022, 132, 155–166. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Li, Q. Multiobjective topology optimization for finite periodic structures. Comput. Struct. 2010, 88, 806–811. [Google Scholar] [CrossRef]
- Marler, R.T.; Arora, J.S. The weighted sum method for multiobjective optimization: New insights. Struct. Multidiscip. Optim. 2010, 41, 853–862. [Google Scholar] [CrossRef]
- Das, I. A preference ordering among various Pareto optimal alternatives. Struct. Multidiscip. Optim. 1999, 18, 30–35. [Google Scholar] [CrossRef]
- Azzouzi, H.; Elhajji, L.; Achchoub, M.; Benbati, M.; Salmaoui, S. Assessment of total phenolic content and antioxidant activity potential of clementine extract obtained by microwave assisted extraction method. Biosci. Biotech. Res. Asia 2021, 18, 779–785. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Extraction of flavanones from immature Citrus unshiu pomace: Process optimization and antioxidant evaluation. Sci. Rep. 2020, 10, 19950. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Arola-Arnal, A. Optimization of a polyphenol extraction method for sweet orange pulp (Citrus sinensis L.) to identify phenolic compounds consumed from sweet oranges. PLoS ONE 2019, 14, e0211267. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.D.; Saini, R.K.; Keum, Y.S. Extraction of antioxidants and flavonoids from yuzu (Citrus junos Sieb ex Tanaka) peels: A response surface methodology study. J. Food Meas. Charact. 2017, 11, 364–379. [Google Scholar] [CrossRef]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Xia, W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Maximising recovery of phenolic compounds and antioxidant properties from banana peel using microwave assisted extraction and water. J. Food Sci. Technol. 2019, 56, 1360–1370. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Kheyar, F.; Kheyar, N.; Amiali, M.; Boulekbache-Makhlouf, L.; Kadi, A.; Benchabane, A.; Bitam, A. Impacts of microwave-assisted extraction parameters on total phenolic compounds yield from Algerian Moringa oleifera leaves, using response surface methodology. Nat. Prod. Res. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sai-Ut, S.; Kingwascharapong, P.; Mazumder, M.A.R.; Rawdkuen, S. Optimization of microwave-assisted extraction of phenolic compounds and antioxidants from Careya sphaerica Roxb. flowers using response surface methodology. Appl. Food Res. 2024, 4, 100379. [Google Scholar] [CrossRef]
