The Influence of Lifestyle on Male Fertility in the Context of Insulin Resistance—Identification of Factors That Influence Semen Quality
Abstract
1. Introduction
2. Materials and Methods
- (a)
- HOMA-IR was calculated as follows: fasting insulin (μIU/mL) × fasting glucose (mmol/mL)/22.5;
- (b)
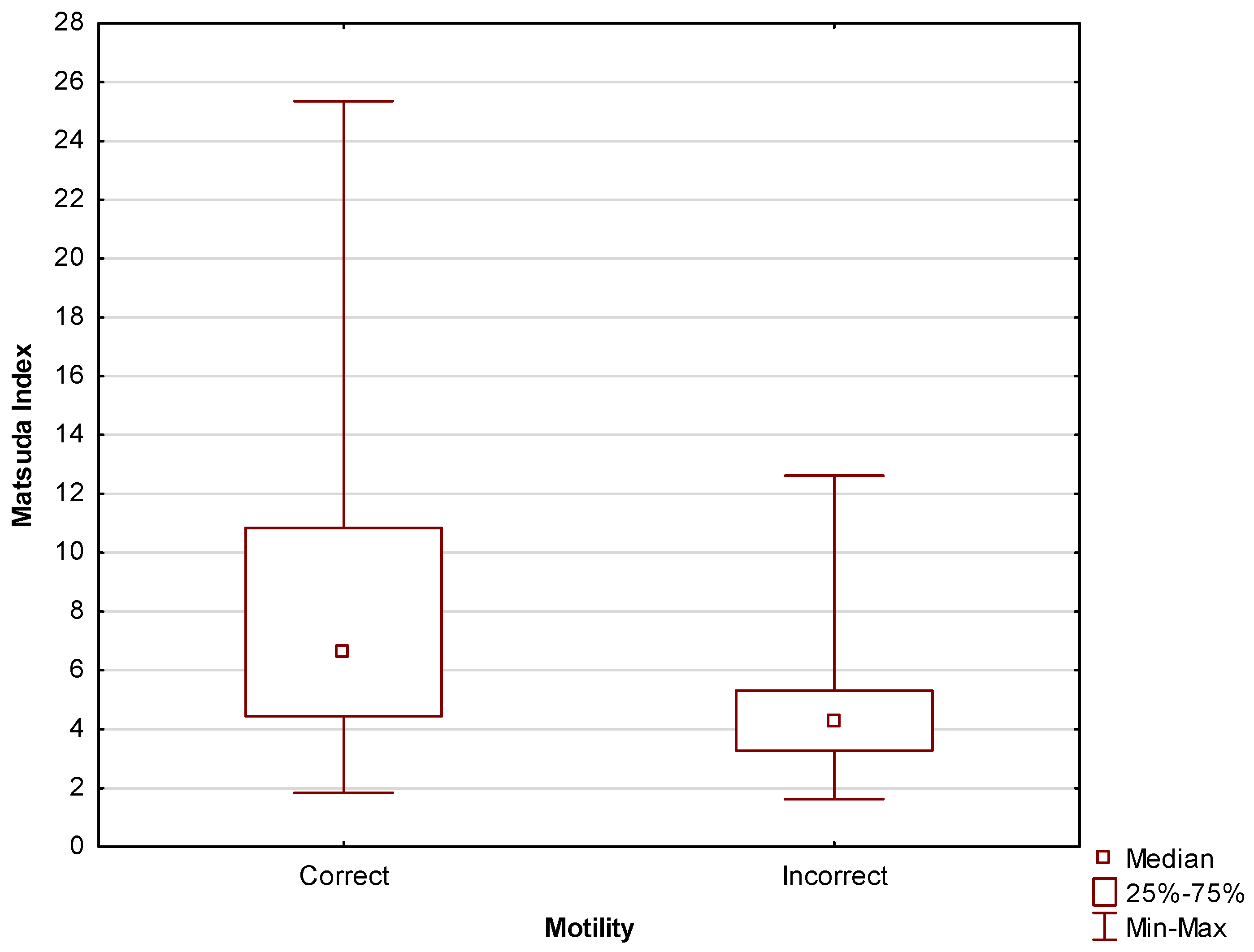
- Matsuda Index was calculated as follows: 10,000/√[fasting glucose (mmol/L) × fasting insulin (pmol/L)] × [mean glucose (mmol/L) × mean insulin (pmol/L) during OGTT] [49].
