Individually Cultured Bovine Zygotes Successfully Develop to the Blastocyst Stage in an Extremely Confined Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oocyte Retrieval and In Vitro Maturation
2.2. Semen Preparation, In Vitro Fertilization and Embryo Culture
2.3. Culture Devices and Loading of Embryos or Presumptive Zygotes
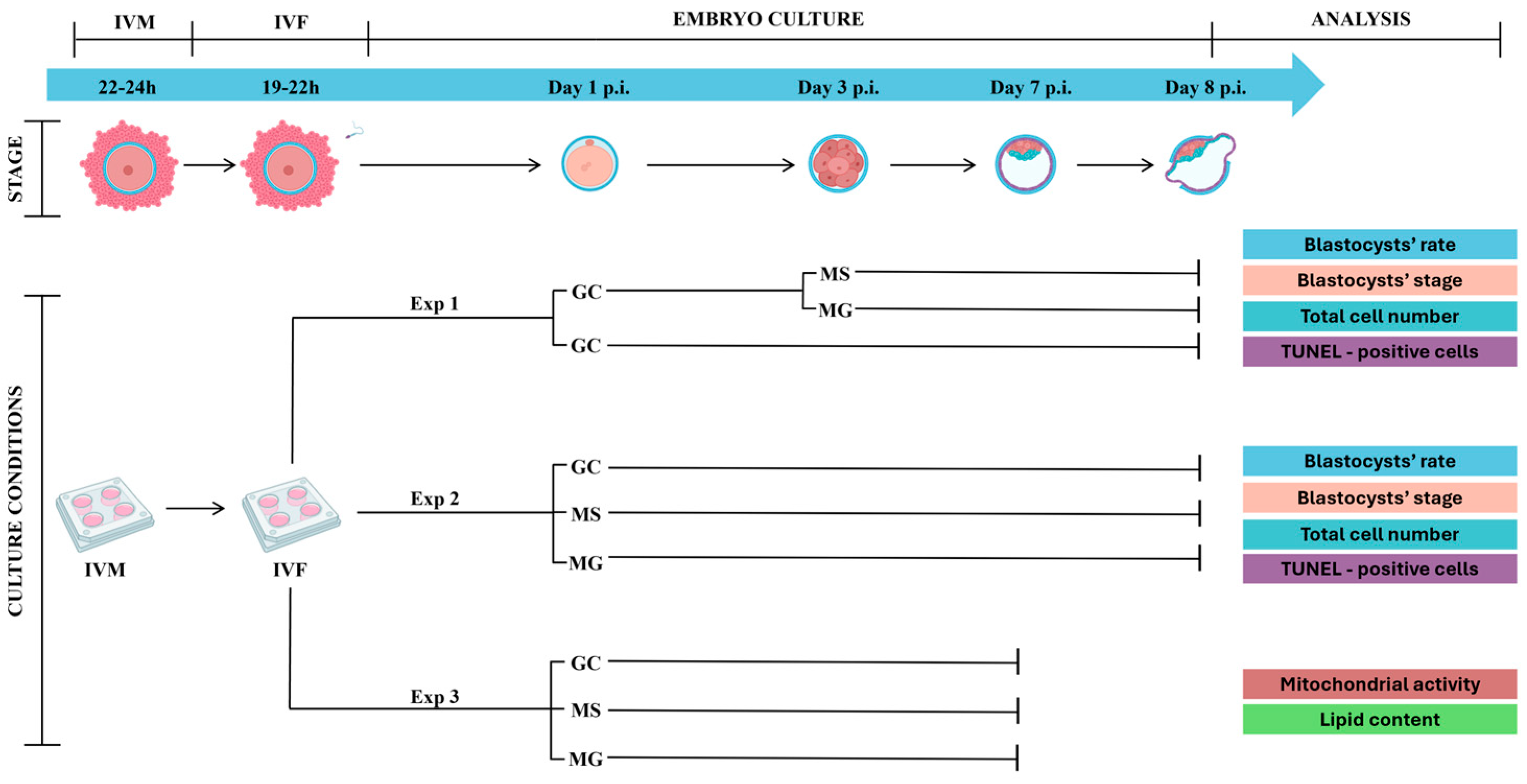
2.4. Experimental Design
2.5. Embryo Development and Quality Assessment
2.5.1. TUNEL Assay
2.5.2. Blastocyst Mitochondrial Activity and Lipid Content
2.6. Statistical Analysis
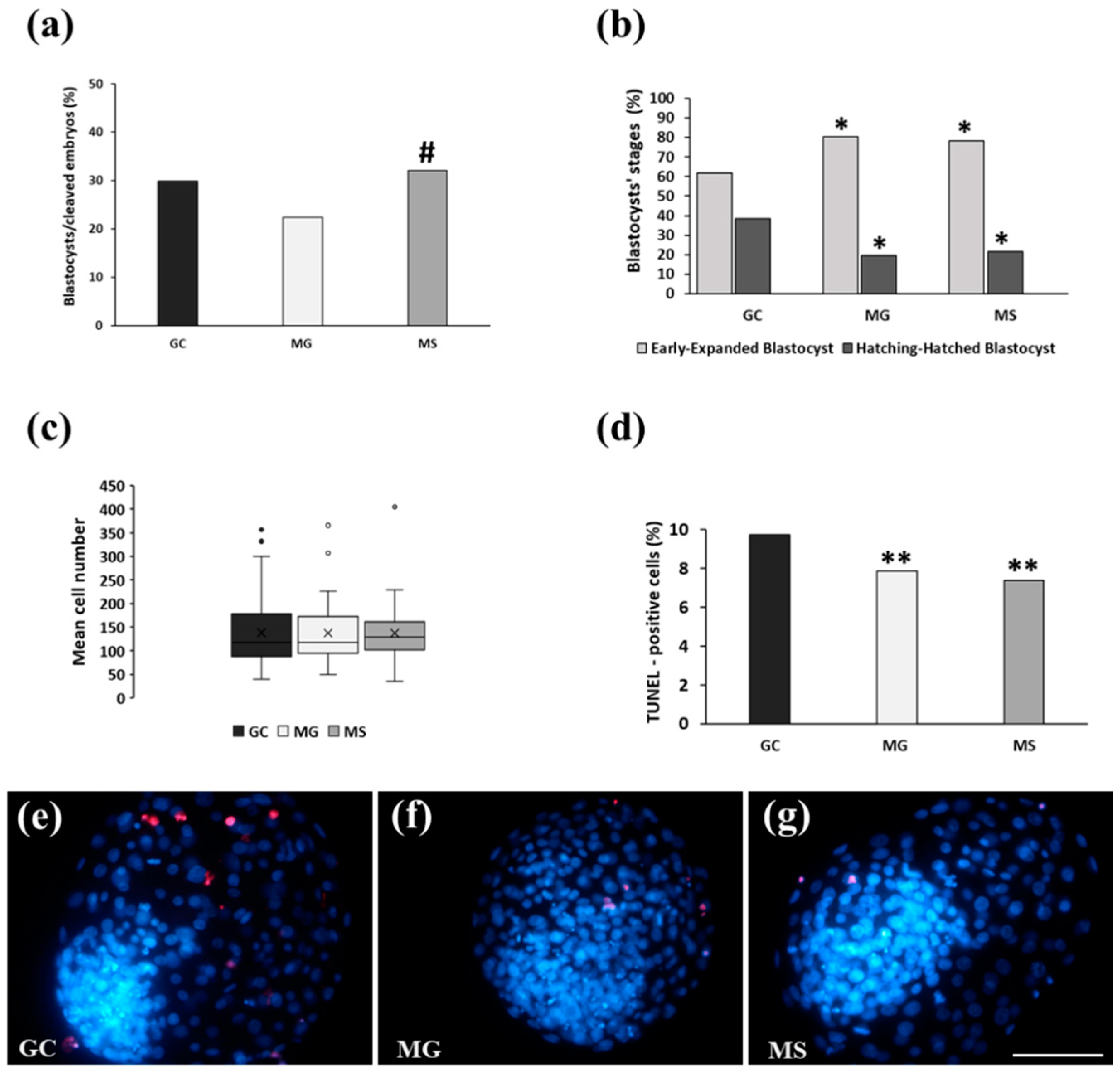
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vajta, G.; Parmegiani, L.; Machaty, Z.; Chen, W.B.; Yakovenko, S. Back to the future: Optimised microwell culture of individual human preimplantation stage embryos. J. Assist. Reprod. Genet. 2021, 38, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Consensus Group, C. ‘There is only one thing that is truly important in an IVF laboratory: Everything’ Cairo Consensus Guidelines on IVF Culture Conditions. Reprod. BioMed. Online 2020, 40, 33–60. [Google Scholar] [CrossRef]
- Vajta, G.; Rienzi, L.; Cobo, A.; Yovich, J. Embryo culture: Can we perform better than nature? Reprod. Biomed. Online 2010, 20, 453–469. [Google Scholar] [CrossRef]
- Tšuiko, O.; Catteeuw, M.; Zamani Esteki, M.; Destouni, A.; Bogado Pascottini, O.; Besenfelder, U.; Havlicek, V.; Smits, K.; Kurg, A.; Salumets, A.; et al. Genome stability of bovine in vivo-conceived cleavage-stage embryos is higher compared to in vitro-produced embryos. Hum. Reprod. 2017, 32, 2348–2357. [Google Scholar] [CrossRef]
- Rizos, D.W.F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Macháty, Z.; Day, B.N.; Prather, R.S. Development of Early Porcine Embryos In Vitro and In Vivo1. Biol. Reprod. 1998, 59, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.K.; Isom, S.C.; Spate, L.D.; Whitworth, K.M.; Spollen, W.G.; Blake, S.M.; Springer, G.K.; Murphy, C.N.; Prather, R.S. Transcriptional Profiling by Deep Sequencing Identifies Differences in mRNA Transcript Abundance in In Vivo-Derived Versus In Vitro-Cultured Porcine Blastocyst Stage Embryos1. Biol. Reprod. 2010, 83, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Jochems, R.; Canedo-Ribeiro, C.; Silvestri, G.; Derks, M.F.L.; Hamland, H.; Zak, L.J.; Knol, E.F.; Handyside, A.H.; Grindflek, E.; Griffin, D.K. Preimplantation Genetic Testing for Aneuploidy (PGT-A) Reveals High Levels of Chromosomal Errors in In Vivo-Derived Pig Embryos, with an Increased Incidence When Produced In Vitro. Cells 2023, 12, 790. [Google Scholar] [CrossRef]
