Mitigation of Breast Cancer Cells’ Invasiveness via Down Regulation of ETV7, Hippo, and PI3K/mTOR Pathways by Vitamin D3 Gold-Nanoparticles
Abstract
1. Introduction
1.1. Breast Cancer
1.2. PI3K/AKT/mTOR
1.3. ETV7
1.4. Hippo
1.5. Vitamin D3-Gold Nanoparticles
1.6. Objective
2. Results
2.1. Characterization of VD3-GNPs
2.1.1. TEM Images
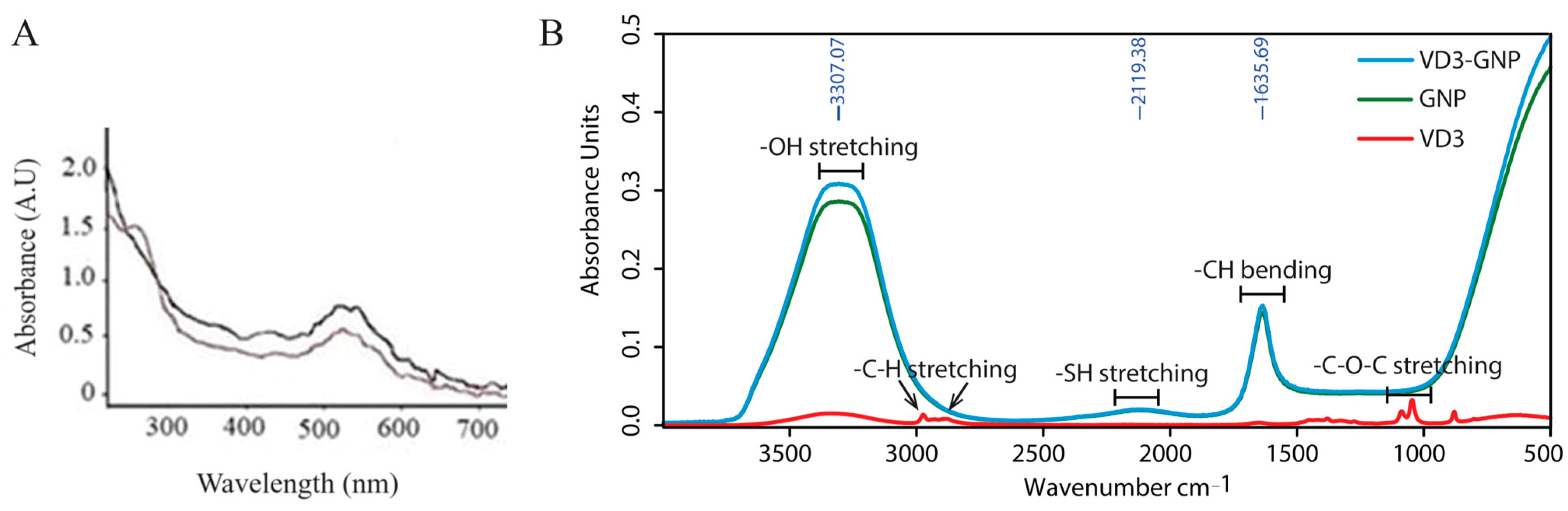
2.1.2. UV–Visible Spectrum
2.1.3. Evaluation of Vitamin D3 Coupling to Gold Nanoparticle Surfaces Using FT-IR
2.1.4. DLS
2.1.5. Zeta Potential Measurement
2.1.6. HPLC Quantitation of VD3 in VD3-GNPs
2.2. Cell-Based Studies
2.2.1. MTT Assay after VD3-GNP Treatment
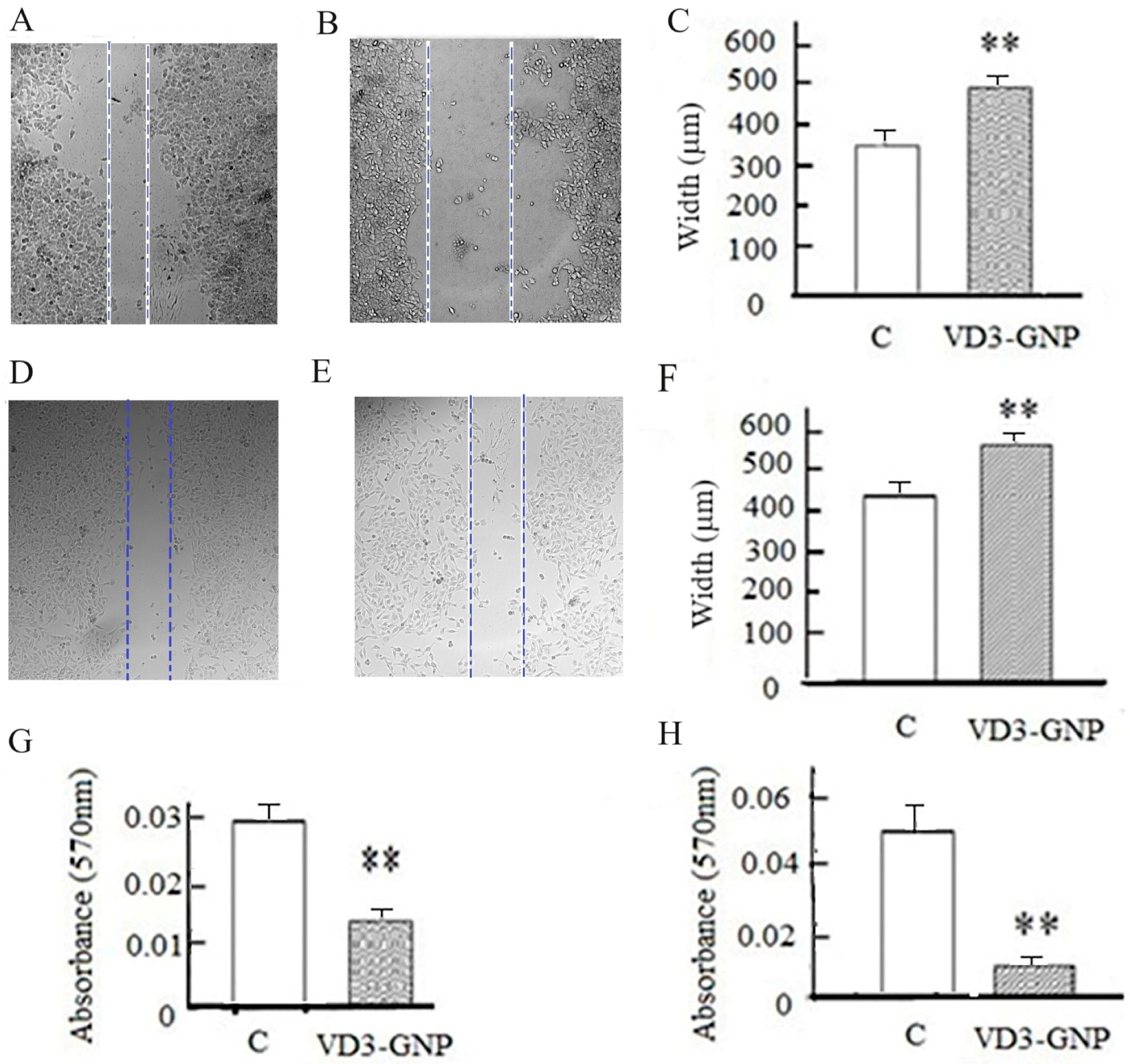
2.2.2. Cell Invasion Assay
2.2.3. Wound Healing Assay
2.2.4. Cancer Cell Static Adhesion Assay
2.2.5. Western Blots of PI3K/AKT/mTOR
2.2.6. Hippo Pathway Western Blots
2.3. PPI Network (STRING Software 12.0) for the Pathway Proteins (PI3K/AKT/MTOR and Hippo)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of GNPs and VD3-GNPs
4.3. VD3-GNPs Characterization Using Biophysical Methods
4.3.1. TEM Microscopy
4.3.2. UV–Visible (UV–Vis) Spectroscopy
4.3.3. FT-IR Spectrum Measurement
4.3.4. Particle Size Measurement Using DLS
4.3.5. Zeta Potential
4.3.6. Quantitation of the Vitamin D3 in VD3-GNPs Using HPLC
4.4. Cell-Based Studies
4.4.1. Breast Cancer Cell Lines’ Maintenance and MTT Assay after VD3-GNP Treatment
4.4.2. Cancer Cell Invasion
4.4.3. Wound Healing Assay
4.4.4. Cancer Cell Static Adhesion Assay
4.4.5. Protein Extraction for Western Blotting
4.4.6. Western Blots
4.5. PPI Network (STRING Software 12.0) for the Pathway Proteins (PI3K/AKT/MTOR and Hippo)
4.5.1. Construction of PPI Networks (STRING)
4.5.2. Network Details
4.6. Statistical Analysis
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronym List
| BRCA | Breast Cancer gene |
| TNBC | Triple-negative breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| VD3-GNPs | Vitamin D3 gold nanoparticles |
| VD3-PEG | Vitamin D3 PEG |
| VD | Vitamin D |
