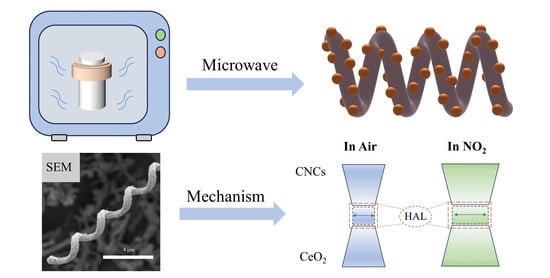
Microwave-Solvothermal Synthesis of Mesoporous CeO2/CNCs Nanocomposite for Enhanced Room Temperature NO2 Detection
Abstract
:1. Introduction
2. Experiments
2.1. Synthesis of CNCs and CeO2/CNCs Nanocomposite
2.2. Characterization
2.3. Fabrication and Measurement of the Gas Sensor
3. Results and Discussion
3.1. Morphological and Structural Characteristics
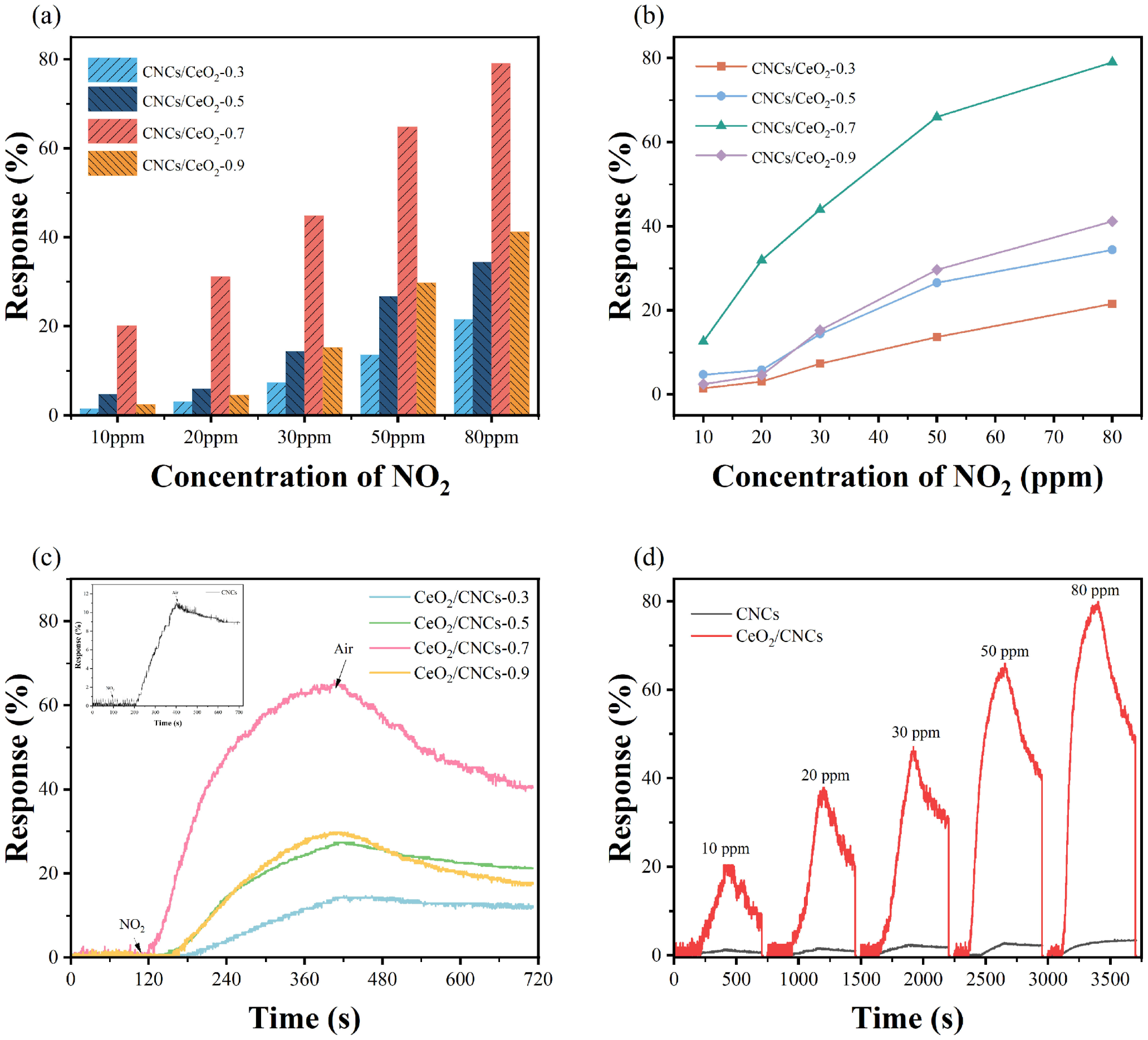
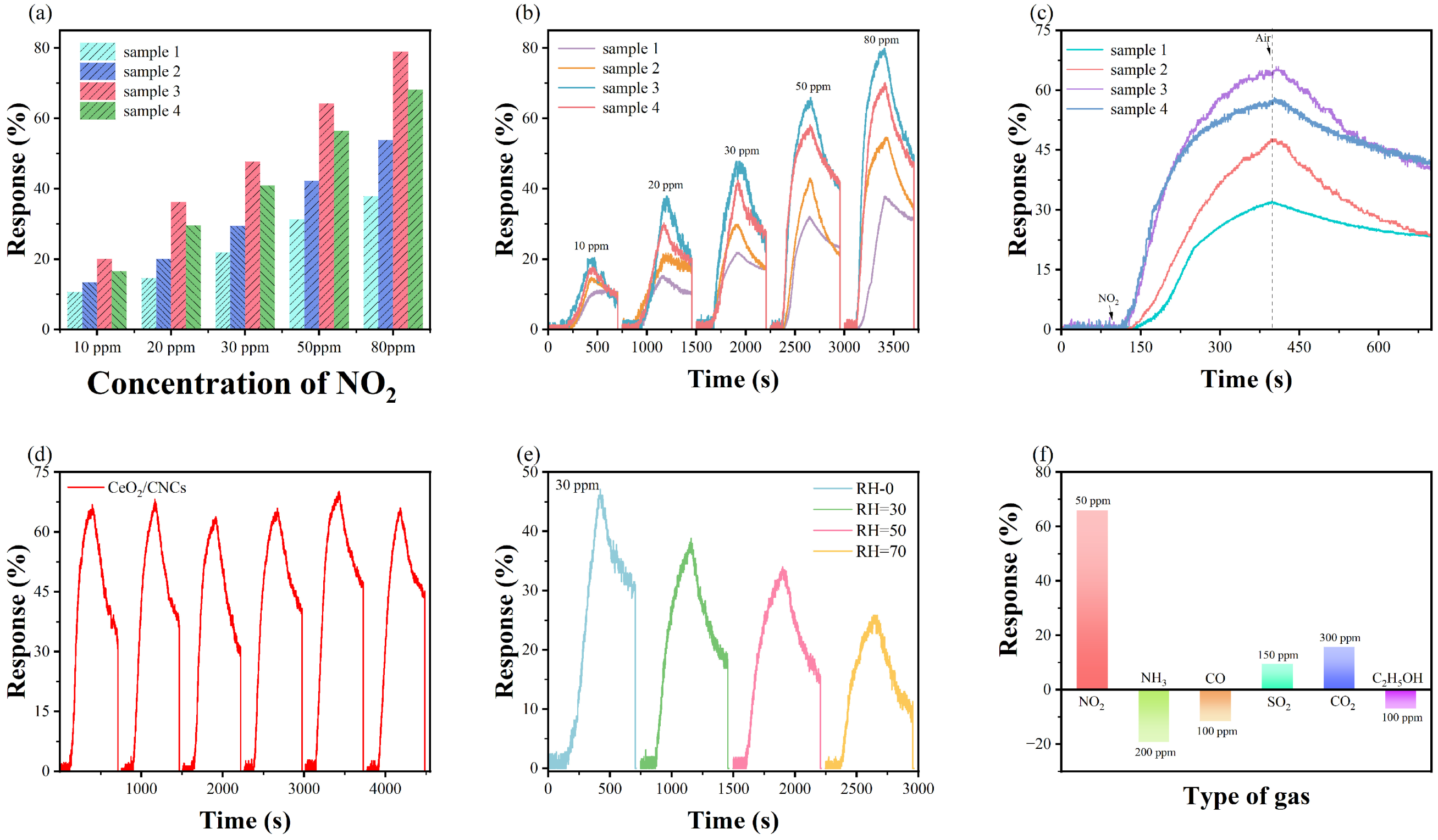
3.2. Gas Sensing Properties
3.3. Gas Sensing Mechanism
3.4. The Effects of Defects on the Gas Sensing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Tobaldi, D.M.; Dvoranová, D.; Lajaunie, L.; Rozman, N.; Figueiredo, B.; Seabra, M.P.; Skapin, A.S.; Calvino, J.J.; Brezová, V.; Labrincha, J.A. Graphene-TiO2 hybrids for photocatalytic aided removal of VOCs and nitrogen oxides from outdoor environment. Chem. Eng. J. 2021, 405, 126651. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Byoun, Y.; Kang, H.; Song, Y.J.; Choi, S.W. ZnO Nanocluster-Functionalized Single-Walled Carbon Nanotubes Synthesized by Microwave Irradiation for Highly Sensitive NO2 Detection at Room Temperature. ACS Omega 2019, 4, 10677–10686. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Lin, T.T.; Lv, X.; Hu, Z.N.; Xu, A.S.; Feng, C.H. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 19020233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, M.; Liu, G.; Gao, Y.; Zhao, L.; Li, S.; Wang, Y.; Liu, F.; Liang, X.; Zhang, T.; et al. Room temperature NO2 gas sensor based on porous Co3O4 slices/reduced graphene oxide hybrid. Sens. Actuators B Chem. 2018, 263, 387–399. [Google Scholar] [CrossRef]
- Chethana, D.M.; Thanuja, T.C.; Mahesh, H.M.; Kiruba, M.S.; Jose, A.S.; Barshilia, H.C.; Manjanna, J. Synthesis, structural, magnetic and NO2 gas sensing property of CuO nanoparticles. Ceram. Int. 2021, 47, 10381–10387. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.W.; Zhu, Q.; Wu, J.J.; Huang, Q.W.; Xie, C.S. Effect of Nickel Vacancies on the Room-Temperature NO2 Sensing Properties of Mesoporous NiO Nanosheets. J. Phys. Chem. C 2016, 120, 3936–3945. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.S.; Kim, S.I.; Baek, S.H.; Chun, D.W.; Jin, C.H.; Lee, K.H. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Saruhan, B.; Yüce, A.; Gönüllü, Y.; Kelm, K. Effect of Al doping on NO2 gas sensing of TiO2 at elevated temperatures. Sens. Actuators B Chem. 2013, 187, 586–597. [Google Scholar] [CrossRef]