- Rambags, B.P.B.; Krijtenburg, P.J.; Drie, H.F.V.; Lazzari, G.; Galli, C.; Pearson, P.L.; Colenbrander, B.; Stout, T.A.E. Numerical chromosomal abnormalities in equine embryos produced in vivo and in vitro. Mol. Reprod. Dev. 2005, 72, 77–87. [Google Scholar] [CrossRef]
- Murray, J.D.; Moran, C.; Boland, M.P.; Nancarrow, C.D.; Sutton, R.; Hoskinson, R.M.; Scaramuzzi, R.J. Polyploid cells in blastocysts and early fetuses from Australian Merino sheep. Reproduction 1986, 78, 439–446. [Google Scholar] [CrossRef]
- Viuff, D.; Rickords, L.; Offenberg, H.; Hyttel, P.; Avery, B.; Greve, T.; Olsaker, I.; Williams, J.L.; Callesen, H.; Thomsen, P.D. A High Proportion of Bovine Blastocysts Produced In Vitro Are Mixoploid1. Biol. Reprod. 1999, 60, 1273–1278. [Google Scholar] [CrossRef]
- O’Neill, C. The potential roles for embryotrophic ligands in preimplantation embryo development. Hum. Reprod. Update 2008, 14, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Gopichandran, N.; Leese, H.J. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006, 131, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Maillo, V.; Sánchez-Calabuig, M.-J.; Lonergan, P. The Consequences of Maternal-Embryonic Cross Talk during the Periconception Period on Subsequent Embryonic Development; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 69–86. [Google Scholar]
- Harris, E.A.; Stephens, K.K.; Winuthayanon, W. Extracellular Vesicles and the Oviduct Function. Int. J. Mol. Sci. 2020, 21, 8280. [Google Scholar] [CrossRef] [PubMed]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the Early Embryonic Microenvironment: Physiology and Regulation of Oviductal Secretions. Int. J. Mol. Sci. 2019, 21, 223. [Google Scholar] [CrossRef] [PubMed]
- Paria, B.C.; Dey, S.K. Preimplantation embryo development in vitro: Cooperative interactions among embryos and role of growth factors. Proc. Natl. Acad. Sci. USA 1990, 87, 4756–4760. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Robichaud, A.; Mercier, J.; Ouellette, R. Influence of group embryo culture strategies on the blastocyst development and pregnancy outcome. J. Assist. Reprod. Genet. 2013, 30, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Minasi, M.G.; Fabozzi, G.; Casciani, V.; Lobascio, A.M.; Colasante, A.; Scarselli, F.; Greco, E. Improved blastocyst formation with reduced culture volume: Comparison of three different culture conditions on 1128 sibling human zygotes. J. Assist. Reprod. Genet. 2015, 32, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Carolan, C.; Lonergan, P.; Khatir, H.; Mermillod, P. In vitro production of bovine embryos using individual oocytes. Mol. Reprod. Dev. 1996, 45, 145–150. [Google Scholar] [CrossRef]
- Miller, K.F.; Goldberg, J.M.; Collins, R.L. Covering embryo cultures with mineral oil alters embryo growth by acting as a sink for an embryotoxic substance. J. Assist. Reprod. Genet. 1994, 11, 342–345. [Google Scholar] [CrossRef]