| GNP | Gold nanoparticle |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Ak strain transforming, Threonine-protein kinase B (PKB) |
| p-AKT | Phospho AKT |
| mTOR | Mammalian target of rapamycin |
| AGC-cAMP | cAMP-dependent, cGMP-dependent and protein kinase C |
| cGMP | dependent and protein kinase C |
| ETV7 | ETS variant transcription factor 7 |
| ETS | Erythroblast Transformation Specific |
| YAP | Yes-associated protein |
| TAZ | Transcriptional co-activator with PDZ-binding motif |
| TEAD | (TEA/ATTS domain) family of transcription factors |
| MST | Mammalian sterile 20-like kinase (MST) |
| miR | MicroRNA |
| LATS1 | Large tumor suppressor kinase 1 |
| SLC7A5 | Solute carrier family 7 member 7 |
| SLC3A2 | Solute carrier family 3 member 2 |
| VD | Vitamin D |
| VDT | Vitamin D toxicity |
| UV–VIS | Ultraviolet–visible |
| TEM | Transmission Electron Microscopy |
| FT-IR | Fourier transform infrared spectroscopy |
| HPLC | High-performance liquid chromatography |
| MTT | 2,5-diphenyl-2H-tetrazolium bromide |
| UALCAN | University of Alabama at Birmingham Cancer data analysis Portal |
| PPI | Protein–protein interactions |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| DLS | Dynamic light Scattering |
| PDI | Polydispersity Index |
| NOS3 | Nitric oxide synthase 3 |
| KRAS-RAS | Kirsten rat sarcoma virus-Rat sarcoma |
| IRS1 | Insulin receptor substrate-1 |
| HRAS | Harvey Rat sarcoma virus |
| MAPKAP1 | MAPK-associated protein 1 |
| MAPK | Mitogen-activated protein kinase |
| ULK1 | Unc-51-like autophagy activating kinase 1 |
| ETS | Erythroblast Transformation Specific |
| KIR3DL1, KIR3DL2 | Killer Cell Immunoglobulin-Like Receptor |
| ETV6 | ETS variant transcription factor 6 |
| ACAD9 | Acyl-CoA dehydrogenase family member 9 |
| ZNF | Zinc-finger proteins |
| TIM | Timeless |
| EGFR | Epidermal growth factor receptor |
| ERBB | Erb-b2 receptor tyrosine kinase |
| DEPTOR | DEP domain containing MTOR interacting protein |
| RICTOR | RPTOR independent companion of MTOR complex |
| EMT | Epithelial–mesenchymal transition |