- (a)
- NOMA-IR index: R = fasting insulinemia (mU/mL) × fasting glycemia (mmol/l)/22.5; R > 0.91 ± 0.38 indicated the presence of insulin resistance;
- (b)
- Matsuda index: 100,000/fasting insulinemia (mU/mL) × fasting glycemia (mg/dl) × mean glycaemia value during OGTT × mean insulinemia value during OGTT; Matsuda index values < 7.3 may indicate the presence of insulin resistance [50].
- (1)
- Vitamin Consumption Index (VCI) (0–10 pts)—each vitamin consumed in amounts specified in the recommendations was awarded 1 pt. The following ten vitamins were considered in the analysis: vitamin E, vitamin A, thiamine, riboflavin, niacin, vitamin B6, vitamin C, folic acid, vitamin D, and vitamin B12.
- (2)
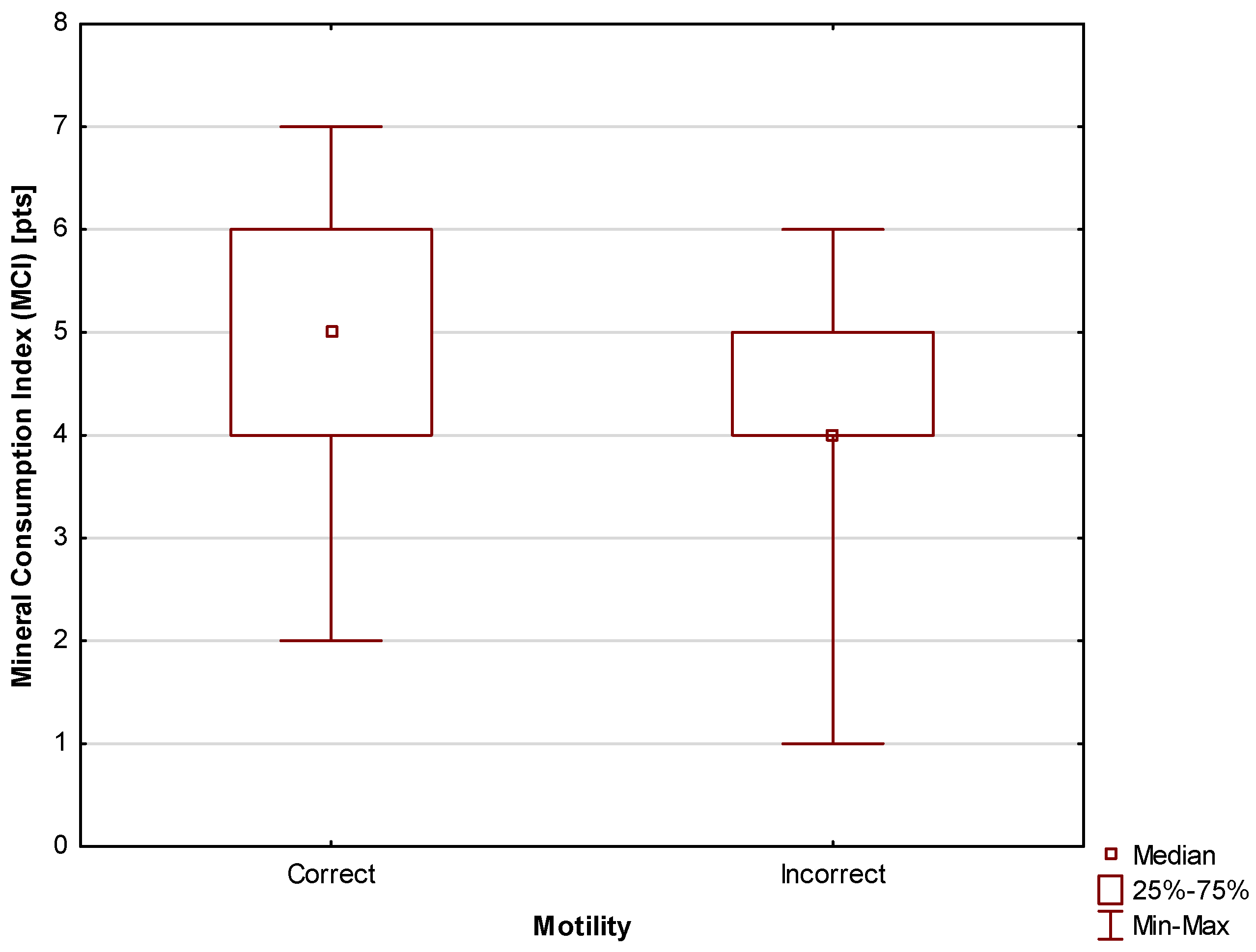
- Mineral Consumption Index (MCI) (0–9 pts)—each mineral consumed in amounts specified in the recommendations was awarded 1 pt. The following nine minerals were considered in the analysis: sodium, potassium, magnesium, phosphorus, calcium, iron, zinc, copper, and manganese.