- Shimada, M.; Kawano, N.; Terada, T. Delay of nuclear maturation and reduction in developmental competence of pig oocytes after mineral oil overlay of in vitro maturation media. Reproduction 2002, 124, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Scarica, C.; Monaco, A.; Borini, A.; Pontemezzo, E.; Bonanni, V.; De Santis, L.; Zacà, C.; Coticchio, G. Use of mineral oil in IVF culture systems: Physico-chemical aspects, management, and safety. J. Assist. Reprod. Genet. 2022, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.F.; Pursel, V.G. Absorption of compounds in medium by the oil covering microdrop cultures. Gamete Res. 1987, 17, 57–61. [Google Scholar] [CrossRef]
- Otsuki, J.; Nagai, Y.; Chiba, K. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil. Steril. 2007, 88, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Nohalez, A.; Parrilla, I.; Motas, M.; Roca, J.; Romero, I.; García-GonzáLez, D.L.; Cuello, C.; Rodriguez-Martinez, H.; Martinez, E.A.; et al. The overlaying oil type influences in vitro embryo production: Differences in composition and compound transfer into incubation medium between oils. Sci. Rep. 2017, 7, 10505. [Google Scholar] [CrossRef] [PubMed]
- Morbeck, D.E.; Khan, Z.; Barnidge, D.R.; Walker, D.L. Washing mineral oil reduces contaminants and embryotoxicity. Fertil. Steril. 2010, 94, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Vajta, G.P.T.T.; Holm, P.; Páldi, A.; Greve, T.; Trounson, A.O.; Callesen, H. New method for culture of zona-included or zona-free embryos: The Well of the Well (WOW) system. Mol. Reprod. Dev. 2000, 55, 256–264. [Google Scholar] [CrossRef]
- Vajta, G.; Korösi, T.; Du, Y.; Nakata, K.; Ieda, S.; Kuwayama, M.; Nagy, Z.P. The Well-of-the-Well system: An efficient approach to improve embryo development. Reprod. Biomed. Online 2008, 17, 73–81. [Google Scholar] [CrossRef]
- Apter, S.; Ebner, T.; Freour, T.; Guns, Y.; Kovacic, B.; Le Clef, N.; Marques, M.; Meseguer, M.; Montjean, D.; Sfontouris, I.; et al. Good practice recommendations for the use of time-lapse technology. Hum. Reprod. Open 2020, 2020, hoaa008. [Google Scholar] [CrossRef] [PubMed]
- Lemmen, J.G.; Agerholm, I.; Ziebe, S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod. Biomed. Online 2008, 17, 385–391. [Google Scholar] [CrossRef]