| ζ | Zeta potential |
| TNF-α | tumor necrosis factor-α |
| DDIT | DNA damage inducible transcript |
| REDD1 | Regulated in DNA Damage and Development 1 |
| CTGF | Cellular communication network factor 2 |
| CYR61 | Cysteine-rich angiogenic inducer 61 |
| MYC | Myelocytomatosis oncogene |
| AXL | AXL receptor tyrosine kinase |
| BIRC5 | Baculoviral IAP repeat containing 5 |
| CCND1 | Cyclin D1 |
| nM | Nanomolar |
| Ser | Serine |
| TCGA | The Cancer Genome Atlas |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | Fetal bovine serum |
| PS | Penicillin and streptomycin |
| DPBS | Dulbecco’s phosphate-buffered saline |
| ATCC | American Tissue Culture Collection |
| RO | Reverse Osmosis |
| DMSO | Dimethyl Sulfoxide |
| ATR | Attenuated total reflection |
| IR | Infrared |
| TBS-T | Tris Buffer Saline–Tween |
| PVDF | Polyvinylidene difluoride |
| BSA | Bovine serum Albumin |
| DSHB | Developmental Studies Hybridoma Bank |
| HRP | Horseradish peroxidase |
| SD | Standard deviation |
| SEM | Standard error of mean |
| ANOVA | Analysis of variance |
References
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Turkoz, F.P.; Solak, M.; Petekkaya, I.; Keskin, O.; Kertmen, N.; Sarici, F.; Arik, Z.; Babacan, T.; Ozisik, Y.; Altundag, K. Association between common risk factors and molecular subtypes in breast cancer patients. Breast 2013, 22, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Black Women Face Three-Fold Increased Risk of Triple Negative Breast Cancers. Penn Medicine, 20 September 2021.
- Eiger, D.; Agostinetto, E.; Saúde-Conde, R.; de Azambuja, E. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers 2021, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Alayev, A.; Holz, M.K. mTOR signaling for biological control and cancer. J. Cell Physiol. 2013, 228, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Raybuck, A.L.; Cho, S.H.; Li, J.; Rogers, M.C.; Lee, K.; Williams, C.L.; Shlomchik, M.; Thomas, J.W.; Chen, J.; Williams, J.V. B Cell-Intrinsic mTORC1 Promotes Germinal Center-Defining Transcription Factor Gene Expression, Somatic Hypermutation, and Memory B Cell Generation in Humoral Immunity. J. Immunol. 