Investigation of Hydrocolloid Plant Polysaccharides as Potential Candidates to Mimic the Functions of MUC5B in Saliva
Abstract
:1. Introduction
2. Materials Methods
2.1. Preparations of Aqueous Extracts/Lyophilized Powders/Aqueous Formulations
2.2. Analysis of Aqueous Plant Extracts
2.2.1. GC-MS Analysis for Monosaccharides after Hydrolysis
Qualitative Analysis of Trimethylsilyl (TMS) Derivatives
Quantification of Neutral Monosaccharides as Alditol Acetates
2.2.2. Quantification of Uronic Acids
2.2.3. Quantification of Proteins
2.2.4. Size-Exclusion Chromatography (SEC)
2.2.5. NMR Analysis
2.3. Investigation of pH, Osmolality and Buffer Capacity
2.4. Investigation of Viscoelastic Behavior
2.5. Investigation of Viscoelastic Interactions with Human Saliva
2.6. Investigation of Microstructure
2.7. Culturing of Human Buccal TR 146 Cells on Aclar Sheets and UWS Collection
2.8. In Vitro Adhesion Studies on TR146 Cells
2.9. Statistical Analysis
3. Results
3.1. Analysis of the Extract Components
3.2. pH, Osmolality, and Buffer Capacity
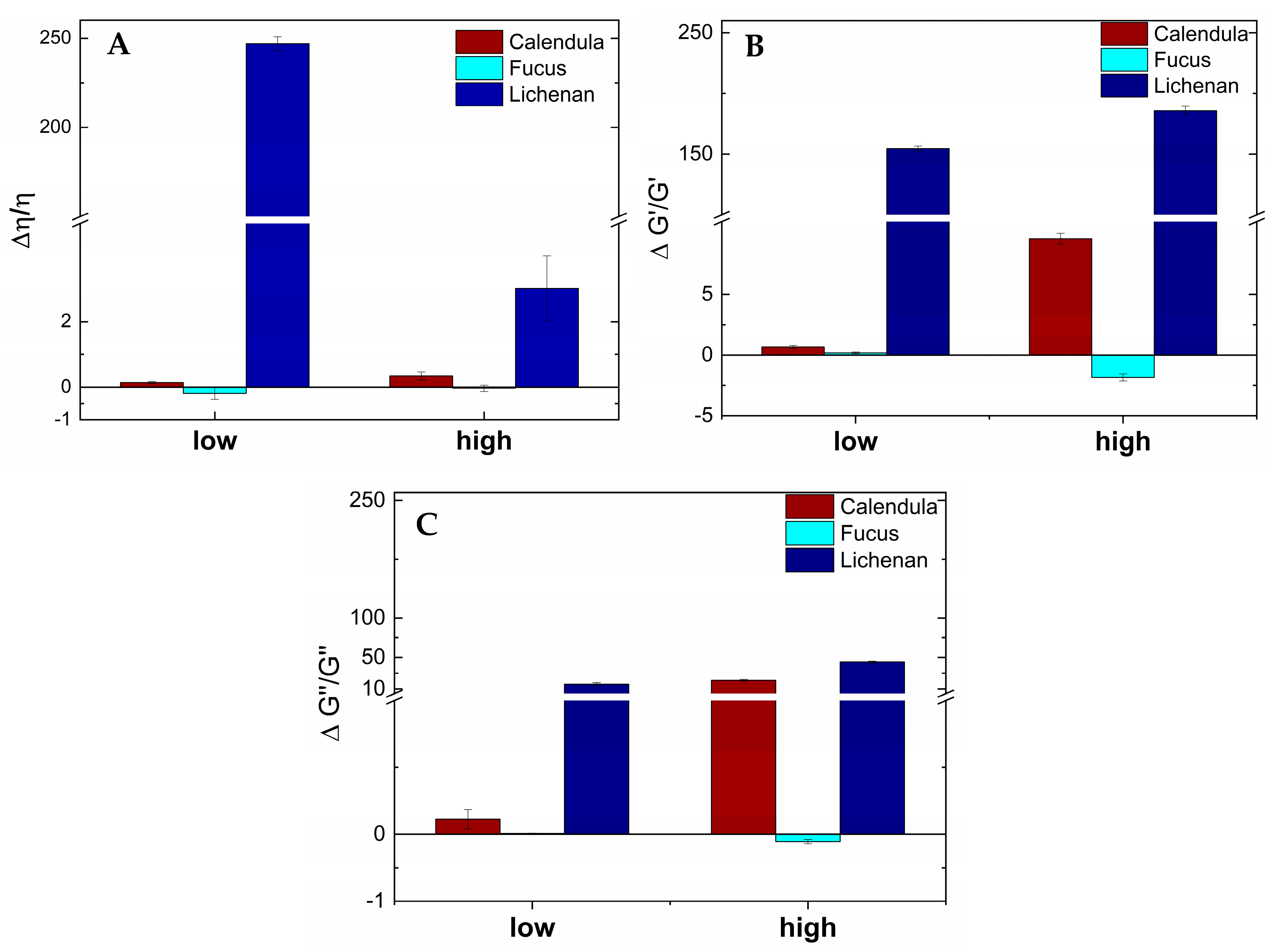
3.3. Viscoelastic Behavior and Viscoelastic Interactions with UWS
3.4. Microstructure of Hydrocolloidal Polysaccharides
3.5. In Vitro Adhesion on TR146 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradway, S.D.; Bergey, E.J.; Scannapieco, F.A.; Ramasubbu, N.; Zawacki, S.; Levine, M.J. Formation of salivary-mucosal pellicle: The role of transglutaminase. Biochem. J. 2015, 284, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What interactions drive the salivary mucosal pellicle formation? Colloids Surf. B Biointerfaces 2014, 120, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Tabak, L.A.; Levine, M.J.; Mandel, I.D.; Ellison, S.A. Role of salivary mucins in the protection of the oral cavity. J. Oral Pathol. 1982, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Haward, S.J.; Odell, J.A.; Berry, M.; Hall, T. Extensional rheology of human saliva. Rheol. Acta 2011, 50, 869–879. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological examination of the mucoadhesive/mucus interaction: The effect of mucoadhesive type and concentration. J. Control. Release 1998, 50, 167–178. [Google Scholar] [CrossRef]
- Roblegg, E.; Coughran, A.; Sirjani, D. Saliva: An all-rounder of our body. Eur. J. Pharm. Biopharm. 2019, 142, 133–141. [Google Scholar] [CrossRef]
- Wickström, C.; Davies, J.R.; Eriksen, G.V.; Veerman, E.C.; Carlstedt, I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: Identification of glycoforms and C-terminal cleavage. Biochem. J. 1998, 334, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, H.L.; Proctor, G.B.; Yakubov, G.E.; Wilson, S.; Carpenter, G.H. Concentration of salivary protective proteins within the bound oral mucosal pellicle. Oral Dis. 2014, 20, 707–713. [Google Scholar] [CrossRef]