- Lee, A.M.; Connell, M.T.; Csokmay, J.M.; Styer, A.K. Elective single embryo transfer- the power of one. Contracept. Reprod. Med. 2016, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Jun, J.H. Non-invasive evaluation of embryo quality for the selection of transferable embryos in human in vitro fertilization-embryo transfer. Clin. Exp. Reprod. Med. 2022, 49, 225–238. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, J.; Dong, H.; Luo, O.; Zheng, X.; Obergfell, C.; Tang, Y.; Bi, J.; O’Neill, R.; Ruan, Y.; et al. Transcriptional profiles of bovine in vivo pre-implantation development. BMC Genom. 2014, 15, 756. [Google Scholar] [CrossRef]
- Ménézo, Y.J.; Hérubel, F. Mouse and bovine models for human IVF. Reprod. Biomed. Online 2002, 4, 170–175. [Google Scholar] [CrossRef]
- Cañón-Beltrán, K.; Cajas, Y.N.; Peréz-Cerezales, S.; Leal, C.L.V.; Agirregoitia, E.; Gutierrez-Adán, A.; González, E.M.; Rizos, D. Nobiletin enhances the development and quality of bovine embryos in vitro during two key periods of embryonic genome activation. Sci. Rep. 2021, 11, 11796. [Google Scholar] [CrossRef] [PubMed]
- Zmuidinaite, R.; Sharara, F.I.; Iles, R.K. Current Advancements in Noninvasive Profiling of the Embryo Culture Media Secretome. Int. J. Mol. Sci. 2021, 22, 2513. [Google Scholar] [CrossRef]
- Egea, R.R.; Puchalt, N.G.; Escrivá, M.M.; Varghese, A.C. OMICS: Current and future perspectives in reproductive medicine and technology. J. Hum. Reprod. Sci. 2014, 7, 73–92. [Google Scholar] [CrossRef]
- Rabel, R.A.C.; Marchioretto, P.V.; Bangert, E.A.; Wilson, K.; Milner, D.J.; Wheeler, M.B. Pre-Implantation Bovine Embryo Evaluation—From Optics to Omics and Beyond. Animals 2023, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Looman, J.; Rodriguez, Z.; Waugh, L.; Gibbons, J. Effects of bovine ova density and culture supplements on cleavage and blastocyst development rates of in vitro embryos. Clin. Theriogenol. 2024, 16. [Google Scholar] [CrossRef]
- Reed, M.L.; Woodward, B.J.; Swain, J.E. Single or group culture of mammalian embryos: The verdict of the literature. J. Reprod. Stem Cell Biotechnol. 2011, 22, 77–87. [Google Scholar] [CrossRef]
- Donnay, I.; Van Langendonckt, A.; Auquier, P.; Grisart, B.; Vansteenbrugge, A.; Massip, A.; Dessy, F. Effects of co-culture and embryo number on the in vitro development of bovine embryos. Theriogenology 1997, 47, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.L.; Gardner, D.K. In vitro culture of individual mouse preimplantation embryos: The role of embryo density, microwells, oxygen, timing and conditioned media. Reprod. BioMed. Online 2017, 34, 441–454. [Google Scholar] [CrossRef]
- Ebner, T.; Shebl, O.; Moser, M.; Mayer, R.B.; Arzt, W.; Tews, G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod. BioMed. Online 2010, 21, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Stokes, P.J.; Abeydeera, L.R.; Leese, H.J. Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Dev. Biol. 2005, 284, 62–71. [Google Scholar] [CrossRef]