- Mattmann, M.; Helbling, T.; Durrer, L.; Roman, C.; Hierold, C.; Pohle, R.; Fleischer, M. Sub-ppm NO2 detection by Al2O3 contact passivated carbon nanotube field effect transistors. Appl. Phys. Lett. 2009, 94, 3125259. [Google Scholar] [CrossRef]
- Nallappan, M.; Gopalan, M. Fabrication of CeO2/PANI composites for high energy density supercapacitors. Mater. Res. Bull. 2018, 106, 357–364. [Google Scholar] [CrossRef]
- Slusser, P.; Kumar, D.; Tiwari, A. Unexpected magnetic behavior of Cu-doped CeO2. Appl. Phys. Lett. 2010, 96, 3383238. [Google Scholar] [CrossRef]
- Fan, Z.H.; Meng, F.M.; Gong, J.F.; Li, H.J.; Hua, Y.D.; Liu, D.R. Enhanced photocatalytic activity of hierarchical flower-like CeO2/TiO2 heterostructures. Mater. Lett. 2016, 175, 36–39. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Gas sensors based on CeO2 nanoparticles prepared by chemical precipitation method and their temperature-dependent selectivity towards H2S and NO2 gases. Appl. Surf. Sci. 2020, 505, 144356. [Google Scholar] [CrossRef]
- Joy, N.A.; Nandasiri, M.I.; Rogers, P.H.; Jiang, W.L.; Varga, T.; Kuchibhatla, S.; Thevuthasan, S.; Carpenter, M.A. Selective Plasmonic Gas Sensing: H2, NO2, and CO Spectral Discrimination by a Single Au-CeO2 Nanocomposite Film. Anal. Chem. 2012, 84, 5025–5034. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Shang, E.; Wang, D.; Ma, X.; Zhao, B.; Han, C.; Zheng, C. A chemiresistive ppt level NO2 gas sensor based on CeO2 nanoparticles modified CuO nanosheets operated at 100 °C. Sens. Actuators B Chem. 2023, 393, 134277. [Google Scholar] [CrossRef]
- Young, S.J.; Lin, Z.D. Ammonia gas sensors with Au-decorated carbon nanotubes. Microsyst. Technol. 2018, 24, 4207–4210. [Google Scholar]