- Thomsson, K.A. The salivary mucin MG1 (MUC5B) carries a repertoire of unique oligosaccharides that is large and diverse. Glycobiology 2002, 12, 1–14. [Google Scholar] [CrossRef]
- Davies, H.S.; Singh, P.; Deckert-Gaudig, T.; Deckert, V.; Rousseau, K.; Ridley, C.E.; Dowd, S.E.; Doig, A.J.; Pudney, P.D.A.; Thornton, D.J.; et al. Secondary structure and glycosylation of mucus glycoproteins by raman spectroscopies. Anal. Chem. 2016, 88, 11609–11615. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. The Secretion, Components, and Properties of Saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Teubl, B.J.; Stojkovic, B.; Docter, D.; Pritz, E.; Leitinger, G.; Poberaj, I.; Prassl, R.; Stauber, R.H.; Fröhlich, E.; Khinast, J.G.; et al. The effect of saliva on the fate of nanoparticles. Clin. Oral Investig. 2018, 22, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Spirk, C.; Hartl, S.; Pritz, E.; Gugatschka, M.; Kolb-Lenz, D.; Leitinger, G.; Roblegg, E. Comprehensive investigation of saliva replacement liquids for the treatment of xerostomia. Int. J. Pharm. 2019, 571, 118759. [Google Scholar] [CrossRef]
- Chaudhury, N.M.A.; Proctor, G.B.; Karlsson, N.G.; Carpenter, G.H.; Flowers, S.A. Reduced Mucin-7 (Muc7) Sialylation and Altered Saliva Rheology in Sjögren’s Syndrome Associated Oral Dryness. Mol. Cell. Proteom. 2016, 15, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Takehara, S.; Yanagishita, M.; Podyma-Inoue, K.A.; Kawaguchi, Y. Degradation of MUC7 and MUC5B in Human Saliva. PLoS ONE 2013, 8, e69059. [Google Scholar] [CrossRef]
- Winter, C.; Keimel, R.; Gugatschka, M.; Kolb, D.; Leitinger, G.; Roblegg, E. Investigation of Changes in Saliva in Radiotherapy-Induced Head Neck Cancer Patients. Int. J. Environ. Res. Public Health 2021, 18, 1629. [Google Scholar] [CrossRef] [PubMed]
- Cassolato, S.F.; Turnbull, R.S. Xerostomia: Clinical aspects and treatment. Gerodontology 2003, 20, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Pang, R.; Yang, G.; Tian, M.; Zhao, T.; Sun, Y.; Lee, E.-S.; Jiang, H.B.; Han, J. Diagnosis, Prevention, and Treatment of Radiotherapy-Induced Xerostomia: A Review. J. Oncol. 2022, 2022, 7802334. [Google Scholar] [CrossRef]
- Furness, S.; Worthington, H.V.; Bryan, G.; Birchenough, S.; McMillan, R. Interventions for the management of dry mouth: Topical therapies. Cochrane Database Syst. Rev. 2011, 7, CD008934. [Google Scholar] [CrossRef]
- Riley, P.; Glenny, A.-M.; Hua, F.; Worthington, H.V. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst. Rev. 2017, 7, CD012744. [Google Scholar] [CrossRef] [PubMed]
- Vera-Llonch, M.; Oster, G.; Hagiwara, M.; Sonis, S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma: Risk factors and clinical consequences. Cancer 2006, 106, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Behr, M.; Handel, G.; Bürgers, R. Saliva substitutes for the treatment of radiation-induced xerostomia—A review. Support. Care Cancer 2009, 17, 1331–1343. [Google Scholar] [CrossRef]