- Ruíz, M.; Santamaría-López, E.; Blasco, V.; Hernáez, M.J.; Caligara, C.; Pellicer, A.; Fernández-Sánchez, M.; Prados, N. Effect of Group Embryo Culture under Low-Oxygen Tension in Benchtop Incubators on Human Embryo Culture: Prospective, Randomized, Controlled Trial. Reprod. Sci. 2020, 27, 1522–1533. [Google Scholar] [CrossRef]
- Spyropoulou, I. A prospective randomized study comparing the outcome of in-vitro fertilization and embryo transfer following culture of human embryos individually or in groups before embryo transfer on day 2. Hum. Reprod. 1999, 14, 76–79. [Google Scholar] [CrossRef]
- Rebollar-Lazaro, I.; Matson, P. The culture of human cleavage stage embryos alone or in groups: Effect upon blastocyst utilization rates and implantation. Reprod. Biol. 2010, 10, 227–234. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum. Reprod. 1992, 7, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Schini, S.A.; Bavister, B.D. Two-Cell Block to Development of Cultured Hamster Embryos is Caused by Phosphate and Glucose1. Biol. Reprod. 1988, 39, 1183–1192. [Google Scholar] [CrossRef]
- O’Doherty, E.M.; Wade, M.G.; Hill, J.L.; Boland, M.P. Effects of culturing bovine oocytes either singly or in groups on development to blastocysts. Theriogenology 1997, 48, 161–169. [Google Scholar] [CrossRef]
- Thouas, G.; Jones, G.; Trounson, A. The ‘GO’ system—A novel method of microculture for in vitro development of mouse zygotes to the blastocyst stage. Reproduction 2003, 126, 161–169. [Google Scholar] [CrossRef]
- Vajta, G.; Lewis, I.M.; Hyttel, P.; Thouas, G.A.; Trounson, A.O. Somatic cell cloning without micromanipulators. Cloning 2001, 3, 89–95. [Google Scholar] [CrossRef]
- Sugimura, S.; Akai, T.; Somfai, T.; Hirayama, M.; Aikawa, Y.; Ohtake, M.; Hattori, H.; Kobayashi, S.; Hashiyada, Y.; Konishi, K.; et al. Time-Lapse Cinematography-Compatible Polystyrene-Based Microwell Culture System: A Novel Tool for Tracking the Development of Individual Bovine Embryos. Biol. Reprod. 2010, 83, 970–978. [Google Scholar] [CrossRef]
- Cockburn, K.; Rossant, J. Making the blastocyst: Lessons from the mouse. J. Clin. Investig. 2010, 120, 995–1003. [Google Scholar] [CrossRef]
- Graf, A.; Krebs, S.; Zakhartchenko, V.; Schwalb, B.; Blum, H.; Wolf, E. Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, 4139–4144. [Google Scholar] [CrossRef]
- Menchero, S.; Rayon, T.; Andreu, M.J.; Manzanares, M. Signaling pathways in mammalian preimplantation development: Linking cellular phenotypes to lineage decisions. Dev. Dyn. 2017, 246, 245–261. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2016, 22, 2–22. [Google Scholar] [CrossRef]
