Immunohistochemical Expression Levels of Epidermal Growth Factor Receptor, Cyclooxygenase-2, and Ki-67 in Canine Cutaneous Squamous Cell Carcinomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Histopathology
2.3. Immunohistochemistry
2.4. Quantification of Immunostaining
2.4.1. Quantification of EGFR Immunostaining
2.4.2. Quantification of Proliferation Index (Ki-67 Immunostaining)
2.4.3. Quantification of Cox-2 Immunostaining
2.5. Statistical Evaluation
3. Results
3.1. Histopathological Evaluation
3.2. Immunohistochemical Evaluation
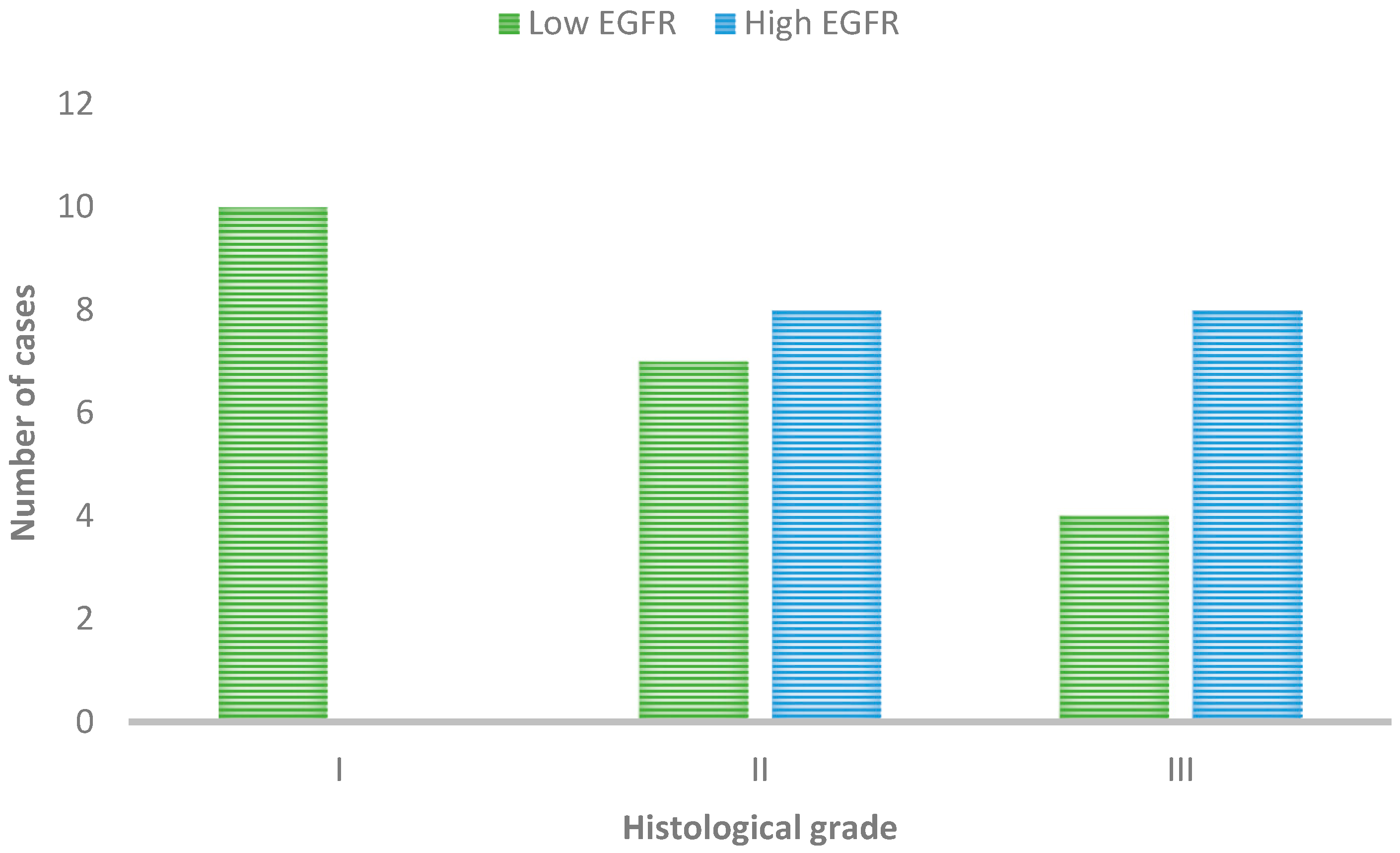
3.2.1. Immunohistochemical Expression of EGFR
3.2.2. Proliferation Index Detected by Ki-67
3.2.3. Immunohistochemical Expression of Cox-2
3.2.4. Associations between the Proliferation Index, the Immunoexpression of EGFR, and Cox-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cocuz, I.G.; Cocuz, M.E.; Repanovici, A.; Sabău, A.-H.; Niculescu, R.; Tinca, A.-C.; Vunvulea, V.; Budin, C.E.; Szoke, A.R.; Popelea, M.C.; et al. Scientific Research Directions on the Histopathology and Immunohistochemistry of the Cutaneous Squamous Cell Carcinoma: A Scientometric Study. Medicina 2022, 58, 1449. [Google Scholar] [CrossRef]
- Sánchez-Danés, A.; Blanpain, C. Deciphering the Cells of Origin of Squamous Cell Carcinomas. Nat. Rev. Cancer 2018, 18, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Gauthier, M.-A.; Malik, A.; Fotiadou, I.; Ostrovski, M.; Dervovic, D.; Ghadban, L.; Tsai, R.; Gish, G.; Loganathan, S.K.; et al. The NOTCH-RIPK4-IRF6-ELOVL4 Axis Suppresses Squamous Cell Carcinoma. Cancers 2023, 15, 737. [Google Scholar] [CrossRef] [PubMed]
- Azin, M.; Demehri, S. Innate Lymphoid Cells: New Targets for Cutaneous Squamous Cell Carcinoma Immunotherapy. J. Investig. Dermatol. 2021, 141, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.K.; Chambers, J.K.; Ong, S.M.; Nakayama, H.; Uchida, K. Hierarchical Cluster Analysis of Cytokeratins and Stem Cell Expression Profiles of Canine Cutaneous Epithelial Tumors. Vet. Pathol. 2018, 55, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Parry, A.T.; Stidworthy, M.F.; Dobson, J.M.; White, R.A.S. Squamous Cell Carcinoma of the Nasal Planum in 17 Dogs. Vet. Rec. 2000, 147, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.R.; Rocha, L.F.; Vargas, T.H.M.; Pulz, L.H.; Huete, G.C.; Cadrobbi, K.G.; Pires, C.G.; Sanches, D.S.; Mota, E.F.F.; Strefezzi, R.F. Relationship of Galectin-3 Expression in Canine Cutaneous Squamous Cell Carcinomas with Histopathological Grading and Proliferation Indices. J. Comp. Pathol. 2020, 178, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Zheng, Z.; Kim, K.-Y.; Xu, X.; Pei, M.; Oh, B.; Kim, S.K.; Chung, K.Y.; Roh, M.R. A Nomogram Combining Clinical Factors and Biomarkers for Predicting the Recurrence of High-Risk Cutaneous Squamous Cell Carcinoma. BMC Cancer 2022, 22, 1126. [Google Scholar] [CrossRef] [PubMed]
- Ortloff, A.; Bustamante, F.A.; Molina, L.; Ojeda, J.; Figueroa, C.D.; Ehrenfeld, P. Kallikrein-Related Peptidase 5 (KLK5) Expression and Distribution in Canine Cutaneous Squamous Cell Carcinoma. J. Comp. Pathol. 2020, 174, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sanz Ressel, B.L.; Massone, A.R.; Barbeito, C.G. Immunohistochemical Expression of Selected Phosphoproteins of the mTOR Signalling Pathway in Canine Cutaneous Squamous Cell Carcinoma. Vet. J. 2019, 245, 41–48. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Thomson, J.; Harwood, C.; Leigh, I. The Role of the Immune System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2009. [Google Scholar] [CrossRef]