2018, 200, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Mcauliffe, F.; Mericbernstam, F.; Mills, G.B.; Gonzalezangulo, A.M. Deciphering the role of PI3K/Akt/MTOR pathway in breast cancer biology and pathogenesis. Clin. Breast Cancer 2010, 10, S59–S65. [Google Scholar] [CrossRef]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell. 2010, 40, 310–322. [Google Scholar] [CrossRef]
- Hare, S.H.; Harvey, A.J. mTOR function and therapeutic targeting in breast cancer. Am. J. Cancer Res. 2017, 7, 383–404. [Google Scholar] [PubMed]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Meškytė, E.M.; Pezzè, L.; Bartolomei, L.; Forcato, M.; Bocci, I.A.; Bertalot, G.; Barbareschi, M.; Oliveira-Ferrer, L.; Bisio, A.; Bicciato, S.; et al. ETV7 reduces inflammatory responses in breast cancer cells by repressing TNFR1/NF-kappaB axis. Cell Death Dis. 2022, 14, 263. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Zheng, S. Comprehensive Analysis Identified ETV7 as a Potential Prognostic Biomarker in Bladder Cancer. Biomed. Res. Int. 2021, 2021, 8530186. [Google Scholar] [CrossRef] [PubMed]
- Carella, C.; Potter, M.; Bonten, J.; Rehg, J.E.; Neale, G.; Grosveld, G.C. The ETS factor TEL2 is a hematopoietic oncoprotein. Blood 2006, 107, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.C.; Fuller, C.; Hogg, T.L.; Dalton, J.; Finkelstein, D.; Lau, C.C.; Chintagumpala, M.; Adesina, A.; Ashley, D.M.; Kellie, S.J.; et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J. Clin. Oncol. 2006, 24, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Piggin, C.L.; Roden, D.L.; Gallego-Ortega, D.; Lee, H.J.; Oakes, S.R.; Ormandy, C.J. ELF5 isoform expression is tissue-specific and significantly altered in cancer. Breast Cancer Res. 2016, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Pezzè, L.; Meškytė, E.M.; Forcato, M.; Pontalti, S.; Badowska, K.A.; Rizzotto, D.; Skvortsova, I.-I.; Bicciato, S.; Ciribilli, Y. ETV7 regulates breast cancer stem-like cell features by repressing IFN-response genes. Cell Death Dis. 2021, 12, 742. [Google Scholar] [CrossRef]