- Dost, F.; Farah, C. Stimulating the discussion on saliva substitutes: A clinical perspective. Aust. Dent. J. 2013, 58, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Preetha, A.; Banerjee, R. Comparison of artificial saliva substitutes. Trends Biomater. Artif. Organs 2005, 18, 178–186. [Google Scholar]
- Authimoolam, S.P.; Dziubla, T.D. Biopolymeric Mucin and Synthetic Polymer Analogs: Their Structure, Function and Role in Biomedical Applications. Polymers 2016, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Petrou, G.; Crouzier, T. Mucins as multifunctional building blocks of biomaterials. Biomater. Sci. 2018, 8, 2282–2297. [Google Scholar] [CrossRef] [PubMed]
- Assery, M.K.A. Efficacy of Artificial Salivary Substitutes in Treatment of Xerostomia: A Systematic Review. J. Pharm. Bioallied Sci. 2019, 11, S1–S12. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Heydarirad, G.; Rezaeizadeh, H.; Choopani, R.; Ghobadi, A.; Gachkar, L. Evaluation of Efficacy of an Herbal Compound on Dry Mouth in Patients with Head and Neck Cancers: A Randomized Clinical Trial. J. Evid.-Based. Complement. Altern. Med. 2016, 21, 30–33. [Google Scholar] [CrossRef]
- Schmidgall, J.; Schnetz, E.; Hensel, A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med. 2000, 66, 48–53. [Google Scholar] [CrossRef]
- De Felice, F.; Scarabelli, M.G.; De Pietro, R.; Chiarello, G.; Di Giammarco, F.; Cattaneo, C.G.; Lombardo, G.; Montinaro, F.R.; Tomaciello, M.; Tombolini, M.; et al. Relationship between Salivary Amylase and Xerostomia in Intensity-Modulated Radiation Therapy for Head and Neck Cancer: A Prospective Pilot Study. Curr. Oncol. 2022, 29, 6564–6572. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.; van Deurs, B.; Pedersen, M.; Rassing, M.R. TR146 cells grown on filters as a model for human buccal epithelium: I. Morphology, growth, barrier properties, and permeability. Int. J. Pharm. 1995, 125, 165–184. [Google Scholar] [CrossRef]
- Franz, G.; Koehler, H. Drogen und Naturstoffe; Springer: Berlin/Heidelberg, Germany, 1992; p. 273. [Google Scholar]
- Sweeley, C.C.; Bentley, R.; Makita, M.; Wells, W.W. Gas-liquid chromatography of trimethylsilyl derivatives of sugars and related substances. J. Am. Chem. Soc. 1963, 85, 2497–2507. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic acid. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.J.; Gershkovich, P. Characterisation of human saliva as a platform for oral dissolution medium development. Eur. J. Pharm. Biopharm. 2015, 91, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Zheng, J.; Zheng, L.; Zhou, Z.R. Influence of centrifugation treatment on the lubricating properties of human whole saliva. Biosurf. Biotribol. 2016, 2, 95–101. [Google Scholar] [CrossRef]