- (3)
- Macronutrient Consumption Index (MacCI) (0–5 pts)—each of the four macronutrients consumed in amounts specified in the recommendations was awarded 1 pt.; 1 pt. was also awarded for adequate caloric intake. The following five parameters were considered in the analysis: fat, carbohydrate, fiber, dietary fiber, and caloric intake.
- (4)
- Dietary Index (DI) (0–24 pts)—this index is calculated as VCI + MCI + MacCI.
- (5)
- Body Composition Index (BCI) (0–3 pts)—each instance of a body composition norm met by the participant was awarded 1 pt. The following three parameters related to body composition were used to calculate the value of the BCI index: Total Body Water (TBW), Body Fat Mass (BFM), and Skeletal Muscle Index (SMI).
3. Results
3.1. Diet
3.2. Body Composition
3.3. Insulin Resistance
3.4. Sexual Abstinence
4. Discussion
5. Conclusions
6. Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dcunha, R.; Hussein, R.S.; Ananda, H.; Kumari, S.; Adiga, S.K.; Kannan, N.; Zhao, Y.; Kalthur, G. Current Insights and Latest Updates in Sperm Motility and Associated Applications in Assisted Reproduction. Reprod. Sci. 2022, 29, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Barratt, C.L. Semen analysis is the cornerstone of investigation for male infertility. Practitioner 2007, 251, 8. [Google Scholar] [PubMed]
- Goyal, R.; Kotru, M.; Gogia, A.; Sharma, S. Qualitative defects with normal sperm counts in a patient attending infertility clinic. Indian J. Pathol. Microbiol. 2018, 61, 233–235. [Google Scholar] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zańko, A.; Siewko, K.; Krętowski, A.J.; Milewski, R. Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility. Int. J. Environ. Res. Public Health 2022, 20, 732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traish, A.M.; Feeley, R.J.; Guay, A. Mechanisms of obesity and related pathologies: Androgen deficiency and endothelial dysfunction may be the link between obesity and erectile dysfunction. FEBS J. 2009, 276, 5755–5767. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.H.; Park, S.Y.; Kim, Y.W. Obesity and erectile dysfunction: From bench to clinical implication. World J. Mens Health 2019, 37, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.H.; Alexandre, E.C.; Calmasini, F.B.; Calixto, M.C.; Antunes, E. Treatment with metformin improves erectile dysfunction in a murine model of obesity associated with insulin resistance. Urology 2015, 86, 423.e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, R.; Huang, Y.; Zheng, F.; Ou, Y.; Tu, X.; Zhang, Y.; Gao, Y.; Chen, X.; Zheng, T.; et al. Insulin resistance is an independent determinate of ED in young adult men. PLoS ONE 2013, 8, e83951. [Google Scholar] [CrossRef]
- Diniz, A.F.A.; de Souza, I.L.L.; Ferreira, E.d.S.; Carvalho, M.T.d.L.; Barros, B.C.; Ferreira, P.B.; Silva, M.d.C.C.; Júnior, F.F.L.; Toscano, L.d.L.T.; Silva, A.S.; et al. Potential therapeutic role of dietary supplementation with spirulina platensis on the erectile function of obese rats fed a hypercaloric diet. Oxid. Med. Cell. Longev. 2020, 2020, 3293065. [Google Scholar] [CrossRef]
- Kondoh, N. Ejaculatory dysfunction as a cause of infertility. Reprod. Med. Biol. 2011, 11, 59–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoleri DTitchenell, P.M. Resolving the paradox of hepatic insulin resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Galmés-Pascual, B.M.; Martínez-Cignoni, M.R.; Morán-Costoya, A.; Bauza-Thorbrügge, M.; Sbert-Roig, M.; Valle, A.; Proenza, A.M.; Lladó, I.; Gianotti, M. 17β-Estradiol ameliorates lipotoxicity-induced hepatic mitochondrial oxidative stress and insulin resistance. Free Radic. Biol. Med. 2020, 150, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Kujawska-Łuczak, M.; Bogdański, P.; Pupek-Muszalik, D. Wskaźnik insulinowrażliwości M i wskaźnik IRI/G w ocenie insulinooporności u pacjentów z nadciśnieniem tętniczym i otyłością. Arter. Hypertens. 2010, 14, 142–150. [Google Scholar]
- Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Piwowar, A. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2019, 163, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Soeters, M.R.; Sauerwein, H.P.; Groener, J.E.; Aerts, J.M.; Ackermans, M.T.; Glatz, J.F.C.; Fliers, E.; Serlie, M.J. Gender-related differences in the metabolic response to fasting. J. Clin. Endocrinol. Metab. 2007, 92, 3646–3652. [Google Scholar] [CrossRef]
- Koska, J.; Stefan, N.; Permana, P.A.; Weyer, C.; Sonoda, M.; Bogardus, C.; Smith, S.R.; Joanisse, D.R.; Funahashi, T.; Krakoff, J.; et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am. J. Clin. Nutr. 2008, 87, 295–302. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhou, B.; Li, Y.; Wu, Z.; Meng, H.; Wang, G. Sex Differences in the Effect of Testosterone on Adipose Tissue Insulin Resistance From Overweight to Obese Adults. J. Clin. Endocrinol. Metab. 2021, 106, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Han, R.-Y.; Mei, X.-A.; Qi, Y.-N.; Ma, J.-Y.; Liu, W.-J.; Wang, S.-S. Correlation of insulin resistance with male reproductive hormone levels and semen parameters. Natl. J. Androl. 2018, 24, 695–699. [Google Scholar]
- Betancourt-Albrecht, M.; Cunningham, G.R. Hypogonadism and diabetes. Int. J. Impot. Res. 2003, 15, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, H.; Horie, H.; Inoue, T.; Kameyama, M.; Kanao, K.; Ishihara, S.; Tsujimura, T.; Nunotani, H.; Minami, T.; Okazaki, Y.; et al. Low testosterone levels in diabetic men and animals: A possible role in testicular impotence. Diabetes Res. Clin. Pract. 1989, 6, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Asare-Anane, H.; Bannison, S.B.; Ofori, E.K.; Ateko, R.O.; Bawah, A.T.; Amanquah, S.D.; Oppong, S.Y.; Gandau, B.B.N.; Ziem, J.B. Tobacco smoking is associated with decreased semen quality. Reprod. Health 2016, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Tsirka, G.; Stavros, S.; Vrachnis, D.; Vrachnis, N.; Potiris, A.; Georgiou, I.; et al. Sperm Mitochondrial Content and Mitochondrial DNA to Nuclear DNA Ratio Are Associated with Body Mass Index and Progressive Motility. Biomedicines 2023, 11, 3014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, C.-Q.; Yang, Y.-Y.; Chen, J.; Feng, L.; Lin, W.-Q. Association Between Sleep Quality and Semen Parameters and Reproductive Hormones: A Cross-Sectional Study in Zhejiang, China. Nat. Sci. Sleep 2020, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Cárceles, A.B.; Mínguez-Alarcón, L.; Mendiola, J.; Vioque, J.; Jørgensen, N.; Árense-Gonzalo, J.J.; Torres-Cantero, A.M.; Chavarro, J. Meat intake in relation to semen quality and reproductive hormone levels among young men in Spain. Br. J. Nutr. 2019, 121, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Jóźków, P.; Rossato, M. The impact of intense exercise on semen quality. Am. J. Men’s Health 2017, 11, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pang, A. Effects of metabolic syndrome on semen quality and circulating sex hormones: A systematic review and meta-analysis. Front. Endocrinol. 2020, 11, 428. [Google Scholar] [CrossRef]
- Orman, D.; Vardi, N.; Ates, B.; Taslidere, E.; Elbe, H. Aminoguanidine mitigates apoptosis, testicular seminiferous tubules damage, and oxidative stress in streptozotocin-induced diabetic rats. Tissue Cell 2015, 47, 284–290. [Google Scholar] [CrossRef]