- Riihilä, P.; Nissinen, L.; Knuutila, J.; Rahmati Nezhad, P.; Viiklepp, K.; Kähäri, V.-M. Complement System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 3550. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Scalvenzi, M. New Emerging Treatment Options for Advanced Basal Cell Carcinoma and Squamous Cell Carcinoma. Adv. Ther. 2022, 39, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Yesantharao, P.; Wang, W.; Ioannidis, N.M.; Demehri, S.; Whittemore, A.S.; Asgari, M.M. Cutaneous Squamous Cell Cancer (cSCC) Risk and the Human Leukocyte Antigen (HLA) System. Hum. Immunol. 2017, 78, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Bobin, C.; Ingrand, P.; Dréno, B.; Rio, E.; Malard, O.; Espitalier, F. Prognostic Factors for Parotid Metastasis of Cutaneous Squamous Cell Carcinoma of the Head and Neck. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.L.; Harwood, C.A.; Crook, T.; Cronin, J.G.; Kelsell, D.P.; Proby, C.M. p16INK4a and p14ARF Tumor Suppressor Genes Are Commonly Inactivated in Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2004, 122, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P.; Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; et al. Guidelines of Care for the Management of Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Luci, C.; Bihl, F.; Bourdely, P.; Khou, S.; Popa, A.; Meghraoui-Kheddar, A.; Vermeulen, O.; Elaldi, R.; Poissonnet, G.; Sudaka, A.; et al. Cutaneous Squamous Cell Carcinoma Development Is Associated with a Temporal Infiltration of ILC1 and NK Cells with Immune Dysfunctions. J. Investig. Dermatol. 2021, 141, 2369–2379. [Google Scholar] [CrossRef]
- Tsang, D.A.; Tam, S.Y.C.; Oh, C.C. Molecular Alterations in Cutaneous Squamous Cell Carcinoma in Immunocompetent and Immunosuppressed Hosts—A Systematic Review. Cancers 2023, 15, 1832. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9.23.1–9.23.17. [Google Scholar] [CrossRef]
- Purkayastha, K.; Dhar, R.; Pethusamy, K.; Srivastava, T.; Shankar, A.; Rath, G.; Karmakar, S. The Issues and Challenges with Cancer Biomarkers. J. Can. Res. Ther. 2023, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Adil, S.; Paracha, R.Z.; Tariq, S.; Nisar, M.; Ijaz, S.; Siddiqa, A.; Hussain, Z.; Amir, A. A Computational Systems Analyses to Identify Biomarkers and Mechanistic Link in Psoriasis and Cutaneous Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 662528. [Google Scholar] [CrossRef]
- Ganguly, A.; Frank, D.; Kumar, N.; Cheng, Y.-C.; Chu, E. Cancer Biomarkers for Integrative Oncology. Curr. Oncol. Rep. 2019, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.; Farina, L.; Petti, M. Networks as Biomarkers: Uses and Purposes. Genes 2023, 14, 429. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, C.; Giampaolo, T. EGFR Antagonists in Cancer Treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar]
- Simond, A.M.; Muller, W.J. In Vivo Modeling of the EGFR Family in Breast Cancer Progression and Therapeutic Approaches. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 147, pp. 189–228. ISBN 978-0-12-820142-8. [Google Scholar]
- Roskoski, R. Small Molecule Inhibitors Targeting the EGFR/ErbB Family of Protein-Tyrosine Kinases in Human Cancers. Pharmacol. Res. 2019, 139, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Zhang, C.; Ma, Z. Epidermal Growth Factor Receptor (EGFR): A Rising Star in the Era of Precision Medicine of Lung Cancer. Oncotarget 2017, 8, 50209–50220. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Moghimi, S.; Toolabi, M.; Foroumadi, A. Pyrimidine-Based EGFR TK Inhibitors in Targeted Cancer Therapy. Eur. J. Med. Chem. 2021, 221, 113523. [Google Scholar] [CrossRef]
