Yarrowia lipolytica Yeast: A Treasure Trove of Enzymes for Biocatalytic Applications—A Review
Abstract
:1. Introduction
2. Whole-Cell Biocatalysis with Y. lipolytica Cells
3. Exploiting the Catalytic Abilities of Y. lipolytica Lipases
4. Versatile Biocatalytic Potential—Other Enzymes
5. Overexpressing of Enzymes in Y. lipolytica and Their Application in the Synthesis of Value-Added Chemicals
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poppe, L.; Vértessy, B.G. The Fourth Wave of Biocatalysis Emerges—The 13 Th International Symposium on Biocatalysis and Biotransformations. Chembiochem 2018, 19, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Abdelraheem, E.M.M.; Busch, H.; Hanefeld, U.; Tonin, F. Biocatalysis Explained: From Pharmaceutical to Bulk Chemical Production. React. Chem. Eng. 2019, 4, 1878–1894. [Google Scholar] [CrossRef]
- Lin, B.; Tao, Y. Whole-Cell Biocatalysts by Design. Microb. Cell Fact. 2017, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.-W.; Chen, X.-Y.; Wang, Y.-T.; Ye, C.; Shi, T.-Q. Application of Adaptive Laboratory Evolution for Yarrowia lipolytica: A Comprehensive Review. Bioresour. Technol. 2024, 391, 129893. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A Beneficious Yeast in Biotechnology as a Rare Opportunistic Fungal Pathogen: A Minireview. World J. Microbiol. Biotechnol. 2019, 35, 10. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of an Extension of Use of Yarrowia lipolytica Yeast Biomass as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2023, 21, e8416. [Google Scholar] [CrossRef] [PubMed]
- Naveira-Pazos, C.; Robles-Iglesias, R.; Fernández-Blanco, C.; Veiga, M.C.; Kennes, C. State-of-the-Art in the Accumulation of Lipids and Other Bioproducts from Sustainable Sources by Yarrowia lipolytica. Rev. Environ. Sci. Biotechnol. 2023, 22, 1131–1158. [Google Scholar] [CrossRef]
- Peters, I.I.; Nelson, F.E. Preliminary Characterization of the Lipase of Mycotorula lipolytica. J. Bacteriol. 1948, 55, 593–600. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; Sales, M.B.; de Castro Bizerra, V.; Nobre, M.M.R.; de Sousa Braz, A.K.; da Silva Sousa, P.; Cavalcante, A.L.G.; Melo, R.L.F.; Gonçalves De Sousa Junior, P.; Neto, F.S.; et al. Lipase from Yarrowia lipolytica: Prospects as an Industrial Biocatalyst for Biotechnological Applications. Fermentation 2023, 9, 581. [Google Scholar] [CrossRef]
- Park, Y.-K.; Ledesma-Amaro, R. What Makes Yarrowia lipolytica Well Suited for Industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef]
- Costa, A.R.; Salgado, J.M.; Lopes, M.; Belo, I. Valorization of By-Products from Vegetable Oil Industries: Enzymes Production by Yarrowia lipolytica through Solid State Fermentation. Front. Sustain. Food Syst. 2022, 6, 1006467. [Google Scholar] [CrossRef]
- Mamaev, D.; Zvyagilskaya, R. Yarrowia lipolytica: A Multitalented Yeast Species of Ecological Significance. FEMS Yeast Res. 2021, 21, foab008. [Google Scholar] [CrossRef]
- Mazloum-Ravasan, S.; Madadi, E.; Mohammadi, A.; Mansoori, B.; Amini, M.; Mokhtarzadeh, A.; Baradaran, B.; Darvishi, F. Yarrowia lipolytica L-Asparaginase Inhibits the Growth and Migration of Lung (A549) and Breast (MCF7) Cancer Cells. Int. J. Biol. Macromol. 2021, 170, 406–414. [Google Scholar] [CrossRef]
- Garzón-Posse, F.; Becerra-Figueroa, L.; Hernández-Arias, J.; Gamba-Sánchez, D. Whole Cells as Biocatalysts in Organic Transformations. Molecules 2018, 23, 1265. [Google Scholar] [CrossRef] [PubMed]
- Łużny, M.; Krzywda, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Effective Hydrogenation of 3-(2”-furyl)- and 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one in Selected Yeast Cultures. Molecules 2019, 24, 3185. [Google Scholar] [CrossRef]