- Morgante, G.; Tosti, C.; Orvieto, R.; Musacchio, M.C.; Piomboni, P.; De Leo, V. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil. Steril. 2011, 95, 2150–2152. [Google Scholar] [CrossRef]
- Tan, O.; Ha, T.; Carr, B.R.; Nakonezny, P.; Doody, K.M.; Doody, K.J. Predictive value of postwashed total progressively motile sperm count using CASA estimates in 6871 non-donor intrauterine insemination cycles. J. Assist. Reprod. Genet. 2014, 31, 1147–1153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeong, M.; Kim, S.K.; Kim, H.; Lee, J.R.; Jee, B.C.; Kim, S.H. Predictive value of sperm motility before and after preparation for the pregnancy outcomes of intrauterine insemination. Clin. Exp. Reprod. Med. 2021, 48, 255–261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andlib, N.; Sajad, M.; Kumar, R.; Thakur, S.C. Abnormalities in sex hormones and sexual dysfunction in males with diabetes mellitus: A mechanistic insight. Acta Histochem. 2023, 125, 151974. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, J. A Grain of Salt: The Science and Pseudoscience of What We Eat; ECW Press: Toronto, ON, Canada, 2019. [Google Scholar]
- Agarwal, A.; Nallella, K.P.; Allamaneni, S.S.; Said, T.M. Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod. Biomed. Online 2004, 8, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bullo, M.; Salas-Salvado, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Nassan, F.L.; Chiu, Y.H.; Minguez-Alarcon, L.; Williams, P.L.; Souter, I.; Hauser, R.; Chavarro, J.E.; EARTH Study Team. Intake of Antioxidants in Relation to Infertility Treatment Outcomes with Assisted Reproductive Technologies. Epidemiology 2019, 30, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Chiu, Y.H.; Gaskins, A.J.; Mínguez-Alarcón, L.; Nassan, F.L.; Williams, P.L.; Petrozza, J.; Hauser, R.; Chavarro, J.E.; for the EARTH Study Team. Men’s Intake of Vitamin C and β-Carotene Is Positively Related to Fertilization Rate but Not to Live Birth Rate in Couples Undergoing Infertility Treatment. J. Nutr. 2019, 149, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Sá, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scarlata, E.; Fernandez, M.C.; O’Flaherty, C. A Novel Combination of γ-Tocopherol-Rich Mixture of Tocopherols and Ascorbic Acid Restores Fertility in Cases of Tyrosine Nitration-Associated Male Infertility in Mice. Antioxidants 2020, 9, 613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef]
- Whillier, S. Exercise and Insulin Resistance. Adv. Exp. Med. Biol. 2020, 1228, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Ibañez-Perez, J.; Santos-Zorrozua, B.; Lopez-Lopez, E.; Matorras, R.; Garcia-Orad, A. An update on the implication of physical activity on semen quality: A systematic review and meta-analysis. Arch Gynecol. Obstet. 2019, 299, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, J.; Rhodes, S.; Sellke, N.; Navarrete, F.; Keller, S.; Gowda, V.; Simon, P.H.G.; Abou Ghayda, R. The Variability of Semen Parameters with Sexual Abstinence Using Mail-in Sperm Testing Is Similar to That Seen with Traditional In-Office Semen Analysis. Am. J. Men’s Health 2023, 17, 15579883231197910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elzanaty, S.; Malm, J.; Giwercman, A. Duration of sexual abstinence: Epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum. Reprod. 2005, 20, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Levitas, E.; Lunenfeld, E.; Weiss, N.; Friger, M.; Har-Vardi, I.; Koifman, A.; Potashnik, G. Relationship between the duration of sexual abstinence and semen quality: Analysis of 9489 semen samples. Fertil. Steril. 2005, 83, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Szurkowska, M.; Szafraniec, K.; Gilis-Januszewska, A. Wskaźniki insulinooporności w badaniu populacyjnym i ich wartość predykcyjna w określeniu zespołu metabolicznego. Przegląd Epidemiol. 2005, 59, 743–752. [Google Scholar]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny: Warsaw, Poland, 2020; ISBN 978-83-65870-28-5. [Google Scholar]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2016, 96, 2–12. [Google Scholar] [CrossRef]