- Katebi, K.; Hassanpour, S.; Eslami, H.; Salehnia, F.; Hosseinifard, H. Effect of Pilocarpine Mouthwash on Salivary Flow Rate in Patients with Xerostomia: A Systematic Review and Meta-Analysis. J. Dent. 2023, 23, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Caramella, C.; Ferrari, F. Rheological analysis and mucoadhesion: A 30 year-old and still active combination. J. Pharm. Biomed. Anal. 2018, 156, 232–238. [Google Scholar] [CrossRef]
- Rossi, S.; Bonferoni, M.C.; Lippoli, G.; Bertoni, M.; Ferrari, F.; Caramella, C.; Conte, U. Influence of mucin type on polymer-mucin rheological interactions. Biomaterials 1995, 16, 1073–1079. [Google Scholar] [CrossRef]
- Poteser, M.; Leitinger, G.; Pritz, E.; Platzer, D.; Frischauf, I.; Romanin, C.; Groschner, K. Live-cell imaging of ER-PM contact architecture by a novel TIRFM approach reveals extension of junctions in response to store-operated Ca2+-entry. Sci. Rep. 2016, 6, 35656. [Google Scholar] [CrossRef]
- Krenceski, M.A.; Johnson, J.F. Shear, tack, and peel of polyisobutylene: Effect of molecular weight and molecular weight distribution. Polym. Eng. Sci. 1989, 29, 36–43. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef]
- Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Bertoni, M.; Caramella, C. Characterization of mucin interaction with three viscosity grades of sodium carboxymethylcellulose. Comparison between rheological and tensile testing. Eur. J. Pharm. Sci. 1996, 4, 189–196. [Google Scholar] [CrossRef]
- Lakrout, H.; Sergot, P.; Creton, C. Direct observation of cavitation and fibrillation in a probe tack experiment on model acrylic pressure-sensitive-adhesives. J. Adhes. 1999, 69, 307–359. [Google Scholar] [CrossRef]
- Hassan, E.E.; Gallo, J.M. A Simple Rheological Method for the in Vitro Assessment of Mucin-Polymer Bioadhesive Bond Strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef]
- Vinke, J.; Kaper, H.J.; Vissink, A.; Sharma, P.K. An ex vivo salivary lubrication system to mimic xerostomic conditions and to predict the lubricating properties of xerostomia relieving agents. Sci. Rep. 2018, 8, 9087. [Google Scholar] [CrossRef]
- Visvanathan, V.; Nix, P. Managing the patient presenting with xerostomia: A review. Int. J. Clin. Pract. 2010, 64, 404–407. [Google Scholar] [CrossRef]
- Plemons, J.M.; Al-Hashimi, I.; Marek, C.L. Managing xerostomia and salivary gland hypofunction. J. Am. Dent. Assoc. 2014, 145, 867–873. [Google Scholar] [CrossRef]
- Varljen, J.; Lipták, A.; Wagner, H. Structural analysis of a rhamnoarabinogalactan and arabinogalactans with immuno-stimulating activity from Calendula officinalis. Phytochemistry 1989, 28, 2379–2383. [Google Scholar] [CrossRef]
- Slavov, A.; Panchev, I.; Kovacheva, D.; Vasileva, I. Physico-chemical characterization of water-soluble pectic extracts from Rosa damascena, Calendula officinalis and Matricaria chamomilla wastes. Food Hydrocoll. 2016, 61, 469–476. [Google Scholar] [CrossRef]