- Guo, S.Y.; Hou, P.X.; Zhang, F.; Liu, C.; Cheng, H.M. Gas Sensors Based on Single-Wall Carbon Nanotubes. Molecules 2022, 27, 27175381. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Glukhova, O.E.; Brzhezinskaya, M.; Stolyarova, D.Y.; Varezhnikov, A.S.; Solomatin, M.A.; Barkov, P.V.; Kirilenko, D.A.; Pavlov, S.I.; et al. Guiding Graphene Derivatization for the On-Chip Multisensor Arrays: From the Synthesis to the Theoretical Background. Adv. Mater. Technol. 2022, 7, 202101250. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yu, L.M.; Guo, F.; Liu, S.; Qi, L.J.; Shan, M.Y.; Fan, X.H. Facial development of high performance room temperature NO2 gas sensors based on ZnO nanowalls decorated rGO nanosheets. Appl. Surf. Sci. 2017, 423, 721–727. [Google Scholar] [CrossRef]
- Deng, C.H.; Sun, Y.M.; Pan, L.J.; Wang, T.Y.; Xie, Y.S.; Liu, J.; Zhu, B.W.; Wang, X.W. Thermal Diffusivity of a Single Carbon Nanocoil: Uncovering the Correlation with Temperature and Domain Size. Acs Nano 2016, 10, 9710–9719. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Wang, C.W.; Pan, L.J.; Fu, X.; Yin, P.H.; Zou, H.L. Electrical conductivity of single polycrystalline-amorphous carbon nanocoils. Carbon 2016, 98, 285–290. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, L.J.; Ma, H.; Wang, T. Structure changes of an individual carbon nanocoil and its field-emission enhancement by laser treatment. Diam. Relat. Mater. 2012, 22, 33–36. [Google Scholar] [CrossRef]
- Zuo, X.Q.; Zhao, Y.P.; Zhang, H.; Huang, H.; Zhou, C.; Cong, T.Z.; Muhammad, J.; Yang, X.; Zhang, Y.F.; Fan, Z.; et al. Surface modification of helical carbon nanocoil (CNC) with N-doped and Co-anchored carbon layer for efficient microwave absorption. J. Colloid Interface Sci. 2022, 608, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, Y.M.; Wu, Z.X.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon Nanocoil-Based Fast-Response and Flexible Humidity Sensor for Multifunctional Applications. Acs Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Dong, Z.; Ding, Z.Z.; Wang, N.; Sun, L.; Wei, H.M.; Wang, G.P. Carbon Nanocoils and Polyvinyl Alcohol Composite Films for Fiber-Optic Fabry-Perot Acoustic Sensors. Coatings 2022, 12, 12101599. [Google Scholar] [CrossRef]
