Whey Protein Isolate as a Substrate to Design Calendula officinalis Flower Extract Controlled-Release Materials
Abstract
:1. Introduction
2. Results
2.1. Characterization of Microparticles
2.2. Materials Characterization
2.2.1. Mechanical Properties
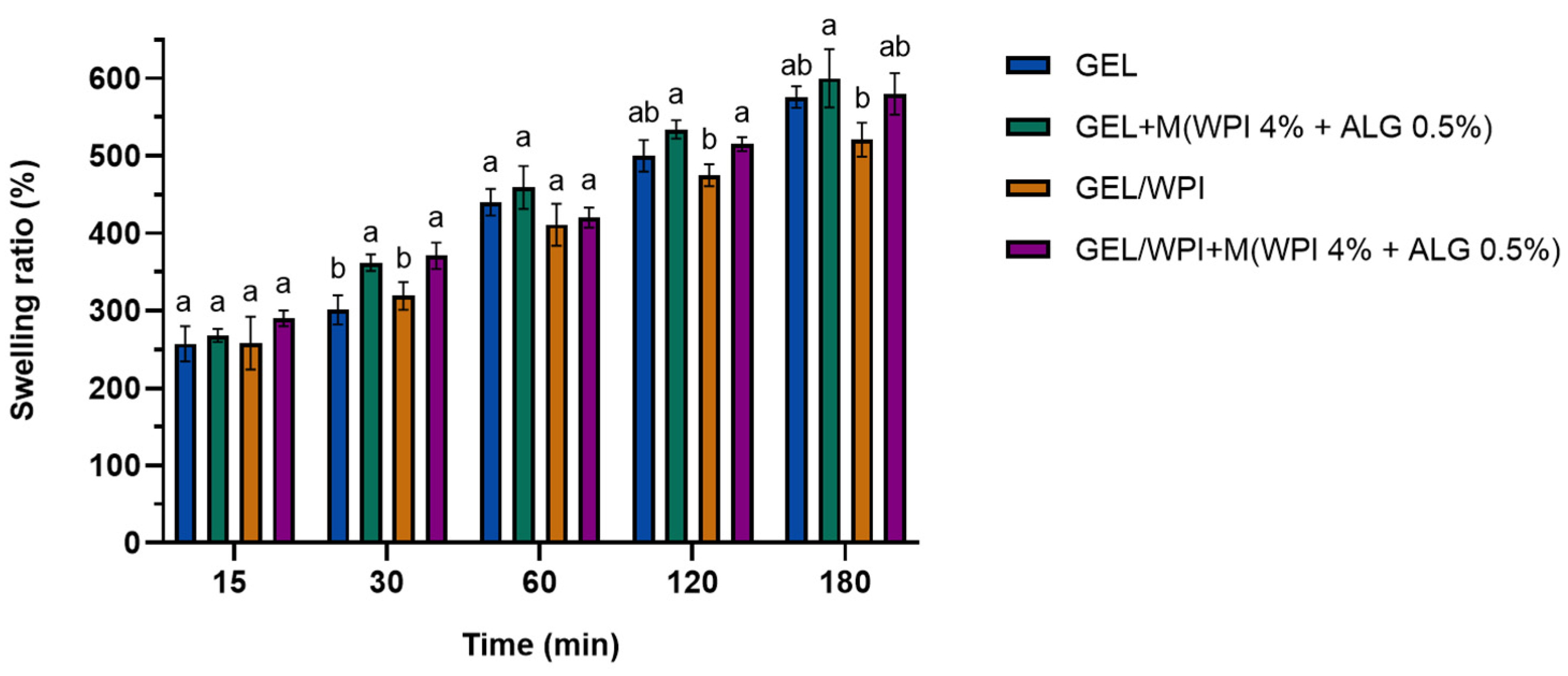
2.2.2. Swelling Tests
2.2.3. Contact Angle Results
2.2.4. Moisture Content
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Microparticles
4.3. Characterization of Microparticles
4.3.1. Imaging of Microparticles
4.3.2. Water Absorption of Microparticles
4.3.3. Loading Capacity of Microspheres
4.3.4. In Vitro Release
4.4. Preparation of Films with Microspheres
4.5. Characterization of Films
4.5.1. Mechanical Tests
4.5.2. Evaluation of Swelling Capacity
4.5.3. Contact Angle Measurements
4.5.4. Moisture Content
4.5.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vieira, S.; Picolotto, R.S.; Wagner, R.; Silvia, N. Elemental (macro- and microelements) and amino acid characterization of milk proteins and their nutritional value. J. Food Nutr. Res. 2015, 3, 430–436. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.G.; Vegarud, G.E.; Skeie, S. Seasonal and regional variation in the composition of whey from Norwegian Cheddar-type and Dutch-type cheeses. Int. Dairy J. 2002, 12, 621–629. [Google Scholar] [CrossRef]
- Smith, T.J.; Foegeding, E.A.; Drake, M.A. Flavor and functional characteristics of whey protein isolates from different whey sources. J. Food Sci. 2016, 81, C849–C857. [Google Scholar] [CrossRef] [PubMed]
- El-Hatmi, H.; Jrad, Z.; Salhi, I.; Aguibi, A.; Nadri, A.; Khorchani, T. Usporedba sastava i profila proteina sirutke majčinog, devinog, magarećeg, kozjeg i kravljeg mlijeka. Mljekarstvo 2015, 65, 159–167. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr. Pharm. Des. 2007, 13, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Tovar Jiménez, X.; Arana Cuenca, A.; Téllez Jurado, A.; Abreu Corona, A.; Muro Urista, C.R. Traditional methods for whey protein isolation and concentration: Effects on nutritional properties and biological activity. J. Mex. Chem. Soc. 2012, 56, 369–377. [Google Scholar] [CrossRef]
- Puyol, P.; Pérez, M.D.; Horne, D.S. Heat-induced gelation of whey protein isolates (WPI): Effect of NaCl and protein concentration. Food Hydrocoll. 2001, 15, 233–237. [Google Scholar] [CrossRef]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of whey proteins during thermal processing: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Kotchabhakdi, A.; Vardhanabhuti, B. Formation of heated whey protein isolate-pectin complexes at pH greater than the isoelectric point with improved emulsification properties. J. Dairy Sci. 2020, 103, 6820–6829. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Vargas, S.A.; Delgado-Macuil, R.J.; Ruiz-Espinosa, H.; Rojas-López, M.; Amador-Espejo, G.G. High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: Evaluation of selected functional properties. Ultrason. Sonochem. 2021, 70, 105340. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Li, C.; Diao, M.; Yan, M.; Wang, C.; Zhang, T. Characterization of interactions between whey protein isolate and hyaluronic acid in aqueous solution: Effects of pH and mixing ratio. Colloids Surf. B Biointerfaces 2021, 203, 111758. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.; Quereshi, D.; Hanh Nguyen, T.T.; Pani, D.; Mohanty, B.; Anis, A.; Maji, S.; Kim, D.; Sarkar, P.; Pal, K. Preparation and characterization of cocoa butter and whey protein isolate based emulgels for pharmaceutical and probiotics delivery applications. J. Dispers. Sci. Technol. 2020, 41, 426–440. [Google Scholar] [CrossRef]
