Qualitative and Quantitative Detection of Typical Reproductive Hormones in Dairy Cows Based on Terahertz Spectroscopy and Metamaterial Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Metamaterial Structure Design
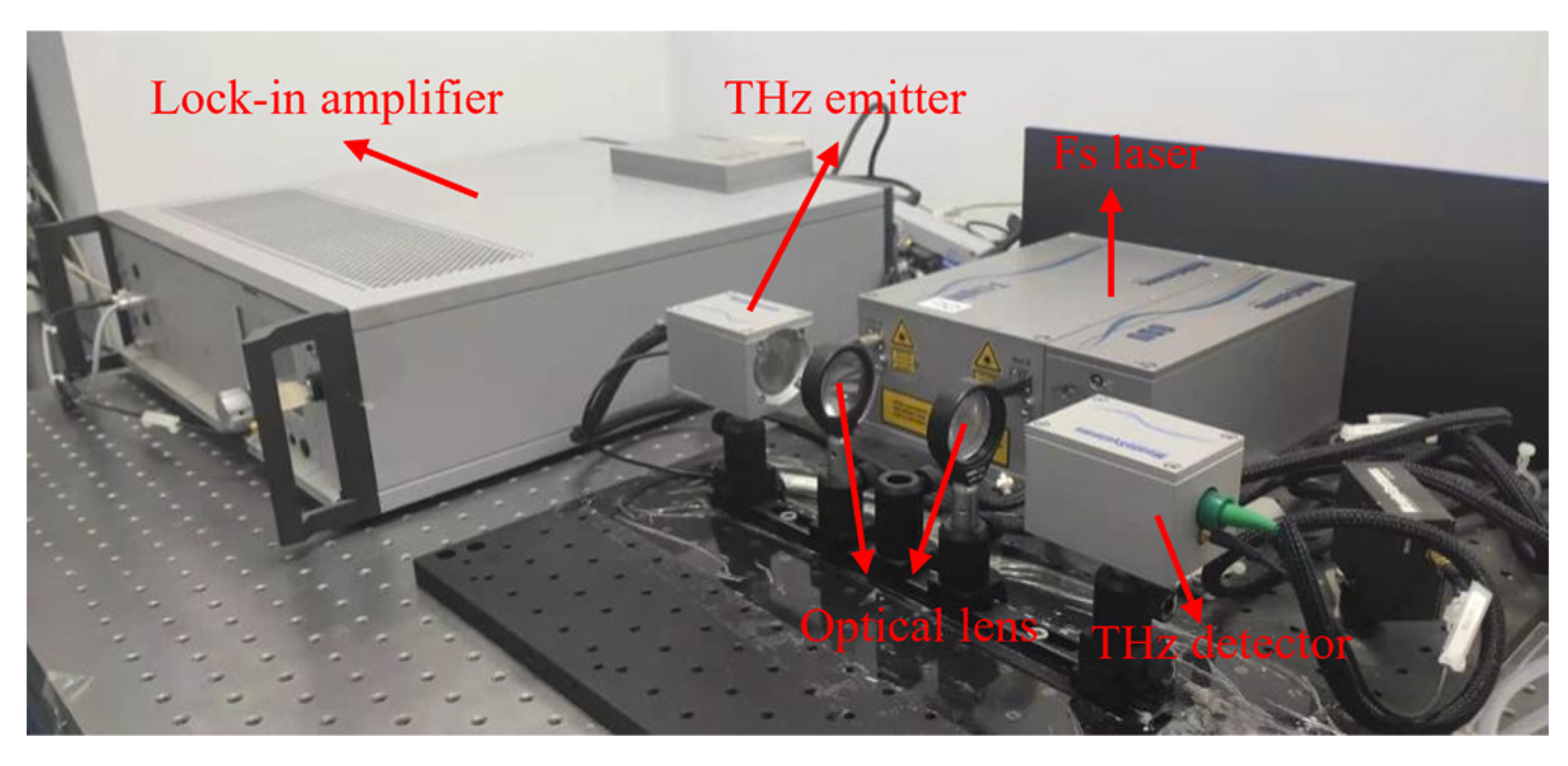
2.3. THz-TDS Measurements
3. Results
3.1. THz Spectroscopy and DFT Analysis of Samples
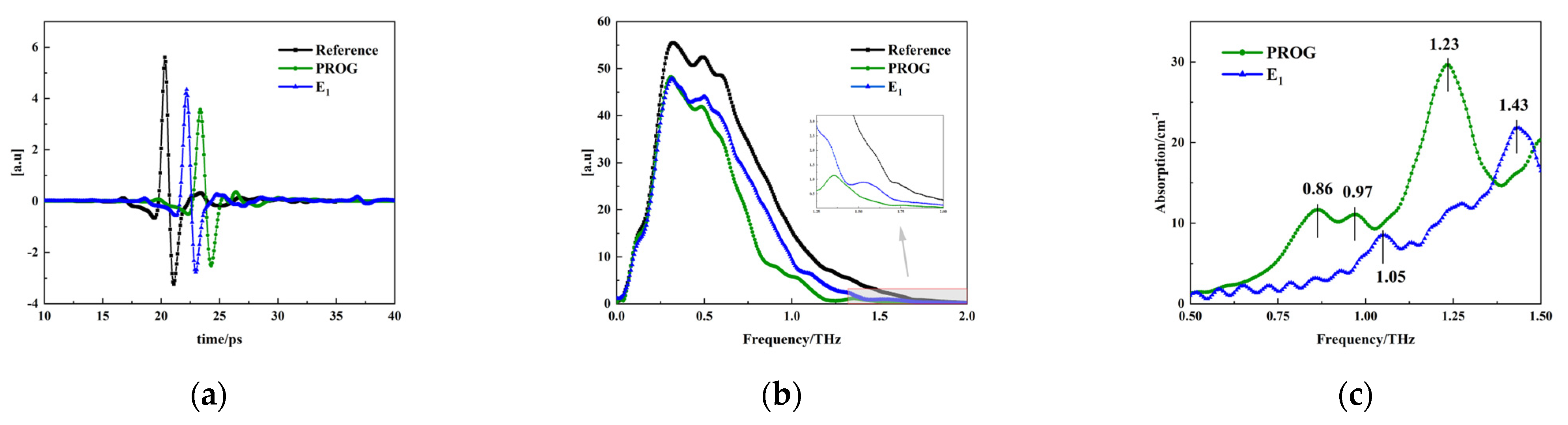
3.1.1. Terahertz Spectral Measurements of the Samples
3.1.2. Comparative Analysis of DFT Calculation and Experimental Results of PROG and E1
3.1.3. Vibration Mode Analysis and Absorption Peak Identification
3.2. Simulation and Verification of Metamaterials
3.3. Measurement and Analysis of PROG and E1 THz Spectra
3.3.1. Measurement of PROG and E1 THz Spectra
3.3.2. Qualitative Analysis of PROG and E1
3.3.3. Quantitative Analysis of PROG and E1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, M.; Wang, J.; Zhu, W.; Wang, M.; Peng, H. The current situation of dairy breeding on large-scale dairy farms in China. Anim. Husb. Vet. Med. 2023, 55, 124–131. [Google Scholar]
- Wang, Z.; Song, H.; Wang, Y.; Hua, Z.; Li, R.; Xu, X. Research Progress and Technology Trend of Intelligent Morning of Dairy Cow Motion Behavior. Smart Agric. 2022, 4, 36–52. [Google Scholar] [CrossRef]
- Randi, F.; McDonald, M.; Duffy, P.; Kelly, A.K.; Lonergan, P. The relationship between external auditory canal temperature and onset of estrus and ovulation in beef heifers. Theriogenology 2018, 110, 175–181. [Google Scholar] [CrossRef]
- Ozaki, R.; Inoue, S.; Yorozui, Y.; Ichikawa, R.; Yamada, N.; Higashi, S.; Matsuyama, S.; Tsukamura, H.; Ohkura, S.; Uenoyama, Y. Capturing temperature changes on the ocular surface along with estrus and ovulation using infrared thermography in Japanese Black cows. J. Reprod. Dev. 2024, 70, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tippenhauer, C.; Plenio, J.-L.; Madureira, A.; Cerri, R.; Heuwieser, W.; Borchardt, S. Factors associated with estrous expression and subsequent fertility in lactating dairy cows using automated activity monitoring. J. Dairy Sci. 2021, 104, 6267–6282. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Bell, M.; Liu, G. Potential of an activity index combining acceleration and location for automated estrus detection in dairy cows. Inf. Process. Agric. 2022, 9, 288–299. [Google Scholar] [CrossRef]
- Wang, Z.; Hua, Z.; Wen, Y.; Zhang, S.; Xu, X.; Song, H. E-YOLO: Recognition of estrus cow based on improved YOLOv8n model. Expert Syst. Appl. 2024, 238, 122212. [Google Scholar] [CrossRef]