- Harwood, F.C.; Geltink, R.I.K.; O’hara, B.P.; Cardone, M.; Janke, L.; Finkelstein, D.; Entin, I.; Paul, L.; Houghton, P.J.; Grosveld, G.C. ETV7 is an essential component of a rapamycin-insensitive mTOR complex in cancer. Sci. Adv. 2018, 4, eaar3938. [Google Scholar] [CrossRef]
- Snigdha, K.; Gangwani, K.S.; Lapalikar, G.V.; Singh, A.; Kango-Singh, M. Hippo Signaling in Cancer: Lessons from Drosophila Models. Front. Cell Dev. Biol. 2019, 7, 85. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.D.; Kim, D.H.; Jeong, H.; Park, H.W. Regulation of TEAD Transcription Factors in Cancer Biology. Cells 2019, 8, 600. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zeng, C.; Ye, S.; Dai, X.; He, Q.; Yang, B.; Zhu, H. Yes-associated protein (YAP) and transcriptional coactivator with a PDZ-binding motif (TAZ): A nexus between hypoxia and cancer. Acta Pharm. Sin. B 2020, 10, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Tumaneng, K.A. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 2012, 12, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, T.V.; Goncharov, D.A.; Pena, A.; Kelly, N.; Vanderpool, R.; Baust, J.; Kobir, A.; Shufesky, W.; Mora, A.L.; Morelli, A.E.; et al. HIPPO-Integrin-linked Kinase Cross-Talk Controls Self-Sustaining Proliferation and Survival in Pulmonary Hypertension. Am. J. Respir. Crit. 2016, 194, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.G.; Ng, Y.L.; Lam, W.L.; Plouffe, S.W.; Guan, K.L. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015, 25, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, N. Vitamin D Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557876/ (accessed on 28 April 2022).
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Bouillon, R. Vitamin D and Extra skeletal Health. UpToDate. 2015. Available online: www.uptodate.com (accessed on 26 January 2024).
