Impact of Titanium Skull Plate on Transcranial Magnetic Stimulation: Analysis of Induced Electric Fields
Abstract
:1. Introduction
2. Materials and Methods
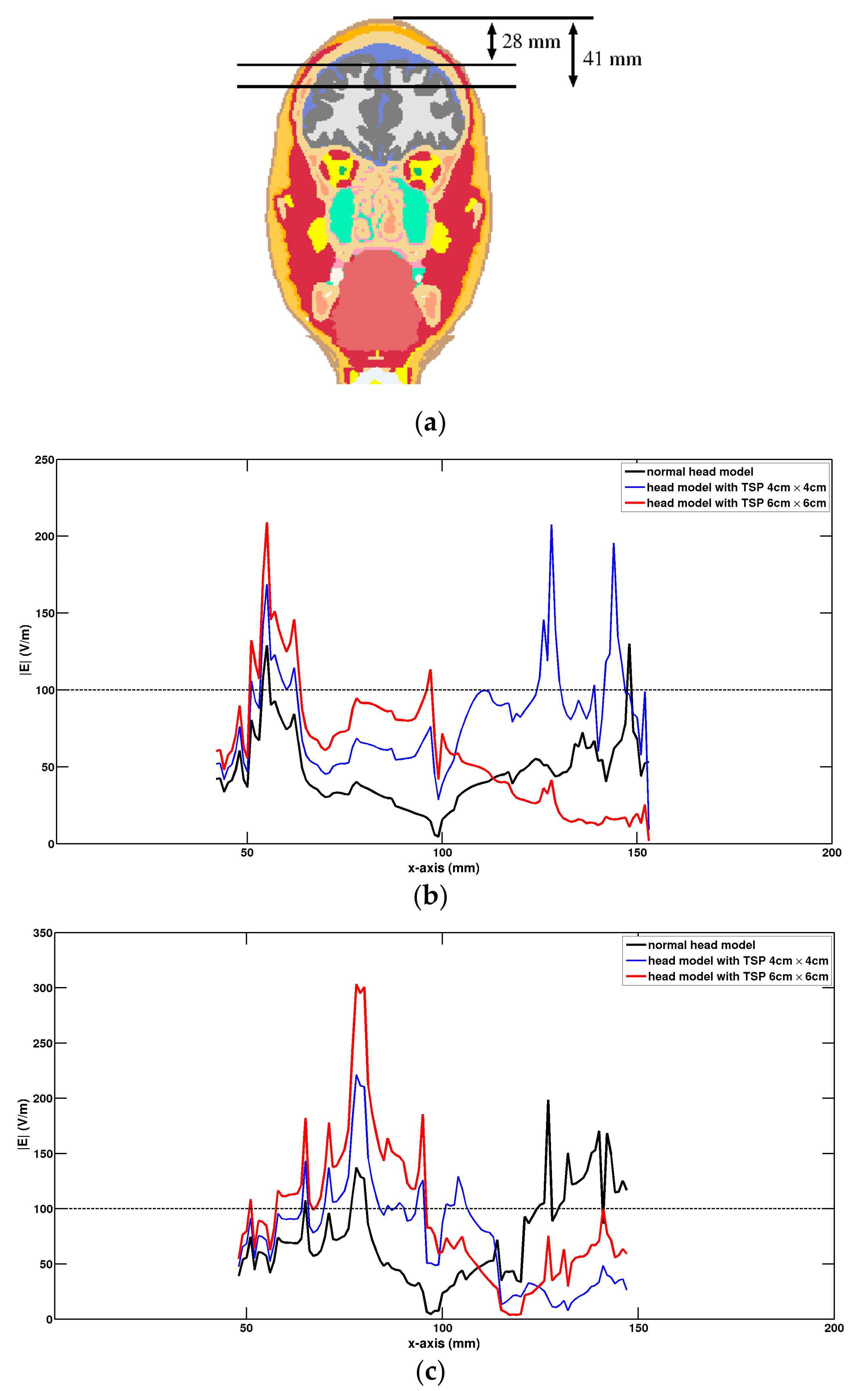
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Tashiro, T.; Harada, K. Localized stimulation of neural tissues in the brain by means of a paired configuration of time-varying magnetic fields. J. Appl. Phys. 1988, 64, 5862–5864. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; MacDonald, W.M. A Clinical Guide to Transcranial Magnetic Stimulation; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Jannati, A.; Oberman, L.M.; Rotenberg, A.; Pascual-Leone, A. Assessing the Mechanisms of Brain Plasticity by Transcranial Magnetic Stimulation. Neuropsychopharmacology 2023, 48, 191–208. [Google Scholar] [CrossRef]
- Marzouk, T.; Winkelbeiner, S.; Azizi, H.; Malhotra, A.K.; Homan, P. Transcranial Magnetic Stimulation for Positive Symptoms in Schizophrenia: A Systematic Review. Neuropsychobiology 2020, 79, 384–396. [Google Scholar] [CrossRef]
- Pelissolo, A.; Harika-Germaneau, G.; Rachid, F.; Gaudeau-Bosma, C.; Tanguy, M.-L.; BenAdhira, R.; Bouaziz, N.; Popa, T.; Wassouf, I.; Saba, G.; et al. Repetitive Transcranial Magnetic Stimulation to Supplementary Motor Area in Refractory Obsessive-Compulsive Disorder Treatment: A Sham-Controlled Trial. Int. J. Neuropsychopharmacol. 2016, 19, pyw025. [Google Scholar] [CrossRef] [PubMed]
- Galhardoni, R.; Correia, G.S.; Araujo, H.; Yeng, L.T.; Fernandes, D.T.; Kaziyama, H.H.; Marcolin, M.A.; Bouhassira, D.; Teixeira, M.J.; De Andrade, D.C. Repetitive Transcranial Magnetic Stimulation in Chronic Pain: A Review of the Literature. Arch. Phys. Med. Rehabil. 2015, 96, S156–S172. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, E.K.; Sohn, E. Repetitive Transcranial Magnetic Stimulation for Neuropathic Pain. Ann. Clin. Neurophysiol. 2022, 24, 53–58. [Google Scholar] [CrossRef]
- Jussi, N.; Anu, H.; Marjo, S.; Teemu, J.; Esa, R.; Antti, K. Repetitive Transcranial Magnetic Stimulation for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Prospective Pilot Study. Int. Neurourol. J. 2020, 24, 144–149. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and Recommendations for TMS Use in Healthy Subjects and Patient Populations, with Updates on Training, Ethical and Regulatory Issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Piazza, M.; Grady, M.S. Cranioplasty. Neurosurg. Clin. N. Am. 2017, 28, 257–265. [Google Scholar] [CrossRef]
- Luo, J.; Morrison, D.A.; Hayes, A.J.; Bala, A.; Watts, G. Single-Piece Titanium Plate Cranioplasty Reconstruction of Complex Defects. J. Craniofac. Surg. 2018, 29, 839–842. [Google Scholar] [CrossRef]
- Cabraja, M.; Klein, M.; Lehmann, T.-N. Long-Term Results Following Titanium Cranioplasty of Large Skull Defects. Neurosurg. Focus 2009, 26, E10. [Google Scholar] [CrossRef]