- Cui, J.; Hu, Y.-F.; Feng, X.-M.; Tian, T.; Guo, Y.-H.; Ma, J.-W.; Nan, K.-J.; Zhang, H.-Y. EGFR Inhibitors and Autophagy in Cancer Treatment. Tumor Biol. 2014, 35, 11701–11709. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, S.; Zhao, W.; Qin, S.; Chu, Q.; Wu, K. EGFR-TKIs Resistance via EGFR-Independent Signaling Pathways. Mol. Cancer 2018, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Quesnelle, K.M.; Boehm, A.L.; Grandis, J.R. STAT-mediated EGFR Signaling in Cancer. J Cell. Biochem. 2007, 102, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Hugo, H.J.; Saunders, C.; Ramsay, R.G.; Thompson, E.W. New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis. J. Mammary Gland Biol. Neoplasia 2015, 20, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Sabichi, A.L.; Lippman, S.M. COX-2 Inhibitors and Other Nonsteroidal Anti-Inflammatory Drugs in Genitourinary Cancer. Semin. Oncol. 2004, 31, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Mason, K.A.; Milas, L. Cyclo-Oxygenase-2 and Its Inhibition in Cancer: Is There a Role? Drugs 2007, 67, 821–845. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Kundu, J.K. Signal Transduction Network Leading to COX-2 Induction: A Road Map in Search of Cancer Chemopreventives. Arch. Pharm. Res. 2005, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Epstein, J.B. Carcinogenesis and Cyclooxygenase: The Potential Role of COX-2 Inhibition in Upper Aerodigestive Tract Cancer. Oral Oncol. 2003, 39, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 Protein as a Tumour Proliferation Marker. Clin. Chim. Acta 2019, 491, 39–45. [Google Scholar] [CrossRef]
- Graefe, C.; Eichhorn, L.; Wurst, P.; Kleiner, J.; Heine, A.; Panetas, I.; Abdulla, Z.; Hoeft, A.; Frede, S.; Kurts, C.; et al. Optimized Ki-67 Staining in Murine Cells: A Tool to Determine Cell Proliferation. Mol. Biol. Rep. 2019, 46, 4631–4643. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Camasses, A.; Parisis, N.; Nicolas, E.; Llères, D.; Gerbe, F.; Prieto, S.; Krasinska, L.; David, A.; et al. The Cell Proliferation Antigen Ki-67 Organises Heterochromatin. eLife 2016, 5, e13722. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 Is a Promising Molecular Target in the Diagnosis of Cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Endl, E.; Gerdes, J. The Ki-67 Protein: Fascinating Forms and an Unknown Function. Exp. Cell Res. 2000, 257, 231–237. [Google Scholar] [CrossRef]
- Remnant, L.; Kochanova, N.Y.; Reid, C.; Cisneros-Soberanis, F.; Earnshaw, W.C. The Intrinsically Disorderly Story of Ki-67. Open Biol. 2021, 11, 210120. [Google Scholar] [CrossRef] [PubMed]
- Anneroth, G.; Hansen, L.S. A Methodologic Study of Histologic Classification and Grading of Malignancy in Oral Squamous Cell Carcinoma. Eur. J. Oral Sci. 1984, 92, 448–468. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, K.A.; Ferreira, E.N.; Lima, A.E.; Bosco, Y.; Silva, L.P.; Barros, A.L.B.; Bertagnolli, A.C.; Cassali, G.D. Relationship between the Expression of Versican and EGFR, HER-2, HER-3 and CD44 in Matrix-Producing Tumours in the Canine Mammary Gland. Histol. Histopathol. 2016, 31, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-Y.; Zheng, H.-H.; Yu, C.; Ye, Y.; Du, C.-T.; Xie, G.-H. Research Progress of Good Markers for Canine Mammary Carcinoma. Mol. Biol. Rep. 2023, 50, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Millanta, F.; Asproni, P.; Canale, A.; Citi, S.; Poli, A. COX-2, mPGES-1 and EP2 Receptor Immunohistochemical Expression in Canine and Feline Malignant Mammary Tumours. Vet. Comp. Oncol. 2016, 14, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ishino, H.; Hara, Y.; Takekoshi, S.; Teshima, T.; Teramoto, A.; Osamura, R.Y.; Tagawa, M. Ki-67 and Minichromosome Maintenance-7 (MCM7) Expression in Canine Pituitary Corticotroph Adenomas. Domest. Anim. Endocrinol. 2011, 41, 207–213. [Google Scholar] [CrossRef]
- Ch’ng, S.; Low, I.; Ng, D.; Brasch, H.; Sullivan, M.; Davis, P.; Tan, S.T. Epidermal Growth Factor Receptor: A Novel Biomarker for Aggressive Head and Neck Cutaneous Squamous Cell Carcinoma. Hum. Pathol. 2008, 39, 344–349. [Google Scholar] [CrossRef]
- Pires, I.; Alves, A.; Queiroga, F.L.; Silva, F.; Lopes, C. Regression of Canine Cutaneous Histiocytoma: Reduced Proliferation or Increased Apoptosis? Anticancer. Res. 2013, 33, 1397–1400. [Google Scholar] [PubMed]