- Reith, S.; Hoy, S. Behavioral signs of estrus and the potential of fully automated systems for detection of estrus in dairy cattle. Animal 2018, 12, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, C.-J.; Simujide, H.; Liu, B.; Chen, Z.; Zhao, P.; Huangfu, M.; Liu, J.; Gao, X.; Wu, Y.; et al. Reproductive Hormones Mediate Intestinal Microbiota Shifts during Estrus Synchronization in Grazing Simmental Cows. Animals 2022, 12, 1715. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Asensio-Ramos, M.; Hernández-Borges, J.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Chromatographic analysis of natural and synthetic estrogens in milk and dairy products. TrAC Trends Anal. Chem. 2013, 44, 58–77. [Google Scholar] [CrossRef]
- Wei, L.; Yan, Y.; Deng, J.; Ma, Y.; Wang, Y.; Wu, X.; Kang, X. Determination of estrogens in milk using polypyrrole fiber-mediated solid-phase extraction followed by high performance liquid chromatography. J. Braz. Chem. Soc. 2018, 29, 2137–2143. [Google Scholar] [CrossRef]
- Yang, Y.; Krin, A.; Cai, X.; Poopari, M.R.; Zhang, Y.; Cheeseman, J.R.; Xu, Y. Conformations of steroid hormones: Infrared and vibrational circular dichroism spectroscopy. Molecules 2023, 28, 771. [Google Scholar] [CrossRef]
- Kim, S.-B.; Nishihara, R.; Paulmurugan, R. Near-Infrared Imaging of Steroid Hormone Activities Using Bright BRET Templates. Int. J. Mol. Sci. 2022, 24, 677. [Google Scholar] [CrossRef]
- Ondieki, A.M.; Birech, Z.; Kaduki, K.A.; Mwangi, P.W.; Juma, M.; Chege, B.M. Label-free assaying of testosterone and growth hormones in blood using surface-enhanced Raman spectroscopy. Vib. Spectrosc. 2023, 129, 103605. [Google Scholar] [CrossRef]
- Ondieki, A.M.; Birech, Z.; Kaduki, K.A.; Kaingu, C.K.; Ndeke, A.N.; Namanya, L. Biomarker Raman bands of estradiol, follicle-stimulating, luteinizing, and progesterone hormones in blood. Vib. Spectrosc. 2022, 122, 103425. [Google Scholar] [CrossRef]
- Bi, H.; You, R.; Bian, X.; Zhao, X.; You, Z. A magnetic control enrichment technique combined with terahertz metamaterial biosensor for detecting SARS-CoV-2 spike protein. Biosens. Bioelectron. 2024, 243, 115763. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qi, L.; Li, B.; Wu, L.; Shi, D.; Uqaili, J.A.; Tao, X. A terahertz metamaterial sensor used for distinguishing glucose concentration. Results Phys. 2021, 26, 104332. [Google Scholar] [CrossRef]