- Brown, R.L. Rate of transport of spermia in human uterus and tubes. Am. J. Obstet. Gynecol. 1944, 47, 407–411. [Google Scholar] [CrossRef]
- Ricci, E.; Al Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Viganò, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. BioMed Online 2017, 34, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.N.; Klock, S.C.; Geoghegan, A.; Travassos, D.E. Relationship between psychological stress and semen quality among in-vitro fertilization patients. Hum. Reprod. 1999, 14, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elmoaty, M.A.; Saleh, R.; Sharma, R.; Agarwal, A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil. Steril. 2010, 94, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Kumar, R. An update on the role of medical treatment including antioxidant therapy in varicocele. Asian J. Androl. 2016, 18, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Guazzone, V.A.; Lustig, L. Varicocele and testicular cord torsion: Immune testicular microenvironment imbalance. Front. Cell Dev. Biol. 2023, 11, 1282579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lane, M.; McPherson, N.O.; Fullston, T.; Spillane, M.; Sandeman, L.; Xian, W.; Zander, D.L. Oxidative stress in mouse sperm impairs embryo development, fetal growth and alters adiposity and glucose regulation in female offspring. PLoS ONE 2014, 9, e100832. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O.P.; Long, K.Z.; Rosado, J.L. Impact of micronutrient deficiencies on obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef]
- McPherson, N.O.; Shehadeh, H.; Fullston, T.; Zander-Fox, D.L.; Lane, M. Dietary Micronutrient Supplementation for 12 Days in Obese Male Mice Restores Sperm Oxidative Stress. Nutrients 2019, 11, 2196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oostingh, E.C.; Steegers-Theunissen, R.P.; de Vries, J.H.; Laven, J.S.; Koster, M.P. Strong adherence to a healthy dietary pattern is associated with better semen quality, especially in men with poor semen quality. Fertil. Steril. 2017, 107, 916–923. [Google Scholar] [CrossRef]
- Wadhwa, L.; Priyadarshini, S.; Fauzdar, A.; Wadhwa, S.N.; Arora, S. Impact of Vitamin D Supplementation on Semen Quality in Vitamin D-Deficient Infertile Males with Oligoasthenozoospermia. J. Obstet. Gynaecol. India 2020, 70, 44–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2022, 5, CD007411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maghsoumi-Norouzabad, L.; Zare Javid, A.; Mansoori, A.; Dadfar, M.; Serajian, A. Evaluation of the effect of vitamin D supplementation on spermatogram, seminal and serum levels of oxidative stress indices in asthenospermia infertile men: A study protocol for a triple-blind, randomized controlled trial. Nutr. J. 2021, 20, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sliwowska, J.H.; Fergani, C.; Gawałek, M.; Skowronska, B.; Fichna, P.; Lehman, M.N. Insulin: Its role in the central control of reproduction. Physiol. Behav. 2014, 133, 197–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alves, M.G.; Martins, A.D.