- Queiroga, F.L.; Pires, I.; Lobo, L.; Lopes, C.S. The Role of Cox-2 Expression in the Prognosis of Dogs with Malignant Mammary Tumours. Res. Vet. Sci. 2010, 88, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.M.; Peñafiel-Verdú, C.; Vilafranca, M.; Ramírez, G.; Méndez-Gallego, M.; Buendía, A.J.; Sánchez, J. Cyclooxygenase-2 Expression Is Related With Localization, Proliferation, and Overall Survival in Canine Melanocytic Neoplasms. Vet. Pathol. 2011, 48, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Uber, R.; Ingle, T.; Li, C.; Liu, Z.; Thakkar, S.; Ning, B.; Wu, L.; Yang, J.; Harris, S.; et al. Study of Pharmacogenomic Information in FDA-Approved Drug Labeling to Facilitate Application of Precision Medicine. Drug Discov. Today 2020, 25, 813–820. [Google Scholar] [CrossRef]
- Fraser, A.R.; Bacci, B.; Le Chevoir, M.A.; Long, S.N. Epidermal Growth Factor Receptor and Ki-67 Expression in Canine Gliomas. Vet. Pathol. 2016, 53, 1131–1137. [Google Scholar] [CrossRef]
- Veloso, E.S.; Gonçalves, I.N.N.; Arantes, J.A.; De Abreu, R.V.S.; Cassali, G.D.; Ferreira, E. Quantification of EGFR Family in Canine Mammary Ductal Carcinomas in Situ: Implications on the Histological Graduation. Vet. Res. Commun. 2019, 43, 123–129. [Google Scholar] [CrossRef]
- Sanz Ressel, B.L.; Massone, A.R.; Barbeito, C.G. Dysregulated Expression of Phosphorylated Epidermal Growth Factor Receptor and Phosphatase and Tensin Homologue in Canine Cutaneous Papillomas and Squamous Cell Carcinomas. J. Comp. Pathol. 2020, 174, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Cañueto, J.; Cardeñoso, E.; García, J.L.; Santos-Briz, Á.; Castellanos-Martín, A.; Fernández-López, E.; Blanco Gómez, A.; Pérez-Losada, J.; Román-Curto, C. Epidermal Growth Factor Receptor Expression Is Associated with Poor Outcome in Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2017, 176, 1279–1287. [Google Scholar] [CrossRef]
- Nichita, M.M.; Giurcăneanu, C.; Mihai, M.M.; Ghigulescu, M.; Beiu, C.; Negoiţă, S.I.; Popa, L.G. The Immunoexpression of Epidermal Growth Factor Receptor in Cutaneous Squamous Cell Carcinoma. Rom. J. Morphol. Embryol. 2021, 62, 201–208. [Google Scholar] [CrossRef]
- Abu-Humaidan, A.H.A.; Ekblad, L.; Wennerberg, J.; Sørensen, O.E. EGFR Modulates Complement Activation in Head and Neck Squamous Cell Carcinoma. BMC Cancer 2020, 20, 121. [Google Scholar] [CrossRef]
- Weiss, J.; Hayes, D.N. Classifying Squamous Cell Carcinoma of the Head and Neck: Prognosis, Prediction and Implications for Therapy. Expert Rev. Anticancer Ther. 2014, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, M.; Morita, K.; Oikawa, Y.; Kayamori, K.; Sakamoto, K.; Ikeda, T.; Harada, H. Co-expression of EGFR and MET Has a Synergistic Effect on the Prognosis of Patients with Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2020, 49, 235–242. [Google Scholar] [CrossRef]
- Doyle, H.A.; Gee, R.J.; Masters, T.D.; Gee, C.R.; Booth, C.J.; Peterson-Roth, E.; Koski, R.A.; Helfand, S.C.; Price, L.; Bascombe, D.; et al. Vaccine-Induced ErbB (EGFR/HER2)-Specific Immunity in Spontaneous Canine Cancer. Transl. Oncol. 2021, 14, 101205. [Google Scholar] [CrossRef] [PubMed]
- Figuerêdo, S.H.; Neto, R.S.C.; Ferreira, E.; Cassali, G.D.; Estrela-Lima, A.; Damasceno, K.A. Expression of VCAN and Its Receptors in Canine Mammary Carcinomas with or without Myoepithelial Proliferation. Res. Vet. Sci. 2021, 140, 56–63. [Google Scholar] [CrossRef]
- Oh, F.; Modiano, J.F.; Bachanova, V.; Vallera, D.A. Bispecific Targeting of EGFR and Urokinase Receptor (uPAR) Using Ligand-Targeted Toxins in Solid Tumors. Biomolecules 2020, 10, 956. [Google Scholar] [CrossRef]
- Inoue, J.; Fujiwara, K.; Hamamoto, H.; Kobayashi, K.; Inazawa, J. Improving the Efficacy of EGFR Inhibitors by Topical Treatment of Cutaneous Squamous Cell Carcinoma with miR-634 Ointment. Mol. Ther. Oncolytics 2020, 19, 294–307. [Google Scholar] [CrossRef]