- Hu, J.; Zhan, C.; Wang, Q.; Shi, H.; He, Y.; Ouyang, A. Research on highly sensitive quantitative detection of aflatoxin b2 solution based on thz metamaterial enhancement. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2023, 300, 122809. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Wang, J.; Liu, H.; Jing, J.; Deng, X.; Wei, D. Low-concentration biological sample detection using an asymmetric split resonator terahertz metamaterial. In Proceedings of the Photonics; 2023; p. 111. [Google Scholar]
- Puc, U.; Abina, A.; Jeglič, A.; Zidanšek, A.; Kašalynas, I.; Venckevičius, R.; Valušis, G. Spectroscopic analysis of melatonin in the terahertz frequency range. Sensors 2018, 18, 4098. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Z.; Zhang, Z.; Guo, C.; Zhang, J.; Ren, G.; Chen, L.; Li, S.; Zhao, H. Ultra-broadband terahertz fingerprint spectrum of melatonin with vibrational mode analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119141. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, J.; Song, Y.; Chang, C.; Ren, G.; Shen, J.; Zhang, Z.; Ji, T.; Chen, M.; Zhao, H. Broadband terahertz signatures and vibrations of dopamine. Analyst 2020, 145, 6006–6013. [Google Scholar] [CrossRef]
- Son, Y.J.; Lee, D.-K.; Son, J.-H. Identification of interpolymorph transformations of progesterone by terahertz time-domain spectroscopy. Curr. Appl. Phys. 2016, 16, 45–50. [Google Scholar] [CrossRef]
- Diskin, M.G.; Sreenan, J.M. Expression and detection of oestrus in cattle. Reprod. Nutr. Dev. 2000, 40, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Huang, G.; Xie, F.; Fu, W.; Yang, X. THz biosensing applications for clinical laboratories: Bottlenecks and strategies. TrAC Trends Anal. Chem. 2023, 163, 117057. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, F.; Xu, Y.; Mauk, M.G.; Qiu, X.; Tian, Z.; Zhang, L. Recent progress in terahertz biosensors based on artificial electromagnetic subwavelength structure. TrAC Trends Anal. Chem. 2023, 158, 116888. [Google Scholar] [CrossRef]
- Bai, J.; Ni, Y.; Li, B.; Wang, H.; Zhu, J.; Dong, C. Quantitative analysis of FQs antibiotics content in FMF using THz spectral and imaging technology. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120284. [Google Scholar] [CrossRef]