- Mandal, C.; Jaiswal, M.; Bosco, S.J.D. Process optimization for ultrasound and microwave-assisted phytonutrients extractions from Averrhoa carambola fruit. Biomass Conv. Bioref. 2023, 1–17. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Fonseca, C.M.; Gaspar-Cunha, A. Methodology to select solutions from the pareto-optimal set: A comparative study. In Proceedings of the 9th Annual Conference on Genetic and Evolutionary Computation, London, UK, 7–11 July 2007. [Google Scholar] [CrossRef]
- Zio, E.; Bazzo, R. A clustering procedure for reducing the number of representative solutions in the Pareto Front of multiobjective optimization problems. Eur. J. Oper. Res. 2011, 210, 624–634. [Google Scholar] [CrossRef]








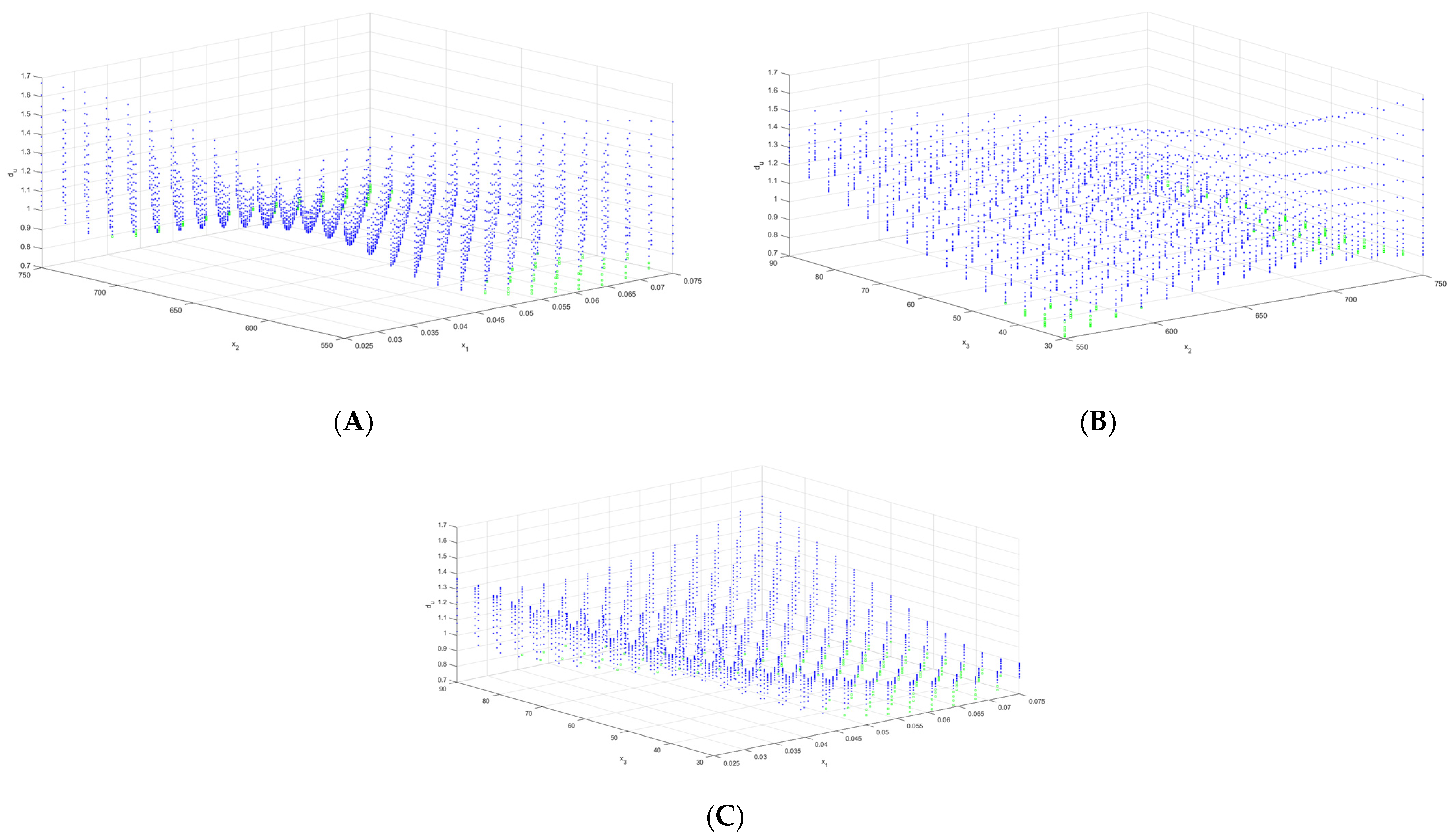


| Criteria | MSE | R2 | Adj R2 | SSE | SSR | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| K1 | 2127.941 | 0.813 | 0.716 | 3.620 × 104 | 8.970 × 104 | 4.6842 | 3.100 × 10−3 |
| K2 | 1606.676 | 0.821 | 0.726 | 2.730 × 104 | 1.250 × 105 | 8.6418 | 8.510 × 10−5 |
| K3 | 16.410 | 0.820 | 0.723 | 278.848 | 1.260 × 103 | 8.5237 | 9.290 × 10−5 |
| K4 | 2.450 | 0.890 | 0.830 | 41.7226 | 3.180 × 102 | 14.3846 | 2.640 × 10−6 |
| x1 (Sample/Solvent Ratio) | x2 (Microwave Power) | x3 (Time) | x1 (Sample/Solvent Ratio) | x2 (Microwave Power) | x3 (Time) |
|---|---|---|---|---|---|
| 0.0250 | 550.0000 | 30.0000 | 0.0607 | 721.4286 | 34.2857 |
| 0.0250 | 550.0000 | 34.2857 | 0.0607 | 735.7143 | 30.0000 |
| 0.0250 | 550.0000 | 38.5714 | 0.0607 | 735.7143 | 34.2857 |
| 0.0250 | 550.0000 | 42.8571 | 0.0607 | 735.7143 | 38.5714 |
| 0.0250 | 550.0000 | 47.1429 | 0.0607 | 735.7143 | 42.8571 |
| 0.0250 | 550.0000 | 51.4286 | 0.0607 | 735.7143 | 60.0000 |
| 0.0250 | 564.2857 | 30.0000 | 0.0607 | 735.7143 | 64.2857 |
| 0.0250 | 564.2857 | 34.2857 | 0.0607 | 735.7143 | 68.5714 |
| 0.0250 | 564.2857 | 38.5714 | 0.0607 | 735.7143 | 72.8571 |
| 0.0250 | 564.2857 | 42.8571 | 0.0607 | 735.7143 | 81.4286 |