- Li, Y.; Yue, L.; Li, Y.; Zhang, Q.; Liang, X. Prognostic Value of Ki-67 in Nasopharyngeal Carcinoma: A Meta-Analysis. Biosci. Rep. 2021, 41, BSR20203334. [Google Scholar] [CrossRef]
- Niotis, A.; Tsiambas, E.; Fotiades, P.P.; Ragos, V. Ki-67 and Topoisomerase IIa Proliferation Markers in Colon Adenocarcinoma. J. BUON 2018, 23, 24–27. [Google Scholar] [PubMed]
- Tian, Y.; Ma, Z.; Chen, Z.; Li, M.; Wu, Z.; Hong, M.; Wang, H.; Svatek, R.; Rodriguez, R.; Wang, Z. Clinicopathological and Prognostic Value of Ki-67 Expression in Bladder Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158891. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Ma, X.; Li, H.; Gu, L.; Gao, Y.; Fan, Y.; Zhang, Y.; Zhang, X. Prognostic and Clinicopathological Role of High Ki-67 Expression in Patients with Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 44281. [Google Scholar] [CrossRef]
- Delgado, L.; Monteiro, L.; Silva, P.; Bousbaa, H.; Garcez, F.; Silva, J.; Brilhante-Simões, P.; Pires, I.; Prada, J. BUBR1 as a Prognostic Biomarker in Canine Oral Squamous Cell Carcinoma. Animals 2022, 12, 3082. [Google Scholar] [CrossRef]
- Alferraly, I.T.; Munir, D.; Putra, I.B.; Sembiring, R.J. Correlation of Ki-67 Expression as Tumor Cell Proliferation Activity Marker with Cutaneous Squamous Cell Carcinoma Grading. Open Access Maced. J. Med. Sci. 2019, 7, 3384–3386. [Google Scholar] [CrossRef] [PubMed]
- Batinac, T.; Zamolo, G.; Coklo, M.; Hadzisejdic, I.; Stemberger, C.; Zauhar, G. Expression of Cell Cycle and Apoptosis Regulatory Proteins in Keratoacanthoma and Squamous Cell Carcinoma. Pathol.—Res. Pract. 2006, 202, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Magliulo, G.; Rubini, C.; Presutti, L.; Re, M. The Clinical Relevance of Ki-67 Expression in Laryngeal Squamous Cell Carcinoma. Eur. Arch. Otorhinolaryngol. 2015, 272, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.S.; Ceausu, A.R.; Comsa, S.; Raica, M. Loss of E-Cadherin Expression Correlates With Ki-67 in Head and Neck Squamous Cell Carcinoma. In Vivo 2022, 36, 1150–1154. [Google Scholar] [CrossRef]
- Gadbail, A.R.; Sarode, S.C.; Chaudhary, M.S.; Gondivkar, S.M.; Tekade, S.A.; Yuwanati, M.; Patil, S. Ki67 Labelling Index Predicts Clinical Outcome and Survival in Oral Squamous Cell Carcinoma. J. Appl. Oral Sci. 2021, 29, e20200751. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a Proliferation Marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Hamilton, N.A.; Liu, T.-C.; Cavatiao, A.; Mawad, K.; Chen, L.; Strasberg, S.S.; Linehan, D.C.; Cao, D.; Hawkins, W.G. Ki-67 Predicts Disease Recurrence and Poor Prognosis in Pancreatic Neuroendocrine Neoplasms. Surgery 2012, 152, 107–113. [Google Scholar] [CrossRef]
- Finkelman, B.S.; Zhang, H.; Hicks, D.G.; Turner, B.M. The Evolution of Ki-67 and Breast Carcinoma: Past Observations, Present Directions, and Future Considerations. Cancers 2023, 15, 808. [Google Scholar] [CrossRef]
- Sato, N.; Yako, Y.; Maruyama, T.; Ishikawa, S.; Kuromiya, K.; Tokuoka, S.M.; Kita, Y.; Fujita, Y. The COX-2/PGE2 Pathway Suppresses Apical Elimination of RasV12-Transformed Cells from Epithelia. Commun. Biol. 2020, 3, 132. [Google Scholar] [CrossRef]
- Valentina Tudor, D.; Bâldea, I.; Lupu, M.; Kacso, T.; Kutasi, E.; Hopârtean, A.; Stretea, R.; Gabriela Filip, A. COX-2 as a Potential Biomarker and Therapeutic Target in Melanoma. Cancer Biol. Med. 2020, 17, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Silveira, T.L.; Pang, L.Y.; Di Domenico, A.; Veloso, E.S.; Silva, I.L.D.; Puerto, H.L.D.; Ferreria, E.; Argyle, D.J. COX-2 Silencing in Canine Malignant Melanoma Inhibits Malignant Behaviour. Front. Vet. Sci. 2021, 8, 633170. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, C.J. COX-1 and COX-2 Inhibitors. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Bardagí, M.; Fondevila, D.; Ferrer, L. Immunohistochemical Detection of COX-2 in Feline and Canine Actinic Keratoses and Cutaneous Squamous Cell Carcinoma. J. Comp. Pathol. 2012, 146, 11–17. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, E.M.P.; Piché, C.; Sirois, J.; Doré, M. Expression of Cyclo-Oxygenase-2 in Naturally Occurring Squamous Cell Carcinomas in Dogs. J. Histochem. Cytochem. 2001, 49, 867–875. [Google Scholar] [CrossRef]