- Van der Meulen, J.J.; Nazir, P.R.; Mathijssen, I.M.; van Adrichem, L.N.; Ongkosuwito, E.; Stolk-Liefferink, S.A.; Vaandrager, M.J. Itemporal Depressions after Cranioplasty for Trigonocephaly: A Long(supra) Orbital Growth in Term Evaluation of 92 Patients. J. Craniofac. Surg. 2008, 19, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.J.; Pascual-Leone, A.; Cohen, L.G.; Hallett, M. The Heating of Metal Electrodes during Rapid-Rate Magnetic Stimulation: A Possible Safety Hazard. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1992, 85, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, A.; Harrington, M.G.; Birnbaum, D.S.; Madsen, J.R.; Glass, I.E.S.; Jensen, F.E.; Pascual-Leone, A. Minimal Heating of Titanium Skull Plates during 1Hz Repetitive Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2007, 118, 2536–2538. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, A.; Pascual-Leone, A. Safety of 1 Hz repetitive transcranial magnetic stimulation (rTMS) in patients with titanium skull plates. Clin. Neurophysiol. 2009, 120, 1417. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Dhamne, S.C.; Chen, J.-J.J.; Carpenter, L.L.; Anastasio, E.M.; Pascual-Leone, A.; Rotenberg, A. Minimal Heating of Aneurysm Clips during Repetitive Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2012, 123, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Stillman, M.; Chandonnet, N.; Davis, L.; Buzan, R.; Wirecki, T. Deep Transcranial Magnetic Stimulation in Patients with Intracranial Aneurysm Clips: A Case Report and Guidelines for Clinicians. Brain Stimul. 2020, 13, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Dhamne, S.C.; Ilmoniemi, R.J.; Tsuboyama, M.; Carpenter, L.L.; Pascual-Leone, A.; Rotenberg, A. Safety of rTMS in patients with intracranial metallic objects. Brain Stimul. 2020, 13, 928–929. [Google Scholar] [CrossRef]
- Datta, A.; Bikson, M.; Fregni, F. Transcranial direct current stimulation in patients with skull defects and skull plates: High-resolution computational FEM study of factors altering cortical current flow. Neuroimage 2010, 52, 1268–1278. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family—Development of Surface-Based Anatomical Models of Two Adults and Two Children for Dosimetric Simulations. Phys. Med. Biol. 2010, 55, N23–N38. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The Dielectric Properties of Biological Tissues: I. Literature Survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: II. Measurements in the Frequency Range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: III. Parametric Models for the Dielectric Spectrum of Tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef]