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 2013, 1, e23992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mai, H.; Ke, J.; Zheng, Z.; Luo, J.; Li, M.; Qu, Y.; Jiang, F.; Cai, S.; Zuo, L. Association of diet and lifestyle factors with semen quality in male partners of Chinese couples preparing for pregnancy. Reprod. Health 2023, 20, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marinaro, J.A. Optimizing outcomes for men with severe infertility. Curr. Opin. Urol. 2023, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.V.; Zeffer, K.B.; Buster, J.E.; Sokol, R.Z. Effect of abstinence on sperm motility in normal men. Am. J. Obstet. Gynecol. 1988, 158 Pt 1, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.V.; Pereira, S.C.; Guerra-Carvalho, B.; Carrageta, D.F.; Pinto, S.; Barros, A.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Hyperoside Supplementation in Preservation Media Surpasses Vitamin C Protection Against Oxidative Stress-Induced Damages in Human Spermatozoa. Cell Physiol. Biochem. 2022, 56, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Fatma, B.A.; Nozha, C.F.; Ines, D.; Hamadi, A.; Basma, H.; Leila, A.K. Sperm quality improvement after date seed oil in vitro supplementation in spontaneous and induced oxidative stress. Asian J. Androl. 2009, 11, 393–398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banihani, S.A. Vitamin B12 and Semen Quality. Biomolecules 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laganà, A.S.; Vitale, S.G.; Ban Frangež, H.; Vrtačnik-Bokal, E.; D’Anna, R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4243–4251. [Google Scholar] [PubMed]
- Salas-Huetos, A.; Babio, N.; Carrell, D.T.; Bulló, M.; Salas-Salvadó, J. Adherence to the Mediterranean diet is positively associated with sperm motility: A cross-sectional analysis. Sci. Rep. 2019, 9, 3389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]






| Parameter | n | Insulin Resistance Median (Q1; Q3) | n | No insulin Resistance Median (Q1; Q3) |
|---|---|---|---|---|
| Age | 48 | 28.00 (25.00; 32.00) | 28 | 26.50 (25.00; 30.00) |
| BMI | 48 | 28.96 (26.74; 33.05) | 28 | 24.41 (22.28; 27.84) |
| Physical activity (hours/week) | 40 | 0.50 (0.50; 3.00) | 19 | 3.00 (0.50; 3.00) |
| TBW (Total Body Water) | 48 | 50.55 (47.20; 55.15) | 28 | 49.95 (45.75; 54.75) |
| BFM (Body Fat Mass) | 48 | 26.70 (21.00; 36.75) | 28 | 14.15 (10.60; 17.50) |
| SMI (Skeletal Muscle Index) | 48 | 41.34 (38.14; 44.02) | 28 | 47.65 (43.71; 49.34) |
| Sperm concentration per mL | 45 | 29.48 (14.37; 46.88) | 28 | 30.66 (12.45; 54.04) |
| Sperm concentration per ejaculate | 45 | 69.53 (40.71; 113.82) | 28 | 101.51 (26.53; 181.96) |
| Progressive sperm motility | 45 | 34.78 (19.04; 47.64) | 28 | 37.45 (29.59; 46.85) |
| Sperm motility | 45 | 48.64 (34.69; 69.02) | 28 | 58.18 (46.83; 70.94) |
| Sperm morphology | 45 | 8.00 (3.00; 13.00) | 28 | 6.00 (2.50; 9.50) |
| Fasting insulin | 48 | 8.90 (7.10; 12.45) | 28 | 4.00 (2.80; 5.55) |
| Cholesterol level | 48 | 179.50 (163.50; 204.00) | 28 | 170.00 (151.00; 182.00) |
| LDL cholesterol level | 48 | 122.00 (103.50; 145.00) | 28 | 105.50 (84.50; 127.50) |
| HDL cholesterol level | 48 | 50.00 (39.00; 56.00) | 28 | 52.50 (44.50; 63.00) |
| Triglyceride level | 48 | 117.00 (83.50; 141.50) | 28 | 75.00 (46.50; 92.50) |
| Parameter | n | Insulin Resistance Median (Q1; Q3) | n | No Insulin Resistance Median (Q1; Q3) |
|---|---|---|---|---|
| Fast progressive motility [%] | 45 | 19.86 (8.89; 31.13) | 28 | 20.13 (9.15; 32.70) |
| Slow progressive motility [%] | 45 | 13.63 (10.18; 11.80) | 28 | 15.68 (10.71; 19.12) |
| Non-progressive motility [%] | 45 | 13.86 (10.66; 17.86) | 28 | 16.58 (12.77; 23.71) |