| 0.0250 | 578.5714 | 30.0000 | 0.0607 | 735.7143 | 85.7143 |
| 0.0250 | 578.5714 | 34.2857 | 0.0607 | 735.7143 | 90.0000 |
| 0.0250 | 578.5714 | 38.5714 | 0.0607 | 750.0000 | 30.0000 |
| 0.0250 | 592.8571 | 30.0000 | 0.0607 | 750.0000 | 34.2857 |
| 0.0250 | 735.7143 | 38.5714 | 0.0607 | 750.0000 | 38.5714 |
| 0.0250 | 735.7143 | 42.8571 | 0.0607 | 750.0000 | 42.8571 |
| 0.0250 | 735.7143 | 47.1429 | 0.0607 | 750.0000 | 47.1429 |
| 0.0250 | 735.7143 | 51.4286 | 0.0607 | 750.0000 | 51.4286 |
| 0.0250 | 735.7143 | 55.7143 | 0.0607 | 750.0000 | 55.7143 |
| 0.0250 | 735.7143 | 60.0000 | 0.0607 | 750.0000 | 60.0000 |
| 0.0250 | 750.0000 | 38.5714 | 0.0607 | 750.0000 | 64.2857 |
| 0.0250 | 750.0000 | 42.8571 | 0.0607 | 750.0000 | 68.5714 |
| 0.0250 | 750.0000 | 47.1429 | 0.0607 | 750.0000 | 72.8571 |
| 0.0250 | 750.0000 | 51.4286 | 0.0607 | 750.0000 | 77.1429 |
| 0.0250 | 750.0000 | 55.7143 | 0.0607 | 750.0000 | 81.4286 |
| 0.0250 | 750.0000 | 60.0000 | 0.0607 | 750.0000 | 85.7143 |
| 0.0250 | 750.0000 | 64.2857 | 0.0607 | 750.0000 | 90.0000 |
| 0.0250 | 750.0000 | 68.5714 | 0.0643 | 550.0000 | 30.0000 |
| 0.0250 | 750.0000 | 72.8571 | 0.0643 | 550.0000 | 34.2857 |
| 0.0250 | 750.0000 | 77.1429 | 0.0643 | 550.0000 | 38.5714 |
| 0.0250 | 750.0000 | 81.4286 | 0.0643 | 550.0000 | 42.8571 |
| 0.0250 | 750.0000 | 85.7143 | 0.0643 | 550.0000 | 47.1429 |
| 0.0250 | 750.0000 | 90.0000 | 0.0643 | 550.0000 | 51.4286 |
| 0.0286 | 550.0000 | 30.0000 | 0.0643 | 564.2857 | 30.0000 |
| 0.0286 | 550.0000 | 34.2857 | 0.0643 | 578.5714 | 30.0000 |
| 0.0286 | 550.0000 | 38.5714 | 0.0643 | 592.8571 | 30.0000 |
| 0.0286 | 550.0000 | 42.8571 | 0.0643 | 607.1429 | 30.0000 |
| 0.0286 | 564.2857 | 30.0000 | 0.0643 | 621.4286 | 30.0000 |
| 0.0286 | 564.2857 | 34.2857 | 0.0643 | 635.7143 | 30.0000 |
| 0.0286 | 564.2857 | 38.5714 | 0.0643 | 650.0000 | 30.0000 |
| 0.0286 | 564.2857 | 42.8571 | 0.0643 | 664.2857 | 30.0000 |
| 0.0286 | 578.5714 | 30.0000 | 0.0643 | 678.5714 | 30.0000 |
| 0.0286 | 578.5714 | 34.2857 | 0.0643 | 692.8571 | 30.0000 |
| 0.0286 | 578.5714 | 38.5714 | 0.0643 | 707.1429 | 30.0000 |
| 0.0286 | 750.0000 | 51.4286 | 0.0643 | 721.4286 | 30.0000 |