- Gomide, R.A.C.; de Oliveira, A.C.S.; Luvizaro, L.B.; Yoshida, M.I.; de Oliveira, C.R.; Borges, S.V. Biopolymeric films based on whey protein isolate/lignin microparticles for waste recovery. J. Food Process Eng. 2021, 44, e13596. [Google Scholar] [CrossRef]
- Schmid, M.; Krimmel, B.; Grupa, U.; Noller, K. Effects of thermally induced denaturation on technological-functional properties of whey protein isolate-based films. J. Dairy Sci. 2014, 97, 5315–5327. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; You, Y.J.; Quek, S.Y.; Wu, W.D.; Chen, X.D. Interplaying effects of wall and core materials on the property and functionality of microparticles for co-encapsulation of vitamin e with coenzyme q10. Food Bioprocess Technol. 2020, 13, 705–721. [Google Scholar] [CrossRef]
- Tavares, L.; Souza, H.K.S.; Gonçalves, M.P.; Rocha, C.M.R. Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate. Food Hydrocoll. 2021, 113, 106471. [Google Scholar] [CrossRef]
- Cabral, B.R.P.; de Oliveira, P.M.; Gelfuso, G.M.; de Souza Cardoso Quintão, T.; Chaker, J.A.; de Oliveira Karnikowski, M.G.; Gris, E.F. Improving stability of antioxidant compounds from Plinia cauliflora (jabuticaba) fruit peel extract by encapsulation in chitosan microparticles. J. Food Eng. 2018, 238, 195–201. [Google Scholar] [CrossRef]
- Li, Y.; Song, F.; Cheng, L.; Qian, J.; Chen, Q. Functionalized large-pore mesoporous silica microparticles for gefitinib and doxorubicin codelivery. Materials 2019, 12, 766. [Google Scholar] [CrossRef] [PubMed]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Production of vitamin B1 microparticles by a spray drying process using different biopolymers as wall materials. Can. J. Chem. Eng. 2020, 98, 1682–1695. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Berling, C.L.; Germer, S.P.M.; Alvim, I.D.; Hubinger, M.D. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: Pigment stability during storage of microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.B.; Azizi, N.; Baffoun, A.; Chevalier, Y.; Majdoub, M. Fragrant microcapsules based on β-cyclodextrin for cosmetotextile application. J. Renew. Mater. 2019, 7, 1347–1362. [Google Scholar] [CrossRef]
- Leon, A.M.; Medina, W.T.; Park, D.J.; Aguilera, J.M. Properties of microparticles from a whey protein isolate/alginate emulsion gel. Food Sci. Technol. Int. 2018, 24, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Dordević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Tavares, L.; Barros, H.L.B.; Vaghetti, J.C.P.; Noreña, C.P.Z. Microencapsulation of garlic extract by complex coacervation using whey protein isolate/chitosan and gum arabic/chitosan as wall materials: Influence of anionic biopolymers on the physicochemical and structural properties of microparticles. Food Bioprocess Technol. 2019, 12, 2093–2106. [Google Scholar] [CrossRef]