- Stoll, F.; Akladios, C.Y.; Mathelin, C. Vitamin D and breast cancer: Is there a link? Gynecol. Obstet. Fertil. 2013, 41, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Šimoliūnas, E.; Rinkūnaitė, I.; Bukelskienė, Ž.; Bukelskienė, V. Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model. Medicina 2019, 55, 265. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity-A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; Hewison, M. Vitamin D A new player in the world of mTOR signaling. Cell Cycle 2011, 10, 1888–1889. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Chojnacki, J.; Szczepanska, J.; Fila, M.; Chojnacki, C. Vitamin D in Triple-Negative and BRCA1-Deficient Breast Cancer-Implications for Pathogenesis and Therapy. Int. J. Mol. Sci. 2020, 21, 3670. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. The pharmacology of vitamin D, including fortification strategies. In Vitamin D; Feldman, D., Pike, J.W., Glorieux, F.H., Eds.; Elsevier Academy Press: New York, NY, USA, 2005; pp. 995–1015. [Google Scholar]
- Audo, I.; Darjatmoko, S.R.; Schlamp, C.L.; Lokken, J.M.; Lindstrom, M.J.; Albert, D.M.; Nickells, R.W. Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Tyner, A.L. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol. Cancer Ther. 2002, 1, 639–649. [Google Scholar] [PubMed]
- Maruyama, R.; Toyota, M.; Suzuki, H.; Sasaki, Y.; Aoki, F.; Shinomura, Y.; Imai, K.; Tokino, T. The functional relation of vitamin D receptor and p53 in cancer cells. Fifth AACR International Conference on Frontiers in Cancer Prevention Research. Cancer Epidemiol. Biomark. Prev. 2006, 15, 12–15. [Google Scholar]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Ramalho, M.; Coelho, M.A.N.; Pereira, M.C. Nanoparticles for Delivery of Vitamin D: Challenges and Opportunities. In A Critical Evaluation of Vitamin D—Clinical Overview; Gowder, S., Ed.; Intechopen Publishing: London, UK, 2017. [Google Scholar]
- Akinc, A.; Battaglia, G. Exploiting endocytosis for nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef]
- Van De Ven, A.L.; Kim, P.; Fakhoury, J.R.; Adriani, G.; Schmulen, J.; Moloney, P.; Hussain, F.; Ferrari, M.; Liu, X.; Yun, S.H. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J. Control. Release 2012, 158, 148–155. [Google Scholar] [CrossRef]
- Iversen, T.G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Nah, H.; Lee, D.; Heo, M.; Lee, J.S.; Lee, S.J.; Heo, D.N.; Seong, J.; Lim, H.-N.; Lee, Y.-H.; Moon, H.-J.; et al. Vitamin D-conjugated gold nanoparticles as functional carriers to enhancing osteogenic differentiation. Sci. Technol. Adv. Mater. 2019, 20, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert. Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef]
- Wang, W.; DingQing, X.; Wang, X.J.; Wang, L.; Lou, X. Zeta-potential data reliability of gold nanoparticle biomolecular conjugates and its application in sensitive quantification of surface absorbed protein. Colloids Surf. B Biointerfaces 2016, 148, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Ottemann, B.M.; Ben Thomas, M.; Mukadam, I.; Nigam, S.; McMillan, J.; Gorantla, S.; Bronich, T.K.; Edagwa, B.; Gendelman, H.E. Neurotheranostics as personalized medicines. Adv. Drug Deliv. Rev. 2019, 148, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Methods in Molecular Biology (Methods and Protocols); McNeil, S., Ed.; Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; p. 697. [Google Scholar]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya SA, H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 8, 649–658. [Google Scholar] [CrossRef]
- Basu, S.; Totty, N.F.; Irwin, M.S.; Sudol, M.; Downward, J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 2003, 11, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, D.; Lorenzoni, P.; Burger, M.M. The role of both specific cellular adhesion and growth promotion in liver colonization by F9 embryonal carcinoma cells. Int. J. Cancer 1991, 48, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Veeresh PK, M.; Basavaraju, C.G.; Dallavalasa, S.; Anantharaju, P.G.; Natraj, S.M.; Sukocheva, O.A.; Madhunapantula, S.V. Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis. Cancers 2023, 15, 4833. [Google Scholar] [CrossRef]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Ganesan, S. In Vitro Cytotoxicity Assay on Gold Nanoparticles with Different Stabilizing Agents. J. Nanomater. 2012, 2012, 734398. [Google Scholar] [CrossRef]