| 0.0286 | 750.0000 | 55.7143 | 0.0643 | 721.4286 | 34.2857 |
| 0.0286 | 750.0000 | 60.0000 | 0.0643 | 721.4286 | 38.5714 |
| 0.0286 | 750.0000 | 64.2857 | 0.0643 | 735.7143 | 34.2857 |
| 0.0286 | 750.0000 | 68.5714 | 0.0643 | 735.7143 | 42.8571 |
| 0.0286 | 750.0000 | 72.8571 | 0.0643 | 735.7143 | 47.1429 |
| 0.0286 | 750.0000 | 77.1429 | 0.0643 | 735.7143 | 51.4286 |
| 0.0286 | 750.0000 | 81.4286 | 0.0643 | 735.7143 | 55.7143 |
| 0.0286 | 750.0000 | 85.7143 | 0.0643 | 735.7143 | 60.0000 |
| 0.0286 | 750.0000 | 90.0000 | 0.0643 | 735.7143 | 64.2857 |
| 0.0321 | 564.2857 | 30.0000 | 0.0643 | 735.7143 | 68.5714 |
| 0.0321 | 578.5714 | 30.0000 | 0.0643 | 735.7143 | 72.8571 |
| 0.0321 | 750.0000 | 51.4286 | 0.0643 | 735.7143 | 85.7143 |
| 0.0321 | 750.0000 | 55.7143 | 0.0643 | 735.7143 | 90.0000 |
| 0.0321 | 750.0000 | 60.0000 | 0.0643 | 750.0000 | 30.0000 |
| 0.0321 | 750.0000 | 64.2857 | 0.0643 | 750.0000 | 34.2857 |
| 0.0321 | 750.0000 | 68.5714 | 0.0643 | 750.0000 | 38.5714 |
| 0.0321 | 750.0000 | 72.8571 | 0.0643 | 750.0000 | 42.8571 |
| 0.0321 | 750.0000 | 77.1429 | 0.0643 | 750.0000 | 47.1429 |
| 0.0321 | 750.0000 | 81.4286 | 0.0643 | 750.0000 | 51.4286 |
| 0.0321 | 750.0000 | 85.7143 | 0.0643 | 750.0000 | 55.7143 |
| 0.0321 | 750.0000 | 90.0000 | 0.0643 | 750.0000 | 60.0000 |
| 0.0357 | 750.0000 | 55.7143 | 0.0643 | 750.0000 | 64.2857 |
| 0.0357 | 750.0000 | 60.0000 | 0.0643 | 750.0000 | 68.5714 |
| 0.0357 | 750.0000 | 64.2857 | 0.0643 | 750.0000 | 72.8571 |
| 0.0357 | 750.0000 | 68.5714 | 0.0643 | 750.0000 | 77.1429 |
| 0.0357 | 750.0000 | 72.8571 | 0.0643 | 750.0000 | 81.4286 |
| 0.0357 | 750.0000 | 77.1429 | 0.0643 | 750.0000 | 85.7143 |
| 0.0357 | 750.0000 | 81.4286 | 0.0643 | 750.0000 | 90.0000 |
| 0.0357 | 750.0000 | 85.7143 | 0.0679 | 550.0000 | 30.0000 |
| 0.0357 | 750.0000 | 90.0000 | 0.0679 | 550.0000 | 34.2857 |
| 0.0393 | 750.0000 | 55.7143 | 0.0679 | 550.0000 | 38.5714 |
| 0.0393 | 750.0000 | 60.0000 | 0.0679 | 550.0000 | 42.8571 |
| 0.0393 | 750.0000 | 64.2857 | 0.0679 | 550.0000 | 47.1429 |
| 0.0393 | 750.0000 | 68.5714 | 0.0679 | 550.0000 | 51.4286 |
| 0.0393 | 750.0000 | 72.8571 | 0.0679 | 564.2857 | 30.0000 |
| 0.0393 | 750.0000 | 77.1429 | 0.0679 | 578.5714 | 30.0000 |