- Poggiani, S.D.S.C.; Hatayde, M.R.; Laufer-Amorim, R.; Werner, J. Expression of Cyclooxygenase-2 and Ki-67 in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma in Dogs. Open J. Vet. Med. 2012, 2, 41–47. [Google Scholar] [CrossRef]
- Millanta, F.; Andreani, G.; Rocchigiani, G.; Lorenzi, D.; Poli, A. Correlation Between Cyclo-Oxygenase-2 and Vascular Endothelial Growth Factor Expression in Canine and Feline Squamous Cell Carcinomas. J. Comp. Pathol. 2016, 154, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Karagece Yalçin, U.; Seçkın, S. The Expression of P53 and COX-2 in Basal Cell Carcinoma, Squamous Cell Carcinoma and Actinic Keratosis Cases. Turk. Patoloji Derg. 2012, 28, 119–127. [Google Scholar] [CrossRef]
- Amirnia, M.; Babaie-Ghazani, A.; Fakhrjou, A.; Khodaeiani, E.; Alikhah, H.; Naghavi-behzad, M.; Zarrintan, A. Immunohistochemical Study of Cyclooxygenase-2 in Skin Tumors. J. Dermatol. Treat. 2014, 25, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Koyuncuer, A. Immunohistochemical Analysis of Cyclooxygenase-2 in Non-Melanocytic Skin Cancer: Correlation With Morphological Subtype and Histologic Grade. World J. Oncol. 2014, 5, 189–195. [Google Scholar] [CrossRef]
- Orehovec, S.S.; Dujmović, A.; Mijatović, D.; Mance, M.; Šarčević, B. Immunohistochemical Expression of Matrix Metalloproteinase-1 and Cyclooxygenase-2 in Cutaneous Squamous Cell and Basal Cell Carcinoma. Acta Dermatovenerol. Croat. 2021, 291, 8–20. [Google Scholar]
- Ciortea, C.D.; Jung, I.; Gurzu, S.; Kövecsi, A.; Turdean, S.G.; Bara, T. Correlation of Angiogenesis with Other Immunohistochemical Markers in Cutaneous Basal and Squamous Cell Carcinomas. Rom. J. Morphol. Embryol. 2015, 56, 665–670. [Google Scholar]
- Jang, T.J. Epithelial to Mesenchymal Transition in Cutaneous Squamous Cell Carcinoma Is Correlated with COX-2 Expression but Not with the Presence of Stromal Macrophages or CD10-Expressing Cells. Virchows Arch. 2012, 460, 481–487. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, G.E.; Cho, N.H.; Pyo, H.R.; Shim, S.J.; Chang, S.K.; Park, H.C.; Suh, C.O.; Park, T.K.; Kim, B.S. Overexpression of Cyclooxygenase-2 Is Associated with a Poor Prognosis in Patients with Squamous Cell Carcinoma of the Uterine Cervix Treated with Radiation and Concurrent Chemotherapy. Cancer 2002, 95, 531–539. [Google Scholar] [CrossRef]
- Eto, S.; Shinada, M.; Saeki, K.; Tsuboi, M.; Kamoto, S.; Yoshitake, R.; Chambers, J.; Uchida, K.; Kato, D.; Nishimura, R.; et al. Pan-Tumour Analysis of COX-2 Expression in Dogs. Vet. J. 2024, 304, 106064. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E. Cyclooxygenase-2 (Cox-2) Blockade in the Chemoprevention of Cancers of the Colon, Breast, Prostate, and Lung. Inflammopharmacology 2009, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, T.H.; Chen, C.P.; Xiang, J.J.; Zhao, X.B.; Gui, R.Y.; Liao, X.H. Targeting COX-2 Potently Inhibits Proliferation of Cancer Cells in Vivo but Not in Vitro in Cutaneous Squamous Cell Carcinoma. Transl. Cancer Res. 2021, 10, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Corchado-Cobos, R.; García-Sancha, N.; González-Sarmiento, R.; Pérez-Losada, J.; Cañueto, J. Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef]
- Kuźbicki, Ł.; Brożyna, A.A. Expression of Cyclooxygenase-2 in Human Epithelial Skin Lesions: A Systematic Review of Immunohistochemical Studies. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 163. [Google Scholar] [CrossRef]
- Cunha, R.M.d.C.; dos Santos Horta, R.; Lavalle, G.E.; Araújo, R.B. Cyclooxygenase-2 Expression in Epithelial Neoplasms and Its Relevance as a Targeted Therapy in Dogs. Cienc. Rural 2016, 46, 1050–1052. [Google Scholar] [CrossRef]