| Immobile sperm [%] | 45 | 47.18 (30.98; 58.22) | 28 | 38.02 (28.65; 50.65) |
| Hyperactive sperm [%] | 45 | 0.00 (0.00; 0.00) | 28 | 0.00 (0.00; 0.00) |
| Mucus penetration [%] | 45 | 33.87 (22.03; 43.78) | 28 | 34.46 (16.95; 43.01) |
| Body Composition Index (BCI) [0–3] | ||||||
|---|---|---|---|---|---|---|
| Motility | 0 | 1 | 2 | 3 | Total | p-Value |
| Incorrect | 8 (50%) | 6 (25%) | 3 (14.29%) | 1 (8.33%) | 18 | 0.04 |
| Correct | 8 (50%) | 18 (75%) | 18 (85.71%) | 11 (91.67%) | 55 | |
| Total | 16 | 24 | 21 | 12 | 73 | |
| HOMA-IR IR Diagnosis | ||||
|---|---|---|---|---|
| Motility | No Insulin Resistance | Insulin Resistance | Total | p-Value |
| Incorrect | 8 (17.02%) | 10 (38.46%) | 18 | 0.04 |
| Correct | 39 (82.98%) | 16 (61.54%) | 55 | |
| Total | 47 | 26 | 73 | |
| Matsuda Index IR Diagnosis | ||||
|---|---|---|---|---|
| Motility | No Insulin Resistance | Insulin Resistance | Total | p-Value |
| Incorrect | 3 (10.71%) | 15 (33.33%) | 18 | 0.03 |
| Correct | 25 (89.29%) | 30 (66.67%) | 55 | |
| Total | 28 | 45 | 73 | |
| Sexual Abstinence | ||||
|---|---|---|---|---|
| Motility | Less than 4 Days | 4 Days and More | Total | p-Value |
| Incorrect | 8 (16.67%) | 9 (50%) | 17 | 0.006 |
| Correct | 40 (83.33%) | 9 (50%) | 49 | |
| Total | 48 | 18 | 66 | |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Matsuda index | 0.2400 | 0.0623 | 0.9243 | 0.04 |
| HOMA-IR index | 0.3282 | 0.1096 | 0.9828 | <0.05 |
| MacCI | 0.5589 | 0.3017 | 1.0353 | 0.06 |
| MCI | 1.6691 | 1.0633 | 2.6200 | 0.03 |
| Tobacco smoking | 1.8667 | 0.4638 | 7.5131 | 0.38 |
| Physical activity | 0.3958 | 0.1156 | 1.3552 | 0.14 |
| BMI | 0.5133 | 0.2438 | 1.0811 | 0.08 |
| Age | 0.9904 | 0.9152 | 1.0718 | 0.81 |
| VCI | 1.5094 | 1.1087 | 2.0551 | 0.009 |
| Duration of sexual abstinence | 0.2000 | 0.0605 | 0.6612 | 0.008 |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Matsuda index | 0.0271 | 0.0016 | 0.4610 | 0.01 |
| VCI | 1.7243 | 1.0680 | 2.7837 | 0.03 |
| Duration of sexual abstinence | 0.0477 | 0.0059 | 0.3863 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zańko, A.; Martynowicz, I.; Citko, A.; Konopka, P.; Paszko, A.; Pawłowski, M.; Szczerbiński, Ł.; Siewko, K.; Krętowski, A.J.; Kuczyński, W.; et al. The Influence of Lifestyle on Male Fertility in the Context of Insulin Resistance—Identification of Factors That Influence Semen Quality. J. Clin. Med. 2024, 13, 2797. https://doi.org/10.3390/jcm13102797
Zańko A, Martynowicz I, Citko A, Konopka P, Paszko A, Pawłowski M, Szczerbiński Ł, Siewko K, Krętowski AJ, Kuczyński W, et al. The Influence of Lifestyle on Male Fertility in the Context of Insulin Resistance—Identification of Factors That Influence Semen Quality. Journal of Clinical Medicine. 2024; 13(10):2797. https://doi.org/10.3390/jcm13102797
Chicago/Turabian StyleZańko, Adrianna, Iwo Martynowicz, Anna Citko, Paulina Konopka, Adam Paszko, Michał Pawłowski, Łukasz Szczerbiński, Katarzyna Siewko, Adam Jacek Krętowski, Waldemar Kuczyński, and et al. 2024. "The Influence of Lifestyle on Male Fertility in the Context of Insulin Resistance—Identification of Factors That Influence Semen Quality" Journal of Clinical Medicine 13, no. 10: 2797. https://doi.org/10.3390/jcm13102797
APA StyleZańko, A., Martynowicz, I., Citko, A., Konopka, P., Paszko, A., Pawłowski, M., Szczerbiński, Ł., Siewko, K., Krętowski, A. J., Kuczyński, W., & Milewski, R. (2024). The Influence of Lifestyle on Male Fertility in the Context of Insulin Resistance—Identification of Factors That Influence Semen Quality. Journal of Clinical Medicine, 13(10), 2797. https://doi.org/10.3390/jcm13102797