| 0.0393 | 750.0000 | 81.4286 | 0.0679 | 592.8571 | 30.0000 |
| 0.0393 | 750.0000 | 85.7143 | 0.0679 | 607.1429 | 30.0000 |
| 0.0393 | 750.0000 | 90.0000 | 0.0679 | 621.4286 | 30.0000 |
| 0.0429 | 735.7143 | 64.2857 | 0.0679 | 635.7143 | 30.0000 |
| 0.0429 | 750.0000 | 51.4286 | 0.0679 | 650.0000 | 30.0000 |
| 0.0429 | 750.0000 | 55.7143 | 0.0679 | 664.2857 | 30.0000 |
| 0.0429 | 750.0000 | 60.0000 | 0.0679 | 678.5714 | 30.0000 |
| 0.0429 | 750.0000 | 64.2857 | 0.0679 | 692.8571 | 30.0000 |
| 0.0429 | 750.0000 | 68.5714 | 0.0679 | 707.1429 | 30.0000 |
| 0.0429 | 750.0000 | 72.8571 | 0.0679 | 721.4286 | 30.0000 |
| 0.0429 | 750.0000 | 77.1429 | 0.0679 | 735.7143 | 30.0000 |
| 0.0429 | 750.0000 | 81.4286 | 0.0679 | 735.7143 | 34.2857 |
| 0.0429 | 750.0000 | 85.7143 | 0.0679 | 735.7143 | 38.5714 |
| 0.0429 | 750.0000 | 90.0000 | 0.0679 | 735.7143 | 42.8571 |
| 0.0464 | 735.7143 | 55.7143 | 0.0679 | 735.7143 | 47.1429 |
| 0.0464 | 750.0000 | 42.8571 | 0.0679 | 735.7143 | 51.4286 |
| 0.0464 | 750.0000 | 47.1429 | 0.0679 | 735.7143 | 55.7143 |
| 0.0464 | 750.0000 | 51.4286 | 0.0679 | 735.7143 | 60.0000 |
| 0.0464 | 750.0000 | 55.7143 | 0.0679 | 735.7143 | 64.2857 |
| 0.0464 | 750.0000 | 60.0000 | 0.0679 | 735.7143 | 68.5714 |
| 0.0464 | 750.0000 | 64.2857 | 0.0679 | 735.7143 | 72.8571 |
| 0.0464 | 750.0000 | 68.5714 | 0.0679 | 750.0000 | 30.0000 |
| 0.0464 | 750.0000 | 72.8571 | 0.0679 | 750.0000 | 34.2857 |
| 0.0464 | 750.0000 | 77.1429 | 0.0679 | 750.0000 | 38.5714 |
| 0.0464 | 750.0000 | 81.4286 | 0.0679 | 750.0000 | 42.8571 |
| 0.0464 | 750.0000 | 85.7143 | 0.0679 | 750.0000 | 47.1429 |
| 0.0464 | 750.0000 | 90.0000 | 0.0679 | 750.0000 | 51.4286 |
| 0.0500 | 550.0000 | 34.2857 | 0.0679 | 750.0000 | 55.7143 |
| 0.0500 | 550.0000 | 38.5714 | 0.0679 | 750.0000 | 60.0000 |
| 0.0500 | 564.2857 | 30.0000 | 0.0679 | 750.0000 | 64.2857 |
| 0.0500 | 735.7143 | 38.5714 | 0.0679 | 750.0000 | 68.5714 |
| 0.0500 | 735.7143 | 47.1429 | 0.0679 | 750.0000 | 72.8571 |
| 0.0500 | 735.7143 | 51.4286 | 0.0679 | 750.0000 | 77.1429 |
| 0.0500 | 735.7143 | 55.7143 | 0.0679 | 750.0000 | 81.4286 |
| 0.0500 | 735.7143 | 60.0000 | 0.0679 | 750.0000 | 85.7143 |