- Zacharski, D.M.; Esch, S.; König, S.; Mormann, M.; Brandt, S.; Ulrich-Merzenich, G.; Hensel, A. β-1,3/1,4-Glucan Lichenan from Cetraria islandica (L.) ACH. induces cellular differentiation of human keratinocytes. Fitoterapia 2018, 129, 226–236. [Google Scholar] [CrossRef]
- Nieuw Amerongen, A.V.; Veerman, E.C.I. Saliva—The defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Simmons, N.L. Epithelial Cell Volume Regulation In Hypotonic Fluids: Studies Using A Model Tissue Culture Renal Epithelial Cell System. Q. J. Exp. Physiol. 1984, 69, 83–95. [Google Scholar] [CrossRef]
- Andersson, R.; Arvidsson, E.; Crossner, C.G.; Holm, A.K.; Mansson, B. The flow rate, pH and buffer effect of mixed saliva in children. J. Int. Assoc. Dent. Child. 1974, 5, 5–12. [Google Scholar]
- Bardow, A.; Moe, D.; Nyvad, B.; Nauntofte, B. The buffer capacity and buffer systems of human whole saliva measured without loss of CO2. Arch. Oral Biol. 2000, 45, 1–12. [Google Scholar] [CrossRef]
- Veerman, E.C.; Valentijn-Benz, M.; Nieuw Amerongen, A.V. Viscosity of human salivary mucins: Effect of pH and ionic strength and role of sialic acid. J. Biol. Buccale 1989, 17, 297–306. [Google Scholar] [PubMed]
- Bradway, S.D.; Bergey, E.J.; Jones, P.C.; Levine, M.J. Oral mucosal pellicle. Adsorption and transpeptidation of salivary components to buccal epithelial cells. Biochem. J. 1989, 261, 887–896. [Google Scholar] [CrossRef]
- Hsein, H.; Garrait, G.; Beyssac, E.; Hoffart, V. Whey protein mucoadhesive properties for oral drug delivery: Mucin–whey protein interaction and mucoadhesive bond strength. Colloids Surf. B Biointerf. 2015, 136, 799–808. [Google Scholar] [CrossRef]
- Meng-Lund, E.; Muff-Westergaard, C.; Sander, C.; Madelung, P.; Jacobsen, J. A mechanistic based approach for enhancing buccal mucoadhesion of chitosan. Int. J. Pharm. 2014, 461, 280–285. [Google Scholar] [CrossRef]
- Shital, G.; Shinkar, D.; Ravindra, S. Mucoadhesive buccal drug delivery: An Overview. J. Adv. Pharm. Edu. Res. 2013, 3, 319–332. [Google Scholar]
- Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems. Pharmaceutics 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Tetyczka, C.; Hartl, S.; Jeitler, R.; Absenger-Novak, M.; Meindl, C.; Fröhlich, E.; Riedl, S.; Zweytick, D.; Roblegg, E. Cytokine-Mediated Inflammation in the Oral Cavity and Its Effect on Lipid Nanocarriers. Nanomaterials 2021, 11, 1330. [Google Scholar] [CrossRef]
- Hägerström, H.; Edsman, K. Limitations of the rheological mucoadhesion method: The effect of the choice of conditions and the rheological synergism parameter. Eur. J. Pharm. Sci. 2003, 18, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef]
- Schimpel, C.; Werzer, O.; Fröhlich, E.; Leitinger, G.; Absenger-Novak, M.; Teubl, B.; Zimmer, A.; Roblegg, E. Atomic force microscopy as analytical tool to study physicomechanical properties of intestinal cells. Beilstein J. Nanotechnol. 2015, 6, 1457–1466. [Google Scholar] [CrossRef]