- Nardi, A.B.; Raposo, T.M.M.; Huppes, R.R.; Daleck, C.R.; Amorim, R.L. COX-2 Inhibitors for Cancer Treatment in Dogs. Pak. Vet. J. 2011, 31, 275–279. [Google Scholar] [CrossRef]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current Biomarkers of Canine Mammary Tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef] [PubMed]
- Rawat, C.; Kukal, S.; Dahiya, U.R.; Kukreti, R. Cyclooxygenase-2 (COX-2) Inhibitors: Future Therapeutic Strategies for Epilepsy Management. J. Neuroinflammation 2019, 16, 197. [Google Scholar] [CrossRef] [PubMed]
- Jaros, E.; Perry, R.; Adam, L.; Kelly, P.; Crawford, P.; Kalbag, R.; Mendelow, A.; Sengupta, R.; Pearson, A. Prognostic Implications of P53 Protein, Epidermal Growth Factor Receptor, and Ki-67 Labelling in Brain Tumours. Br. J. Cancer 1992, 66, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Meert, A.P.; Martin, B.; Verdebout, J.M.; Feoli, F.; Mascaux, C.; Ninane, V.; Sculier, J.P. EGFR, c-erbB-2 and Ki-67 in NSCLC and Preneoplastic Bronchial Lesions. Anticancer Res. 2006, 26, 135–138. [Google Scholar]
- Sakuma, K.; Fujimori, T.; Hirabayashi, K.; Terano, A. Cyclooxygenase (COX)-2 Immunoreactivity and Relationship to P53 and Ki-67 Expression in Colorectal Cancer. J. Gastroenterol. 1999, 34, 189–194. [Google Scholar] [CrossRef]
- Escobar, E.; Gómez-Valenzuela, F.; Peñafiel, C.; Hormazábal-Hevia, A.; Herrera-Fuentes, C.; Mori-Aliaga, D. Immunohistochemical Expression of COX-2, Ki-67, Bcl-2, Bax, VEGF and CD105 According to Histological Grading in Oral Squamous Cell Carcinoma. Rev. Española De Patol. 2023, 56, 147–157. [Google Scholar] [CrossRef]
- Rodrigues, P.; Bangali, H.; Hammoud, A.; Mustafa, Y.F.; Al-Hetty, H.R.A.K.; Alkhafaji, A.T.; Deorari, M.M.; Al-Taee, M.M.; Zabibah, R.S.; Alsalamy, A. COX 2-Inhibitors; a Thorough and Updated Survey into Combinational Therapies in Cancers. Med. Oncol. 2024, 41, 41. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined Chemotherapy with Cyclooxygenase-2 (COX-2) Inhibitors in Treating Human Cancers: Recent Advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef]
- Sandler, A.B.; Dubinett, S.M. COX-2 Inhibition and Lung Cancer. Semin. Oncol. 2004, 31, 45–52. [Google Scholar] [CrossRef]
- Jalili, A.; Pinc, A.; Pieczkowski, F.; Karlhofer, F.M.; Stingl, G.; Wagner, S.N. Combination of an EGFR Blocker and a COX-2 Inhibitor for the Treatment of Advanced Cutaneous Squamous Cell Carcinoma. JDDG J. Der Dtsch. Dermatol. Ges. 2008, 6, 1066–1069. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Description | Grade I | Grade II | Grade III |
|---|---|---|---|---|
| Keratinization/Differentiation | The proportion of tumor cells exhibiting keratinization | >50% keratinized cells | 20–50% keratinized cells | 0–20% keratinized cells |
| Nuclear Pleomorphism | Maturity of cells | Minimal; >75% mature cells | Moderate; 50–75% mature cells | Marked nuclear pleomorphism |
| Mitotic Count | Mitoses per ten high-power fields (HPF) | 0 to 1 mitosis/HPF | 2 to 3 mitoses/HPF | ≥4 mitoses/HPF |
| Invasion Pattern | The pattern of tumor invasion | Well-defined with pushing borders | Infiltration by solid cords, bands, and strands | Infiltration by small groups, strands, or individual cells |
| Invasion Stage | The extent of tumor invasion | Carcinoma in situ or questionable invasion | Apparent invasion limited to the lamina propria | Invasion beyond the lamina propria involving muscle |
| Lymphoplasmacytic Infiltration | Level of lymphoplasmacytic infiltration | Marked | Moderate | Mild to absent |
| Histological Characteristic | Classification | n | % |
|---|---|---|---|
| Ulceration | Absent | 4 | 10.8% |
| Present | 33 | 89.2% | |
| Necrosis | Absent | 1 | 2.7% |
| Present | 36 | 97.3% | |
| Emboli | Absent | 30 | 81.1% |
| Present | 7 | 18.9% | |
| Histological grade of malignancy | I | 10 | 27.03% |
| II | 15 | 40.54% | |