- Campelo-Felix, P.H.; Souza, H.J.B.; Figueiredo, J.D.A.; De Barros Fernandes, R.V.; Botrel, D.A.; De Oliveira, C.R.; Yoshida, M.I.; Borges, S.V. Prebiotic carbohydrates: Effect on reconstitution, storage, release, and antioxidant properties of lime essential oil microparticles. J. Agric. Food Chem. 2017, 65, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lee, Y. Encapsulation of tributyrin with whey protein isolate (WPI) by spray-drying with a three-fluid nozzle. J. Food Eng. 2020, 281, 109992. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Wang, X.; Yao, J.; Xu, J. Structural characterization and properties of polyols plasticized chitosan films. Int. J. Biol. Macromol. 2019, 135, 240–245. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Biodegradable films based on gelatin and papaya peel microparticles with antioxidant properties. Food Bioprocess Technol. 2018, 11, 536–550. [Google Scholar] [CrossRef]
- Dammak, I.; Bittante, A.M.Q.B.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of gelatin-based films incorporated with chitosan-coated microparticles charged with rutin. Int. J. Biol. Macromol. 2017, 101, 643–652. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Jan, N. Calendula Officinalis-An important medicinal plant with potential biological properties. Proc. Indian Natl. Sci. Acad. 2017, 93, 769–787. [Google Scholar] [CrossRef]
- Butnariu, M.; Coradini, C.Z. Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem. Cent. J. 2012, 6, 35. [Google Scholar] [CrossRef]
- Verma, P.K.; Raina, R.; Agarwal, S.; Kour, H. Phytochemical ingredients and pharmacological potential of Calendula officinalis Linn. Pharm. Biomed. Res. 2018, 4, 1–17. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Hajaratul Najwa, M.; Norsyazwani Solehah, N. Encapsulation of protein within alginate-inulin matrix for targeted drug delivery system. J. Phys. Conf. Ser. 2020, 1532, 012013. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Nardella, M.; Chiarini, M.; Valbonetti, L.; Neri, L.; Difonzo, G.; Pittia, P. Structuring alginate beads with different biopolymers for the development of functional ingredients loaded with olive leaves phenolic extract. Food Hydrocoll. 2020, 108, 105849. [Google Scholar] [CrossRef]
- Moghaddam, N.; Seyed Dorraji, M.S.; Mousavi, S.N.; Chiti, H.; Rasoulifard, M.H.; Pourmansouri, Z. Application of whey protein-alginate particles coated by black seed oil as a biocompatible carrier of quercetin at treating non-alcoholic fatty liver disease. J. Funct. Foods 2021, 86, 104728. [Google Scholar] [CrossRef]
- Sahiner, N.; Sengel, S.B. Tannic acid decorated poly(methacrylic acid) micro and nanoparticles with controllable tannic acid release and antioxidant properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 30–38. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Michailidou, G.; Lazaridou, M.; Christodoulou, E.; Gounari, E.; Ofrydopoulou, A.; Lambropoulou, D.A.; Vergkizi-Nikolakaki, S.; Lykidou, S.; Nikolaidis, N. Innovative skin product emulsions with enhanced antioxidant, antimicrobial and UV protection properties containing nanoparticles of pure and modified chitosan with encapsulated fresh pomegranate juice. Polymers 2020, 12, 1542. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Chai, Z.; Leng, X. Study of the physical properties of whey protein isolate and gelatin composite films. J. Agric. Food Chem. 2010, 58, 5100–5108. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Krochta, J.M. Denaturation time and temperature effects on solubility, tensile properties, and oxygen. J. Food Sci. 2001, 66, 705–710. [Google Scholar] [CrossRef]
- Bekhit, M.; Arab-Tehrany, E.; Kahn, C.J.F.; Cleymand, F.; Fleutot, S.; Desobry, S.; Sánchez-González, L. Bioactive films containing alginate-pectin composite microbeads with Lactococcus lactis subsp. Lactis: Physicochemical characterization and antilisterial activity. Int. J. Mol. Sci. 2018, 19, 574. [Google Scholar] [CrossRef]
- Lim, L.T.; Mine, Y.; Tung, M.A. Barrier and tensile properties of transglutaminase cross-linked gelatin films as affected by relative humidity, temperature, and glycerol content. J. Food Sci. 1999, 64, 616–622. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and quantification of molecular interactions in protein films: A review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Gounga, M.E.; Xu, S.Y.; Wang, Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J. Food Eng. 2007, 83, 521–530. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghadertaj, A.; Mehryar, L. Whey protein isolate-based films incorporated with nanoemulsions of orange peel (Citrus sinensis) essential oil: Preparation and characterization. J. Food Process. Preserv. 2021, 45, e15196. [Google Scholar] [CrossRef]
- Esteghlal, S.; Niakousari, M.; Hosseini, S.M.H. Physical and mechanical properties of gelatin-CMC composite films under the influence of electrostatic interactions. Int. J. Biol. Macromol. 2018, 114, 1–9. [Google Scholar] [CrossRef]
- Bai, H.; Xu, J.; Liao, P.; Liu, X. Mechanical and water barrier properties of soy protein isolate film incorporated with gelatin. J. Plast. Film Sheeting 2013, 29, 174–188. [Google Scholar] [CrossRef]
- de Jesus, G.L.; Baldasso, C.; Marcílio, N.R.; Tessaro, I.C. Demineralized whey–gelatin composite films: Effects of composition on film formation, mechanical, and physical properties. J. Appl. Polym. Sci. 2020, 137, 49282. [Google Scholar] [CrossRef]
- Shams, B.; Ebrahimi, N.G.; Khodaiyan, F. Development of antibacterial nanocomposite: Whey protein-gelatin-nanoclay films with orange peel extract and tripolyphosphate as potential food packaging. Adv. Polym. Technol. 2019, 2019, 1973184. [Google Scholar] [CrossRef]
- Shaw, N.B.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M. Physical properties of WPI films plasticized with glycerol, xylitol, or sorbitol. J. Food Sci. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, J.; Wu, J.L.; Chen, S.F.; Zhang, Q.Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Kowalonek, J.; Stachowiak, N.; Bolczak, K.; Richert, A. Physicochemical and antibacterial properties of alginate films containing tansy (Tanacetum vulgare L.) essential oil. Polymers 2023, 15, 260. [Google Scholar] [CrossRef]