- Sun, Y.M.; Ding, Z.Z.; Zhang, Y.P.; Dong, Z.; Sun, L.; Wang, N.; Yin, M.J.; Zhang, J.; Wang, G.P. Carbon nanocoils decorated with scale-like mesoporous NiO nanosheets for ultrasensitive room temperature ppb-level NO2 sensing. Phys. Chem. Chem. Phys. 2023, 25, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.L.; Wu, K.D.; Zhang, D.Z.; Debliquy, M.; Zhang, C. Microwave-assisted hydrothermal synthesis of copper oxide-based gas-sensitive nanostructures. Rare Met. 2021, 40, 1477–1493. [Google Scholar] [CrossRef]
- Gao, Y.W.; Chen, D.L.; Hou, X.H.; Zhang, Y.; Yi, S.S.; Ji, H.P.; Wang, Y.; Yin, L.; Sun, J. Microwave-assisted synthesis of hierarchically porous Co3O4/rGO nanocomposite for low-temperature acetone detection. J. Colloid Interface Sci. 2021, 594, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; Fang, Q.L.; Huang, Y.H.; Xu, K.W.; Ma, F.; Chu, P.K. Facet-engineered CeO2/graphene composites for enhanced NO2 gas-sensing. J. Mater. Chem. C 2017, 5, 6973–6981. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.W.; Sun, J.B.; Zhang, X.T. 3D Fe3O4 nanoparticle/graphene aerogel for NO2 sensing at room temperature. Rsc Adv. 2015, 5, 73699–73704. [Google Scholar] [CrossRef]
- Yang, W.; Wan, P.; Zhou, X.D.; Hu, J.M.; Guan, Y.F.; Feng, L. Additive-Free Synthesis of In2O3 Cubes Embedded into Graphene Sheets and Their Enhanced NO2 Sensing Performance at Room Temperature. Acs Appl. Mater. Interfaces 2014, 6, 21093–21100. [Google Scholar] [CrossRef] [PubMed]
- Rui, K.; Wang, X.S.; Du, M.; Zhang, Y.; Wang, Q.Q.; Ma, Z.Y.; Zhang, Q.; Li, D.S.; Huang, X.; Sun, G.Z.; et al. Dual-Function Metal-Organic Framework-Based Wearable Fibers for Gas Probing and Energy Storage. Acs Appl. Mater. Interfaces 2018, 10, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Liu, X.Y.; Yuan, Z.; Jiang, Y.D.; Su, Y.J.; Ma, J.Y.; Tai, H.L. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B Chem. 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Cui, S.M.; Wen, Z.H.; Mattson, E.C.; Mao, S.; Chang, J.B.; Weinert, M.; Hirschmugl, C.J.; Gajdardziska-Josifovska, M.; Chen, J.H. Indium-doped SnO2 nanoparticle-graphene nanohybrids: Simple one-pot synthesis and their selective detection of NO2. J. Mater. Chem. A 2013, 1, 4462–4467. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Fang, Q.L.; Huang, Y.H.; Xu, K.W.; Chu, P.K.; Ma, F. Oxygen Vacancy Enhanced Gas-Sensing Performance of CeO2/Graphene Heterostructure at Room Temperature. Anal. Chem. 2018, 90, 9821–9829. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, S.J.; Liu, M.M.; Zhang, C.M.; Chen, W. Three-Dimensional Mesoporous Graphene Aerogel-Supported SnO2 Nanocrystals for High-Performance NO2 Gas Sensing at Low Temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Yin, L.W.; Zhang, L.Y.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Tai, H.L.; Ye, Z.B.; Yuan, Z.; Liu, C.H.; Su, Y.J.; Jiang, Y.D. Novel p-n heterojunction-type rGO/CeO2 bilayer membrane for room-temperature nitrogen dioxide detection. Mater. Lett. 2017, 186, 49–52. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, L.X.; Xing, Y.; Zhang, P.; Chen, J.J.; Yin, J.; Bie, L.J. Morphology-controlled synthesis of CeO2 nanocrystals and their facet-dependent gas sensing properties. Sens. Actuators B Chem. 2021, 330, 129374. [Google Scholar] [CrossRef]
- Molina, A.; Al-Sardar, M.; Rodriguez-Gonzalez, V.; Escobar-Barrios, V.; Zakhidov, A.A.; Mtz-Enriquez, A.I.; Encinas, A.; Oliva, J. Efficient NO2 detection and the sensing mechanism of stretchable/ biodegradable MWCNT based sensors decorated with CeO2 nanoparticles. Synth. Met. 2022, 287, 117091. [Google Scholar] [CrossRef]
- Khan, A.S.; Pan, L.J.; Farid, A.; Javid, M.; Huang, H.; Zhao, Y.P. Carbon nanocoils decorated with a porous NiCo2O4 nanosheet array as a highly efficient electrode for supercapacitors. Nanoscale 2021, 13, 11943–11952. [Google Scholar] [CrossRef] [PubMed]
- Osorio, A.G.; Silveira, I.C.L.; Bueno, V.L.; Bergmann, C.P. H2SO4/HNO3/HCl-Functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl. Surf. Sci. 2008, 255, 2485–2489. [Google Scholar] [CrossRef]
- Mittal, M.; Kumar, A. Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens. Actuators B Chem. 2014, 203, 349–362. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Shen, X.P.; Li, M.Z.; Zhou, H.; Zhu, G.X.; Chen, K.M. Synthesis of reduced graphene oxide/CeO2 nanocomposites and their photocatalytic properties. Nanotechnology 2013, 24, 115603. [Google Scholar] [CrossRef] [PubMed]