- De Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- López-Damián, E.P.; Jiménez-Medina, J.A.; Lammoglia, M.A.; Pimentel, J.A.; Agredano-Moreno, L.T.; Wood, C.; Galina, C.S.; Fiordelisio, T. Lipid droplets in clusters negatively affect Bos indicus embryos during cryopreservation. Anat. Histol. Embryol. 2018, 47, 435–443. [Google Scholar] [CrossRef]
- Li, R.; Hosoe, M.; Shioya, Y.; Bou, S. The preliminary research on freezing viability of bovine in vitro fertilized embryos. Chin. J. Sci. Agric. Sin. 2002, 35, 1125–1129. [Google Scholar]
- Nagashima, H.; Kashiwazaki, N.; Ashman, R.J.; Grupen, C.G.; Seamark, R.F.; Nottle, M.B. Removal of Cytoplasmic Lipid Enhances the Tolerance of Porcine Embryos to Chilling. Biol. Reprod. 1994, 51, 618–622. [Google Scholar] [CrossRef]
- Kajdasz, A.; Warzych, E.; Derebecka, N.; Madeja, Z.E.; Lechniak, D.; Wesoly, J.; Pawlak, P. Lipid Stores and Lipid Metabolism Associated Gene Expression in Porcine and Bovine Parthenogenetic Embryos Revealed by Fluorescent Staining and RNA-seq. Int. J. Mol. Sci. 2020, 21, 6488. [Google Scholar] [CrossRef]
- Sudano, M.J.; Rascado, T.D.S.; Tata, A.; Belaz, K.R.A.; Santos, V.G.; Valente, R.S.; Mesquita, F.S.; Ferreira, C.R.; Araújo, J.P.; Eberlin, M.N.; et al. Lipidome signatures in early bovine embryo development. Theriogenology 2016, 86, 472–484.e1. [Google Scholar] [CrossRef]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef]
- Leaver, M.; Wells, D. Non-invasive preimplantation genetic testing (niPGT): The next revolution in reproductive genetics? Hum. Reprod. Update 2020, 26, 16–42. [Google Scholar] [CrossRef]
- Salmerón, A.M.; Abreu, A.C.; Vilches-Ferrón, M.; Fernández, I. Solution NMR in human embryo culture media as an option for assessment of embryo implantation potential. NMR Biomed. 2021, 34, e4536. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travaglione, A.; Candela, A.; De Gregorio, V.; Genovese, V.; Cimmino, M.; Barbato, V.; Talevi, R.; Gualtieri, R. Individually Cultured Bovine Zygotes Successfully Develop to the Blastocyst Stage in an Extremely Confined Environment. Cells 2024, 13, 868. https://doi.org/10.3390/cells13100868
Travaglione A, Candela A, De Gregorio V, Genovese V, Cimmino M, Barbato V, Talevi R, Gualtieri R. Individually Cultured Bovine Zygotes Successfully Develop to the Blastocyst Stage in an Extremely Confined Environment. Cells. 2024; 13(10):868. https://doi.org/10.3390/cells13100868
Chicago/Turabian StyleTravaglione, Angela, Andrea Candela, Vincenza De Gregorio, Vincenzo Genovese, Mario Cimmino, Vincenza Barbato, Riccardo Talevi, and Roberto Gualtieri. 2024. "Individually Cultured Bovine Zygotes Successfully Develop to the Blastocyst Stage in an Extremely Confined Environment" Cells 13, no. 10: 868. https://doi.org/10.3390/cells13100868