- Santos, J.M.; Khan, Z.S.; Munir, M.T.; Tarafdar, K.; Rahman, S.M.; Hussain, F. Vitamin D3 decreases glycolysis and invasiveness, and increases cellular stiffness in breast cancer cells. J. Nutr. Biochem. 2018, 53, 111–120. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Dandagi, P.M.; Rath, S.P.; Gadad, A.P.; Mastiholimath, V.S. Taste masked quinine sulphate loaded solid lipid nanoparticles for flexible pediatric dosing. Indian J. Pharm. Educ. Res. 2014, 48, 93–99. [Google Scholar] [CrossRef]
- Desai, S.K.; Bera, S.; Mondal, D. Gelatin Nanoparticles as Carrier for Effective Antituberculosis Drug Delivery in Combination Therapy. BioNanoScience 2024, 1–10. [Google Scholar] [CrossRef]
- Temova, Z.; Roškar, R. Stability-Indicating HPLC–UV Method for Vitamin D3 Determination in Solutions, Nutritional Supplements and Pharmaceuticals. J. Chromatogr. Sci. 2016, 54, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Theodossiou, T.A.; Ali, M.; Grigalavicius, M.; Grallert, B.; Dillard, P.; Schink, K.O.; Olsen, C.E.; Wälchli, S.; Inderberg, E.M.; Kubin, A.; et al. Simultaneous defeat of MCF7 and MDA-MB-231 resistances by a hypericin PDT–tamoxifen hybrid therapy. NPJ Breast Cancer 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Lunetti, P.; Giacomo, M.D.; Vergara, D.; Domenico, S.; De Maffia, M.; Zara, V.; Capobianco, L.L.; Ferramosca, A. Metabolic reprogramming in breast cancer results in distinct mitochondrial bioenergetics between luminal and basal subtypes. FEBS J. 2019, 286, 688–709. [Google Scholar] [CrossRef] [PubMed]
- Gest, C.; Joimel, U.; Huang, L.; Pritchard, L.-L.; Petit, A.; Dulong, C.; Buquet, C.; Hu, C.-Q.; Mirshahi, P.; Laurent, M.; et al. Rac3 induces a molecular pathway triggering breast cancer cell aggressiveness: Differences in MDA-MB-231 and MCF-7 breast cancer cell lines. BMC Cancer 2013, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Jaiswal, P.; Verma, R.; Sahu, K.; Majumder, S.K.; Chakraborty, S.; Jha, H.C.; Singh Parmar, H.S. Therapeutic influence of simvastatin on MCF-7 and MDA-MB-231 breast cancer cells via mitochondrial depletion and improvement in chemosensitivity of cytotoxic drugs. Adv. Cancer Biol.-Metastasis 2023, 9, 2667–3940. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, L.; Duan, Y.; Zhang, M.; Wang, G.; Zhang, J.; Zhao, Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014, 14, 126. [Google Scholar] [CrossRef]










| Nanoparticles | DLS (nm) | PDI |
|---|---|---|
| VD3-GNP | 34.6 ± 0.6 | 1.622 |
| GNP | 27.05 ± 1.8 | 1.505 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, M.; Hussain, F. Mitigation of Breast Cancer Cells’ Invasiveness via Down Regulation of ETV7, Hippo, and PI3K/mTOR Pathways by Vitamin D3 Gold-Nanoparticles. Int. J. Mol. Sci. 2024, 25, 5348. https://doi.org/10.3390/ijms25105348
Roy M, Hussain F. Mitigation of Breast Cancer Cells’ Invasiveness via Down Regulation of ETV7, Hippo, and PI3K/mTOR Pathways by Vitamin D3 Gold-Nanoparticles. International Journal of Molecular Sciences. 2024; 25(10):5348. https://doi.org/10.3390/ijms25105348
Chicago/Turabian StyleRoy, Moumita, and Fazle Hussain. 2024. "Mitigation of Breast Cancer Cells’ Invasiveness via Down Regulation of ETV7, Hippo, and PI3K/mTOR Pathways by Vitamin D3 Gold-Nanoparticles" International Journal of Molecular Sciences 25, no. 10: 5348. https://doi.org/10.3390/ijms25105348
APA StyleRoy, M., & Hussain, F. (2024). Mitigation of Breast Cancer Cells’ Invasiveness via Down Regulation of ETV7, Hippo, and PI3K/mTOR Pathways by Vitamin D3 Gold-Nanoparticles. International Journal of Molecular Sciences, 25(10), 5348. https://doi.org/10.3390/ijms25105348