- Orcutt, N.; Gandhi, O.P. A 3-D Impedance Method to Calculate Power Deposition in Biological Bodies Subjected to Time Varying Magnetic Fields. IEEE Trans. Biomed. Eng. 1988, 35, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Ueno, S. Deep Transcranial Magnetic Stimulation Using Figure-of-Eight and Halo Coils. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar]
- Lu, M.; Ueno, S. Safety Assessment of H-Coil for Nursing Staff in Deep Transcranial Magnetic Stimulation. IEEE Magn. Lett. 2022, 13, 1–5. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Sommer, M.; Thickbroom, G.; Hamada, M.; Pascual-Leonne, A.; Paulus, W.; Classen, J.; Peterchev, A.V.; Zangen, A.; Ugawa, Y. Consensus: New Methodologies for Brain Stimulation. Brain Stimul. 2009, 2, 2–13. [Google Scholar] [CrossRef]





| Tissue | Conductivity (S/m) | Tissue | Conductivity (S/m) |
|---|---|---|---|
| Artery | 7.00 × 10−1 | Hypothalamus | 5.26 × 10−1 |
| Blood Vessel | 3.10 × 10−1 | Mandible | 2.03 × 10−2 |
| Cartilage | 1.75 × 10−1 | Marrow—bone | 2.44 × 10−3 |
| Cerebellum | 1.24 × 10−1 | MO*3 | 4.65 × 10−1 |
| CSF | 2.00 × 100 | Midbrain | 4.65 × 10−1 |
| CA*1 | 6.44 × 10−2 | Mucosa | 8.46 × 10−4 |
| CP*2 | 6.44 × 10−2 | Muscle | 3.31 × 10−1 |
| Connective Tissue | 2.04 × 10−1 | Nerve | 3.04 × 10−2 |
| Ear—cartilage | 1.75 × 10−1 | Pineal—body | 5.26 × 10−1 |
| Ear—skin | 2.00 × 10−4 | Pons | 4.65 × 10−1 |
| Eye—cornea | 4.25 × 10−1 | Skin | 2.00 × 10−4 |
| Eye—lens | 3.31 × 10−1 | Skull | 2.03 × 10−2 |
| Eye—sclera | 5.07 × 10−1 | Spinal Cord | 3.04 × 10−2 |
| Eye—vitreous humor | 1.50 × 100 | Teeth | 2.03 × 10−2 |
| FAT | 2.32 × 10−2 | Thalamus | 1.04 × 10−1 |
| Gray matter | 1.04 × 10−1 | Tongue | 2.76 × 10−1 |
| Hippocampus | 1.04 × 10−1 | titanium | 5.00 × 105 |
| Hypophysis | 5.26 × 10−1 | White Matter | 6.44 × 10−2 |
| Tissue | Head Model with TSP-4cm | Head Model with TSP-6cm | Normal Head Model |
|---|---|---|---|
| Cerebellum | 22.5 | 25 | 20.4 |
| Commissure—Anterior | 18.3 | 21.1 | 14.1 |
| Commissure—Posterior | 10.3 | 16.7 | 2.94 |
| Hippocampus | 30.3 | 45.9 | 16.8 |
| Hypophysis | 54.3 | 67.6 | 33.9 |
| Hypothalamus | 30.9 | 39.5 | 19.13 |
| Medulla Oblongata | 3.7 | 4.9 | 2.52 |
| Midbrain | 14.9 | 20.6 | 8.89 |
| Pineal—Body | 5.3 | 7.8 | 3.55 |
| Pons | 8.8 | 10.9 | 6.46 |
| Thalamus | 29.7 | 41.2 | 16.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Ueno, S. Impact of Titanium Skull Plate on Transcranial Magnetic Stimulation: Analysis of Induced Electric Fields. Life 2024, 14, 642. https://doi.org/10.3390/life14050642
Lu M, Ueno S. Impact of Titanium Skull Plate on Transcranial Magnetic Stimulation: Analysis of Induced Electric Fields. Life. 2024; 14(5):642. https://doi.org/10.3390/life14050642
Chicago/Turabian StyleLu, Mai, and Shoogo Ueno. 2024. "Impact of Titanium Skull Plate on Transcranial Magnetic Stimulation: Analysis of Induced Electric Fields" Life 14, no. 5: 642. https://doi.org/10.3390/life14050642