| III | 12 | 32.43% | |
| Total | 37 | 100% |
| Case Number | Breed | Gender | Age | Ulceration | Necrosis | Grade | Embolus | EGFR | Ki-67 | Cox-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Indeterminate | female | 10 | 1 | 1 | 3 | 1 | 1 | 1 | 1 |
| 2 | Boxer | male | 8 | 1 | 1 | 2 | 0 | 1 | 0 | 1 |
| 3 | Indeterminate | female | 11 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 4 | Portuguese Pointer | male | 11 | 1 | 1 | 2 | 0 | 1 | 0 | 1 |
| 5 | Golden Retriever | male | 9 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| 6 | Dalmatian | female | 7 | 1 | 1 | 3 | 0 | 0 | 1 | 1 |
| 7 | Doberman | female | 6 | 1 | 1 | 2 | 0 | 1 | 1 | 2 |
| 8 | Estrela Mountain Dog | female | 4 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| 9 | Basset Hound | female | 14 | 1 | 1 | 2 | 0 | 0 | 1 | 2 |
| 10 | Labrador Retriever | female | 7 | 1 | 1 | 2 | 0 | 1 | 1 | 2 |
| 11 | West Highland Terrier | male | 9 | 1 | 1 | 2 | 0 | 0 | 0 | 1 |
| 12 | Dalmatian | male | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 13 | Portuguese Cattle Dog | male | 9 | 1 | 1 | 2 | 0 | 1 | 1 | 1 |
| 14 | Dalmatian | male | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| 15 | Podengo | male | 11 | 1 | 1 | 3 | 0 | 1 | 0 | 2 |
| 16 | Setter | male | 9 | 1 | 1 | 2 | 0 | 0 | 0 | 1 |
| 17 | Indeterminate | female | 8 | 1 | 1 | 3 | 0 | 0 | 1 | 2 |
| 18 | Boxer | male | 8 | 1 | 1 | 3 | 0 | 0 | 1 | 2 |
| 19 | Shar Pei | female | 10 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| 20 | Boxer | male | 7 | 1 | 1 | 2 | 0 | 0 | 0 | 1 |
| 21 | Pitbull | male | 9 | 0 | 1 | 3 | 1 | 1 | 1 | 2 |
| 22 | Indeterminate | female | 17 | 1 | 1 | 2 | 0 | 1 | 1 | 1 |
| 23 | Giant Schnauzer | male | 7 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| 24 | Boxer | male | 10 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 25 | Boxer | male | 9 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| 26 | Cocker Spaniel | female | 10 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 27 | Poodle | female | 12 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 28 | Boxer | female | 8 | 1 | 1 | 2 | 0 | 1 | 1 | 2 |
| 29 | German Shepherd | male | 2 | 1 | 1 | 2 | 0 | 1 | 1 | 2 |
| 30 | Boxer | female | 9 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 31 | Poodle | female | 10 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 32 | Pekingese | female | 6 | 1 | 1 | 2 | 0 | 0 | 1 | 1 |
| 33 | Scent hound | female | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 34 | Boxer | female | 7 | 1 | 1 | 2 | 0 | 0 | 0 | 1 |
| 35 | Bichon | male | 9 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| 36 | Poodle | female | 11 | 1 | 1 | 2 | 0 | 0 | 0 | 1 |
| 37 | Partridge | female | 7 | 1 | 1 | 3 | 0 | 0 | 0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, J.M.; Files, R.; Cardoso, C.; Pimenta, J.; Maia, G.; Silva, F.; Queiroga, F.L.; Prada, J.; Pires, I. Immunohistochemical Expression Levels of Epidermal Growth Factor Receptor, Cyclooxygenase-2, and Ki-67 in Canine Cutaneous Squamous Cell Carcinomas. Curr. Issues Mol. Biol. 2024, 46, 4951-4967. https://doi.org/10.3390/cimb46050297
Luís JM, Files R, Cardoso C, Pimenta J, Maia G, Silva F, Queiroga FL, Prada J, Pires I. Immunohistochemical Expression Levels of Epidermal Growth Factor Receptor, Cyclooxygenase-2, and Ki-67 in Canine Cutaneous Squamous Cell Carcinomas. Current Issues in Molecular Biology. 2024; 46(5):4951-4967. https://doi.org/10.3390/cimb46050297
Chicago/Turabian StyleLuís, João Miguel, Rita Files, Cláudia Cardoso, José Pimenta, Gabriela Maia, Filipe Silva, Felisbina L. Queiroga, Justina Prada, and Isabel Pires. 2024. "Immunohistochemical Expression Levels of Epidermal Growth Factor Receptor, Cyclooxygenase-2, and Ki-67 in Canine Cutaneous Squamous Cell Carcinomas" Current Issues in Molecular Biology 46, no. 5: 4951-4967. https://doi.org/10.3390/cimb46050297