- Lindh, L.; Svendsen, I.E.; Svensson, O.; Cárdenas, M.; Arnebrant, T. The salivary mucin MUC5B and lactoperoxidase can be used for layer-by-layer film formation. J. Colloid Interface Sci. 2007, 310, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Rump, A.; Tetyczka, C.; Littringer, E.; Kromrey, L.M.; Bülow, R.; Roblegg, E.; Weitschies, W.; Grimm, M. In Vitro and In Vivo Evaluation of Carbopol 71G NF-Based Mucoadhesive Minitablets as a Gastroretentive Dosage Form. Mol. Pharm. 2023, 20, 1624–1630. [Google Scholar] [CrossRef]





| Monosaccharides [mg/g] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extract | Ara (#) | Fuc | Gal | Glu | Man | Rha | Rib | Xyl |
| Calendula | 18.9 | 5.3 | 61.1 | 59.4 | 5.1 | 29.5 | 0.5 | 4.2 |
| Fucus | n.d. (##) | 111.7 | 18.5 | 165.0 | 43.0 | 1.0 | n.d. | 15.7 |
| Protein | MW [Da] | Retention Time [min] | Retention Volume [mL] | log MW | |
|---|---|---|---|---|---|
| Calendula | Peak 1 | 265,759.6 | 21.1 | 8.4 | 5.4 |
| Peak 2 | 4155.2 | 41.2 | 16.5 | 3.6 | |
| Peak 3 | 179.2 | 56.4 | 22.6 | 2.3 | |
| Fucus | Peak 1 | 225,192.0 | 21.9 | 8.8 | 5.4 |
| Peak 2 | 3720.8 | 41.7 | 16.7 | 3.6 | |
| Peak 3 | 96.3 | 59.4 | 23.8 | 2.0 |
| Plant Extract | Without K2HPO4 | With 0.02 M K2HPO4 | |||
|---|---|---|---|---|---|
| pH | Osmolality [osmol/kg] | pH | Osmolality [osmol/kg] | Buffer Capacity [mmol H+/L] | |
| Calendula | 4.26 * ± 0.08 | - (#) | 7.02 ± 0.09 | - (#) | 7.90 * ± 0.82 |
| Fucus | 4.62 * ± 0.06 | 0.007 * ± 0.004 | 7.41 ± 0.09 | 0.053 ± 0.006 | 8.41 * ± 0.31 |
| Lichenan | 6.74 ± 0.09 | 0.001 * ± 0.001 | 8.91 * ± 0.08 | 0.052 ± 0.006 | 5.50 ± 0.22 |
| UWS [14] | 6.80 ± 0.17 | 0.052 ± 0.003 | - | - | 5.34 ± 1.70 (##) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, C.; Tetyczka, C.; Pham, D.T.; Kolb, D.; Leitinger, G.; Schönfelder, S.; Kunert, O.; Gerlza, T.; Kungl, A.; Bucar, F.; et al. Investigation of Hydrocolloid Plant Polysaccharides as Potential Candidates to Mimic the Functions of MUC5B in Saliva. Pharmaceutics 2024, 16, 682. https://doi.org/10.3390/pharmaceutics16050682
Winter C, Tetyczka C, Pham DT, Kolb D, Leitinger G, Schönfelder S, Kunert O, Gerlza T, Kungl A, Bucar F, et al. Investigation of Hydrocolloid Plant Polysaccharides as Potential Candidates to Mimic the Functions of MUC5B in Saliva. Pharmaceutics. 2024; 16(5):682. https://doi.org/10.3390/pharmaceutics16050682
Chicago/Turabian StyleWinter, Christina, Carolin Tetyczka, Duy Toan Pham, Dagmar Kolb, Gerd Leitinger, Sandra Schönfelder, Olaf Kunert, Tanja Gerlza, Andreas Kungl, Franz Bucar, and et al. 2024. "Investigation of Hydrocolloid Plant Polysaccharides as Potential Candidates to Mimic the Functions of MUC5B in Saliva" Pharmaceutics 16, no. 5: 682. https://doi.org/10.3390/pharmaceutics16050682