- Li, D.; Yang, Z.; Fu, A.; Chen, T.; Chen, L.; Tang, M.; Zhang, H.; Mu, N.; Wang, S.; Liang, G.; et al. Detecting melanoma with a terahertz spectroscopy imaging technique. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 234, 118229. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, X.; Zan, J.; Li, M.; Liu, Y.; Chen, J. Terahertz spectra and weak intermolecular interactions of nucleosides or nucleoside drugs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120344. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Li, S.; Zou, T.; Yu, Z.; Zhang, B.; Wang, C.; Zhang, J.; He, M.; Zhao, H. Terahertz spectra of L-phenylalanine and its monohydrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 19–23. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, D.; Choi, M.H.; Son, J.-H.; Seo, M. Highly sensitive and selective detection of steroid hormones using terahertz molecule-specific sensors. Anal. Chem. 2019, 91, 6844–6849. [Google Scholar] [CrossRef]
- Tantiwanichapan, K.; Durmaz, H. Herbicide/pesticide sensing with metamaterial absorber in THz regime. Sens. Actuators A Phys. 2021, 331, 112960. [Google Scholar] [CrossRef]
- Liang, W.; Zuo, J.; Zhou, Q.; Zhang, C. Quantitative determination of glycerol concentration in aqueous glycerol solutions by metamaterial-based terahertz spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 270, 120812. [Google Scholar] [CrossRef] [PubMed]










| PROG | 0.819 THz | 1.394 THz | |
|---|---|---|---|
| Frequency Shift/GHz | Amplitude Offset | Amplitude Offset | |
| 5 mg/mL | 13 | 0.0152 | 0.0259 |
| 10 mg/mL | 19 | 0.0251 | 0.0323 |
| 20 mg/mL | 44 | 0.0544 | 0.0855 |
| 40 mg/mL | 75 | 0.0729 | 0.1136 |
| E1 | 0.819 THz | 1.394 THz |
|---|---|---|
| Amplitude Offset | Amplitude Offset | |
| 5 mg/mL | 0.0105 | 0.0104 |
| 10 mg/mL | 0.0122 | 0.0320 |
| 20 mg/mL | 0.0208 | 0.0566 |
| 40 mg/mL | 0.0280 | 0.0983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Zhao, J.; Zhao, W.; Jia, N.; Zhang, Z.; Li, B. Qualitative and Quantitative Detection of Typical Reproductive Hormones in Dairy Cows Based on Terahertz Spectroscopy and Metamaterial Technology. Molecules 2024, 29, 2366. https://doi.org/10.3390/molecules29102366
Liang S, Zhao J, Zhao W, Jia N, Zhang Z, Li B. Qualitative and Quantitative Detection of Typical Reproductive Hormones in Dairy Cows Based on Terahertz Spectroscopy and Metamaterial Technology. Molecules. 2024; 29(10):2366. https://doi.org/10.3390/molecules29102366
Chicago/Turabian StyleLiang, Shuang, Jingbo Zhao, Wenwen Zhao, Nan Jia, Zhiyong Zhang, and Bin Li. 2024. "Qualitative and Quantitative Detection of Typical Reproductive Hormones in Dairy Cows Based on Terahertz Spectroscopy and Metamaterial Technology" Molecules 29, no. 10: 2366. https://doi.org/10.3390/molecules29102366