- Takte, M.A.; Ingle, N.N.; Dole, B.N.; Tsai, M.L.; Hianik, T.; Shirsat, M.D. A stable and highly-sensitive flexible gas sensor based on Ceria (CeO2) nano-cube decorated rGO nanosheets for selective detection of NO2 at room temperature. Synth. Met. 2023, 297, 117411. [Google Scholar] [CrossRef]
- Liu, H.L.; He, H.L.; Chen, L.Q.; Pan, Q.J.; Zhang, G. Flower-like Co3O4 sensor with rich oxygen vacancy defects for enhancing room temperature NOx sensing performances. J. Alloys Compd. 2021, 868, 159180. [Google Scholar] [CrossRef]
- Liu, L.Z.; Gao, H.L.; Zhao, J.J.; Lu, J.P. Quantum conductance of armchair carbon nanocoils: Roles of geometry effects. Sci. China-Phys. Mech. Astron. 2011, 54, 841–845. [Google Scholar] [CrossRef]
- Sobaszek, M.; Brzhezinskaya, M.; Olejnik, A.; Mortet, V.; Alam, M.; Sawczak, M.; Ficek, M.; Gazda, M.; Weiss, Z.; Bogdanowicz, R. Highly Occupied Surface States at Deuterium-Grown Boron-Doped Diamond Interfaces for Efficient Photoelectrochemistry. Small 2023, 19, 202208265. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Bauman, Y.I.; Maksimova, T.A.; Stoyanovskii, V.O.; Vedyagin, A.A.; Mishakov, I.V.; Shubin, Y.V.; Gerasimov, E.Y. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Belenkov, E.A.; Greshnyakov, V.A.; Yalovega, G.E.; Bashkin, I.O. New aspects in the study of carbon-hydrogen interaction in hydrogenated carbon nanotubes for energy storage applications. J. Alloys Compd. 2019, 792, 713–720. [Google Scholar] [CrossRef]
- Sun, K.; Zhan, G.H.; Zhang, L.; Wang, Z.L.; Lin, S.W. Highly sensitive NO2 gas sensor based on ZnO nanoarray modulated by oxygen vacancy with Ce doping. Sens. Actuators B Chem. 2023, 379, 133294. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.K.; Si, G.K.; Li, Y.; Zhang, S.W.; Deng, X.L.; Xu, X.J. Oxygen vacancy defects engineering on Ce-doped α-Fe2O3 gas sensor for reducing gases. Sens. Actuators B Chem. 2020, 302, 127165. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhou, N.; Wang, H.Y.; Peng, Z.G.; Li, H.Y.; Tang, Y.G.; Liu, K. Synergistically enhanced oxygen reduction activity of MnOx-CeO2/Ketjenblack composites. Chem. Commun. 2015, 51, 10123. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.J.; Xue, Y.; Zhang, M.; Li, P.W.; Lian, K.; Zhuiykov, S.; Zhang, W.D.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sens. Actuators B Chem. 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Duy, L.T.; Kim, D.J.; Trung, T.Q.; Dang, V.Q.; Kim, B.Y.; Moon, H.K.; Lee, N.E. High Performance Three-Dimensional Chemical Sensor Platform Using Reduced Graphene Oxide Formed on High Aspect-Ratio Micro-Pillars. Adv. Funct. Mater. 2015, 25, 883–890. [Google Scholar] [CrossRef]







| Samples | SBET (m2 g−1) | Micropore Volume (cm3 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|---|
| CNCs | 38.8186 | 0.000158 | 0.078161 | 8.054 |
| CeO2/CNCs | 83.6895 | 0.020243 | 0.083183 | 7.2005 |
| Material | Method | Concentration (ppm) | Response/Recovery Time (s) | Response | LOD (ppm) | Ref |
|---|---|---|---|---|---|---|
| CeO2/graphene | Hydrothermal | 50 | 30/85 | 24.82% | - | [32] |
| NiO/CNCs | Hydrothermal | 60 | 126/- | 11.9% | 60.3 ppb | [29] |
| Fe3O4/rGO | Hydrothermal | 400 | 275/738 | 24.2% | 30 | [33] |
| In2O3 cubes/rGO | Hydrothermal | 5 | 149/243 | 37.81% | - | [34] |
| Co3O4/MWCNT | Hydrothermal | 1000 | -/- | 32% | 0.1 | [35] |
| PPy/N-MWCNT | In-situ self-assembly/annealing | 5 | 65/668 | 24.82% | [36] | |
| ZnO/SWCNTs | MW-irradiation | 1 | -/- | 5.03 | 88 ppb | [3] |
| In-SnO2-RGO | Hydrothermal | 100 | -/- | 11 | - | [37] |
| CeO2/CNCs | MWS method | 50 | 180/- | 65.4% | 710.3 ppb | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Lu, X.; Huang, Y.; Wang, G. Microwave-Solvothermal Synthesis of Mesoporous CeO2/CNCs Nanocomposite for Enhanced Room Temperature NO2 Detection. Nanomaterials 2024, 14, 812. https://doi.org/10.3390/nano14100812
Sun Y, Lu X, Huang Y, Wang G. Microwave-Solvothermal Synthesis of Mesoporous CeO2/CNCs Nanocomposite for Enhanced Room Temperature NO2 Detection. Nanomaterials. 2024; 14(10):812. https://doi.org/10.3390/nano14100812
Chicago/Turabian StyleSun, Yanming, Xiaoying Lu, Yanchen Huang, and Guoping Wang. 2024. "Microwave-Solvothermal Synthesis of Mesoporous CeO2/CNCs Nanocomposite for Enhanced Room Temperature NO2 Detection" Nanomaterials 14, no. 10: 812. https://doi.org/10.3390/nano14100812