| x1 (Sample/Solvent Ratio) | x2 (Microwave Power) | x2 (Time) | x1 (Sample/Solvent Ratio) | x2 (Microwave Power) | x3 (Time) |
|---|---|---|---|---|---|
| 0.0536 | 750 | 77.1429 | 0.0607 | 750 | 85.7143 |
| 0.0536 | 750 | 81.4286 | 0.0643 | 750 | 64.2857 |
| 0.0536 | 750 | 85.7143 | 0.0643 | 750 | 68.5714 |
| 0.0571 | 750 | 72.8571 | 0.0643 | 750 | 72.8571 |
| 0.0571 | 750 | 77.1429 | 0.0643 | 750 | 77.1429 |
| 0.0571 | 750 | 81.4286 | 0.0643 | 750 | 81.4286 |
| 0.0571 | 750 | 85.7143 | 0.0643 | 750 | 85.7143 |
| 0.0607 | 750 | 68.5714 | 0.0679 | 750 | 64.2857 |
| 0.0607 | 750 | 72.8571 | 0.0679 | 750 | 68.5714 |
| 0.0607 | 750 | 77.1429 | 0.0679 | 750 | 72.8571 |
| 0.0607 | 750 | 81.4286 | 0.0679 | 750 | 77.1429 |
| x1 (Sample/Solvent Ratio) | x2 (Microwave Power) | x3 (Time) | x1 (Sample/Solvent Ratio) | x2 (Microwave Power) | x3 (Time) |
|---|---|---|---|---|---|
| 0.0357 | 750.0000 | 85.7143 | 0.0643 | 564.2857 | 30.0000 |
| 0.0357 | 750.0000 | 90.0000 | 0.0643 | 564.2857 | 34.2857 |
| 0.0393 | 750.0000 | 72.8571 | 0.0643 | 564.2857 | 38.5714 |
| 0.0393 | 750.0000 | 77.1429 | 0.0643 | 578.5714 | 30.0000 |
| 0.0393 | 750.0000 | 81.4286 | 0.0643 | 578.5714 | 34.2857 |
| 0.0393 | 750.0000 | 85.7143 | 0.0643 | 578.5714 | 38.5714 |
| 0.0393 | 750.0000 | 90.0000 | 0.0643 | 592.8571 | 30.0000 |
| 0.0429 | 750.0000 | 68.5714 | 0.0643 | 721.4286 | 47.1429 |
| 0.0429 | 750.0000 | 72.8571 | 0.0643 | 721.4286 | 51.4286 |
| 0.0429 | 750.0000 | 77.1429 | 0.0643 | 721.4286 | 55.7143 |
| 0.0429 | 750.0000 | 81.4286 | 0.0643 | 735.7143 | 42.8571 |
| 0.0429 | 750.0000 | 85.7143 | 0.0643 | 735.7143 | 47.1429 |
| 0.0429 | 750.0000 | 90.0000 | 0.0643 | 735.7143 | 51.4286 |
| 0.0464 | 550.0000 | 30.0000 | 0.0643 | 735.7143 | 55.7143 |
| 0.0464 | 550.0000 | 34.2857 | 0.0643 | 735.7143 | 60.0000 |
| 0.0464 | 750.0000 | 64.2857 | 0.0643 | 750.0000 | 38.5714 |
| 0.0464 | 750.0000 | 68.5714 | 0.0643 | 750.0000 | 42.8571 |
| 0.0464 | 750.0000 | 72.8571 | 0.0643 | 750.0000 | 47.1429 |
| 0.0464 | 750.0000 | 77.1429 | 0.0643 | 750.0000 | 51.4286 |
| 0.0464 | 750.0000 | 81.4286 | 0.0643 | 750.0000 | 55.7143 |
| 0.0464 | 750.0000 | 85.7143 | 0.0643 | 750.0000 | 60.0000 |
| 0.0500 | 550.0000 | 30.0000 | 0.0643 | 750.0000 | 64.2857 |