- Déat-Lainé, E.; Hoffart, V.; Cardot, J.M.; Subirade, M.; Beyssac, E. Development and in vitro characterization of insulin loaded whey protein and alginate microparticles. Int. J. Pharm. 2012, 439, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Prus-Walendziak, W.; Stachowiak, N.; Bajek, A.; Kazmierski, L.; Tylkowski, B. Modification of collagen/gelatin/hydroxyethyl cellulose-based materials by addition of herbal extract-loaded microspheres made from gellan gum and xanthan gum. Materials 2020, 13, 3507. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Batista, P.; Castro, P.; Madureira, A.R.; Sarmento, B.; Pintado, M. Development and characterization of chitosan microparticles-in-films for buccal delivery of bioactive peptides. Pharmaceuticals 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shen, Y.; Ao, P.; Dai, L.; Liu, Z.; Zhou, C. The improvement of hemostatic and wound healing property of chitosan by halloysite nanotubes. RSC Adv. 2014, 4, 23540–23553. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef]




| Microparticles | Particle Size (µm) | Water Absorption (%) | Loading Capacity (mg/g) | |
|---|---|---|---|---|
| Swollen | Dry | |||
| M(WPI 4% + ALG 0.5%) | 2183 ± 53 bc | 986 ± 32 a | 784 ± 45 a | 262 ± 7 b |
| M(WPI 4% + ALG 1%) | 2245 ± 43 ab | 993 ± 39 a | 669 ± 57 a | 169 ± 5 d |
| M(WPI 5% + ALG 0.5%) | 2339 ± 56 a | 982 ± 16 a | 829 ± 43 a | 293 ± 6 a |
| M(WPI 5% + ALG 1%) | 2201 ± 61 abc | 990 ± 30 a | 723 ± 38 a | 234 ± 9 c |
| Films | Young’s Modulus (MPa) | Tensile Strength (N) | Elongation at Break (%) | |||
|---|---|---|---|---|---|---|
| Dry | Soaked | Dry | Soaked | Dry | Soaked | |
| GEL | 497 ± 78 abc | 1.5 ± 0.21 a | 29 ± 2 abc | 0.34 ± 0.04 b | 17 ± 2 b | 63 ± 3 abc |
| GEL + M(WPI 4% + ALG 0.5%) | 474 ± 62 bc | 1.4 ± 0.52 a | 23 ± 3 bc | 0.33 ± 0.05 b | 21 ± 1 a | 70 ± 3 a |
| GEL/WPI | 669 ± 83 a | 1.1 ± 0.07 a | 33 ± 4 a | 0.54 ± 0.05 a | 4 ± 1 c | 57 ± 4 bc |
| GEL/WPI + M(WPI 4% + ALG 0.5%) | 518 ± 43 ab | 0.83 ± 0.05 a | 30 ± 3 ab | 0.42 ± 0.02 b | 7 ± 1 c | 68 ± 7 ab |
| Sample | Contact Angle (°) | Surface Free Energy (γs) (mJ/m2) | Dispersive and Polar Components (mJ/m2) | ||
|---|---|---|---|---|---|
| G | D | γsd | γsp | ||
| GEL | 75.8 ± 0.4 a | 52.8 ± 1.4 a | 33.2 | 28.0 | 5.2 |
| GEL/WPI | 71.4 ± 0.4 b | 50.4 ± 0.8 a | 35.3 | 28.5 | 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachowiak-Trojanowska, N.; Walendziak, W.; Douglas, T.E.L.; Kozlowska, J. Whey Protein Isolate as a Substrate to Design Calendula officinalis Flower Extract Controlled-Release Materials. Int. J. Mol. Sci. 2024, 25, 5325. https://doi.org/10.3390/ijms25105325
Stachowiak-Trojanowska N, Walendziak W, Douglas TEL, Kozlowska J. Whey Protein Isolate as a Substrate to Design Calendula officinalis Flower Extract Controlled-Release Materials. International Journal of Molecular Sciences. 2024; 25(10):5325. https://doi.org/10.3390/ijms25105325
Chicago/Turabian StyleStachowiak-Trojanowska, Natalia, Weronika Walendziak, Timothy E. L. Douglas, and Justyna Kozlowska. 2024. "Whey Protein Isolate as a Substrate to Design Calendula officinalis Flower Extract Controlled-Release Materials" International Journal of Molecular Sciences 25, no. 10: 5325. https://doi.org/10.3390/ijms25105325