- Łużny, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Highly Effective, Regiospecific Hydrogenation of Methoxychalcone by Yarrowia lipolytica Enables Production of Food Sweeteners. Catalysts 2020, 10, 1135. [Google Scholar] [CrossRef]
- Fuganti, C.; Grasselli, P.; Mendozza, M.; Servi, S.; Zucchi, G. Microbially-aided Preparation of (S)-2-methoxycyclohexanone Key Intermediate in the Synthesis of Sanfetrinem. Tetrahedron 1997, 53, 2617–2624. [Google Scholar] [CrossRef]
- Martínez, F.; Del Campo, C.; Sinisterra, J.V.; Llama, E.F. Preparation of Halohydrin β-Blocker Precursors Using Yeast-Catalysed Reduction. Tetrahedron Asymmetry 2000, 11, 4651–4660. [Google Scholar] [CrossRef]
- Lagos, F.M.; Del Campo, C.; Llama, E.F.; Sinisterra, J.V. New Yeast Strains for Enantioselective Production of Halohydrin Precursor of (S)-Propranolol. Enzyme Microb. Technol. 2002, 30, 895–901. [Google Scholar] [CrossRef]
- Martinez-Lagos, F.; Carballeira, J.D.; Bermúdez, J.L.; Alvarez, E.; Sinisterra, J.V. Highly Stereoselective Reduction of Haloketones Using Three New Yeasts: Application to the Synthesis of (S)-Adrenergic β-Blockers Related to Propranolol. Tetrahedron Asymmetry 2004, 15, 763–770. [Google Scholar] [CrossRef]
- Martinez-Lagos, F.; Sinisterra, J.V. Enantioselective Production of Halohydrin Precursor of Propranolol Catalysed by Immobilized Yeasts. J. Mol. Catal. B Enzym. 2005, 36, 1–7. [Google Scholar] [CrossRef]
- Olejniczak, T. Chemoenzymatic Synthesis of 2-Oxabicyclo[3.3.1]Nonan-3-One Enantiomers via Microbial Reduction by Absidia coerulea AM 93. J. Mol. Catal. B Enzym. 2010, 63, 1–10. [Google Scholar] [CrossRef]
- Furuta, M.; Shoji, M.; Sugai, T. Stereoselective Approach to (2R,3S)- and (2R,3R)-1,2-(Cyclohexylidenedioxy)Hept-6-En-3-Ol by Microbial Reduction. J. Mol. Catal. B Enzym. 2012, 82, 8–11. [Google Scholar] [CrossRef]
- Janeczko, T.; Kostrzewa-Susłow, E. Enantioselective Reduction of Propiophenone Formed from 3-Chloropropiophenone and Stereoinversion of the Resulting Alcohols in Selected Yeast Cultures. Tetrahedron Asymmetry 2014, 25, 1264–1269. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M.; Żarowska, B. Yeast-Mediated Stereoselective Reduction of α-Acetylbutyrolactone. Appl. Sci. 2018, 8, 1334. [Google Scholar] [CrossRef]
- Palmerín-Carreño, D.M.; Rutiaga-Quiñones, O.M.; Verde Calvo, J.R.; Prado-Barragán, A.; Huerta-Ochoa, S. Screening of Microorganisms for Bioconversion of (+)-Valencene to (+)-Nootkatone. LWT 2015, 64, 788–793. [Google Scholar] [CrossRef]
- Palmerín-Carreño, D.M.; Castillo-Araiza, C.O.; Rutiaga-Quiñones, O.M.; Verde Calvo, J.R.; Trejo-Aguilar, G.M.; Dutta, A.; Huerta-Ochoa, S. Whole Cell Bioconversion of (+)-valencene to (+)-nootkatone by Yarrowia lipolytica Using a Three Phase Partitioning Bioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 1164–1172. [Google Scholar] [CrossRef]
- Janeczko, T.; Dymarska, M.; Siepka, M.; Gniłka, R.; Leśniak, A.; Popłoński, J.; Kostrzewa-Susłow, E. Enantioselective Reduction of Flavanone and Oxidation of Cis- and Trans-Flavan-4-ol by Selected Yeast Cultures. J. Mol. Catal. B Enzym. 2014, 109, 47–52. [Google Scholar] [CrossRef]
- Fantin, G.; Fogagnolo, M.; Medici, A.; Pedrini, P.; Fontana, S. Kinetic Resolution of Racemic Secondary Alcohols via Oxidation with Yarrowia lipolytica Strains. Tetrahedron Asymmetry 2000, 11, 2367–2373. [Google Scholar] [CrossRef]
- Verma, N.K.; Kumar, S.; Basotra, S.D.; Jain, A.; Vij, M.; Prasad, G.S.; Bhattacharyya, M.S. Biocatalytic Reduction of Prochiral Ketones to Enantiopure Alcohols by Novel Yeast Isolates from Unique Biodiversity. Biocatal. Agric. Biotechnol. 2021, 31, 101547. [Google Scholar] [CrossRef]