| 0.0500 | 550.0000 | 34.2857 | 0.0679 | 550.0000 | 30.0000 |
| 0.0500 | 550.0000 | 38.5714 | 0.0679 | 550.0000 | 34.2857 |
| 0.0500 | 564.2857 | 30.0000 | 0.0679 | 550.0000 | 38.5714 |
| 0.0500 | 750.0000 | 60.0000 | 0.0679 | 564.2857 | 30.0000 |
| 0.0500 | 750.0000 | 64.2857 | 0.0679 | 564.2857 | 34.2857 |
| 0.0500 | 750.0000 | 68.5714 | 0.0679 | 564.2857 | 38.5714 |
| 0.0500 | 750.0000 | 72.8571 | 0.0679 | 578.5714 | 30.0000 |
| 0.0500 | 750.0000 | 77.1429 | 0.0679 | 578.5714 | 34.2857 |
| 0.0500 | 750.0000 | 81.4286 | 0.0679 | 721.4286 | 42.8571 |
| 0.0536 | 550.0000 | 30.0000 | 0.0679 | 721.4286 | 47.1429 |
| 0.0536 | 550.0000 | 34.2857 | 0.0679 | 721.4286 | 51.4286 |
| 0.0536 | 550.0000 | 38.5714 | 0.0679 | 721.4286 | 55.7143 |
| 0.0536 | 564.2857 | 30.0000 | 0.0679 | 735.7143 | 38.5714 |
| 0.0536 | 564.2857 | 34.2857 | 0.0679 | 735.7143 | 42.8571 |
| 0.0536 | 564.2857 | 38.5714 | 0.0679 | 735.7143 | 47.1429 |
| 0.0536 | 750.0000 | 55.7143 | 0.0679 | 735.7143 | 51.4286 |
| 0.0536 | 750.0000 | 60.0000 | 0.0679 | 735.7143 | 55.7143 |
| 0.0536 | 750.0000 | 64.2857 | 0.0679 | 735.7143 | 60.0000 |
| 0.0536 | 750.0000 | 68.5714 | 0.0679 | 750.0000 | 34.2857 |
| 0.0536 | 750.0000 | 72.8571 | 0.0679 | 750.0000 | 38.5714 |
| 0.0571 | 550.0000 | 30.0000 | 0.0679 | 750.0000 | 42.8571 |
| 0.0571 | 550.0000 | 34.2857 | 0.0679 | 750.0000 | 47.1429 |
| 0.0571 | 550.0000 | 38.5714 | 0.0679 | 750.0000 | 51.4286 |
| 0.0571 | 550.0000 | 42.8571 | 0.0679 | 750.0000 | 55.7143 |
| 0.0571 | 564.2857 | 30.0000 | 0.0679 | 750.0000 | 60.0000 |
| 0.0571 | 564.2857 | 34.2857 | 0.0679 | 750.0000 | 64.2857 |
| 0.0571 | 564.2857 | 38.5714 | 0.0714 | 550.0000 | 30.0000 |
| 0.0571 | 578.5714 | 30.0000 | 0.0714 | 550.0000 | 34.2857 |
| 0.0571 | 578.5714 | 34.2857 | 0.0714 | 564.2857 | 30.0000 |
| 0.0571 | 735.7143 | 55.7143 | 0.0714 | 564.2857 | 34.2857 |
| 0.0571 | 735.7143 | 60.0000 | 0.0714 | 721.4286 | 38.5714 |
| 0.0571 | 750.0000 | 51.4286 | 0.0714 | 721.4286 | 42.8571 |
| 0.0571 | 750.0000 | 55.7143 | 0.0714 | 721.4286 | 47.1429 |
| 0.0571 | 750.0000 | 60.0000 | 0.0714 | 721.4286 | 51.4286 |
| 0.0571 | 750.0000 | 64.2857 | 0.0714 | 735.7143 | 38.5714 |
| 0.0571 | 750.0000 | 68.5714 | 0.0714 | 735.7143 | 42.8571 |
| 0.0607 | 550.0000 | 30.0000 | 0.0714 | 735.7143 | 47.1429 |
| 0.0607 | 550.0000 | 34.2857 | 0.0714 | 735.7143 | 51.4286 |
| 0.0607 | 550.0000 | 38.5714 | 0.0714 | 735.7143 | 55.7143 |
| 0.0607 | 550.0000 | 42.8571 | 0.0714 | 735.7143 | 60.0000 |
| 0.0607 | 564.2857 | 30.0000 | 0.0714 | 750.0000 | 34.2857 |
| 0.0607 | 564.2857 | 34.2857 | 0.0714 | 750.0000 | 38.5714 |
| 0.0607 | 564.2857 | 38.5714 | 0.0714 | 750.0000 | 42.8571 |
| 0.0607 | 578.5714 | 30.0000 | 0.0714 | 750.0000 | 47.1429 |
| 0.0607 | 578.5714 | 34.2857 | 0.0714 | 750.0000 | 51.4286 |
| 0.0607 | 578.5714 | 38.5714 | 0.0714 | 750.0000 | 55.7143 |
| 0.0607 | 735.7143 | 47.1429 | 0.0714 | 750.0000 | 60.0000 |
| 0.0607 | 735.7143 | 51.4286 | 0.0714 | 750.0000 | 64.2857 |
| 0.0607 | 735.7143 | 55.7143 | 0.0750 | 735.7143 | 38.5714 |
| 0.0607 | 735.7143 | 60.0000 | 0.0750 | 735.7143 | 42.8571 |
| 0.0607 | 750.0000 | 42.8571 | 0.0750 | 735.7143 | 47.1429 |
| 0.0607 | 750.0000 | 47.1429 | 0.0750 | 735.7143 | 51.4286 |
| 0.0607 | 750.0000 | 51.4286 | 0.0750 | 750.0000 | 34.2857 |
| 0.0607 | 750.0000 | 55.7143 | 0.0750 | 750.0000 | 38.5714 |
| 0.0607 | 750.0000 | 60.0000 | 0.0750 | 750.0000 | 42.8571 |
| 0.0607 | 750.0000 | 64.2857 | 0.0750 | 750.0000 | 47.1429 |
| 0.0607 | 750.0000 | 68.5714 | 0.0750 | 750.0000 | 51.4286 |
| 0.0643 | 550.0000 | 30.0000 | 0.0750 | 750.0000 | 55.7143 |
| 0.0643 | 550.0000 | 34.2857 | 0.0750 | 750.0000 | 60.0000 |
| 0.0643 | 550.0000 | 38.5714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plawgo, M.; Kocira, S.; Bohata, A. Optimizing Microwave-Assisted Extraction from Levisticum officinale WDJ Koch Roots Using Pareto Optimal Solutions. Processes 2024, 12, 1026. https://doi.org/10.3390/pr12051026
Plawgo M, Kocira S, Bohata A. Optimizing Microwave-Assisted Extraction from Levisticum officinale WDJ Koch Roots Using Pareto Optimal Solutions. Processes. 2024; 12(5):1026. https://doi.org/10.3390/pr12051026
Chicago/Turabian StylePlawgo, Michał, Sławomir Kocira, and Andrea Bohata. 2024. "Optimizing Microwave-Assisted Extraction from Levisticum officinale WDJ Koch Roots Using Pareto Optimal Solutions" Processes 12, no. 5: 1026. https://doi.org/10.3390/pr12051026