- Botezatu, A.V.; Horincar, G.; Ghinea, I.O.; Furdui, B.; Bahrim, G.-E.; Barbu, V.; Balanescu, F.; Favier, L.; Dinica, R.-M. Whole-Cells of Yarrowia lipolytica Applied in “One Pot” Indolizine Biosynthesis. Catalysts 2020, 10, 629. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A.; Wołoszynowska, M.; Białecka-Florjańczyk, E. Synthesis of Flavor Compound Ethyl Hydrocinnamate by Yarrowia lipolytica Lipases. Biocatal. Biotransform. 2021, 39, 455–464. [Google Scholar] [CrossRef]
- Vatsal, A.; Potdar, C.; Zinjarde, S.S.; Ravi Kumar, V.; Kulkarni, B.D.; Ravi Kumar, A. Role of Aliasing and Interacting Factors in the Enhanced Production of Dehalogenase from Yarrowia lipolytica for Degradation of Brominated Compounds. J. Ind. Eng. Chem. 2016, 41, 114–121. [Google Scholar] [CrossRef]
- Vatsal, A.A.; Zinjarde, S.S.; RaviKumar, A. Phenol Is the Initial Product Formed during Growth and Degradation of Bromobenzene by Tropical Marine Yeast, Yarrowia lipolytica NCIM 3589 via an Early Dehalogenation Step. Front. Microbiol. 2017, 8, 1165. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Son, H.F.; Hwang, S.; Gong, G.; Ko, J.K.; Um, Y.; Han, S.O.; Lee, S.-M. Improving Lipid Production of Yarrowia lipolytica by the Aldehyde Dehydrogenase-Mediated Furfural Detoxification. Int. J. Mol. Sci. 2022, 23, 4761. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Castagna, A.; Marzorati, S.; Valentino, M. Biotransformation of the Proteogenic Amino Acids Phenylalanine, Tyrosine and Tryptophan by Yarrowia Species: An Application to the Preparative Synthesis of Natural Phenylacetic Acid. Catalysts 2022, 12, 1638. [Google Scholar] [CrossRef]
- Zieniuk, B.; Groborz, K.; Wołoszynowska, M.; Ratusz, K.; Białecka-Florjańczyk, E.; Fabiszewska, A. Enzymatic Synthesis of Lipophilic Esters of Phenolic Compounds, Evaluation of Their Antioxidant Activity and Effect on the Oxidative Stability of Selected Oils. Biomolecules 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guzmán, C.; Prado-Barragán, A.; Gimeno, M.; Román-Guerrero, A.; Rutiaga-Quiñones, O.M.; Rocha Guzmán, N.E.; Huerta-Ochoa, S. Whole-Cell Bioconversion of Naringenin to High Added Value Hydroxylated Compounds Using Yarrowia lipolytica 2.2ab in Surface and Liquid Cultures. Bioprocess Biosyst. Eng. 2020, 43, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Grabarczyk, M.; Mączka, W.; Żarowska, B.; Maciejewska, G.; Dancewicz, K.; Gabryś, B.; Szumny, A.; Anioł, M. Biotransformation of Bicyclic Halolactones with a Methyl Group in the Cyclohexane Ring into Hydroxylactones and Their Biological Activity. Molecules 2016, 21, 1453. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. Enzyme-Catalyzed Processes in Organic Solvents. Proc. Natl. Acad. Sci. USA 1985, 82, 3192–3196. [Google Scholar] [CrossRef]
- Borowiecki, P. Przemysłowe zastosowania lipaz w syntezie związków o wysokiej wartości dodanej—85 lat katalizy enzymatycznej lipazami. Część 1. Wiadomości Chem. 2015, 69, 67–106. [Google Scholar]
- da Silva, J.R.; de Souza, C.E.C.; Valoni, E.; de Castro, A.M.; Coelho, M.A.Z.; Ribeiro, B.D.; Henriques, C.A.; Langone, M.A.P. Biocatalytic Esterification of Fatty Acids Using a Low-Cost Fermented Solid from Solid-State Fermentation with Yarrowia lipolytica. 3 Biotech 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.; Penha, A.; Pereira, A.d.S.; Silva, K.; Akil, E.; Torres, A.; Amaral, P. Use of Yarrowia lipolytica Lipase Immobilized in Cell Debris for the Production of Lipolyzed Milk Fat (LMF). Int. J. Mol. Sci. 2018, 19, 3413. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Gandolfi, R.; Aragozzini, F. Microbial Catalyzed Esterification of Primary and Secondary Alcohols in Organic Solvent. Biotechnol. Tech. 1996, 10, 103–108. [Google Scholar] [CrossRef]
- Białecka-Florjańczyk, E.; Krzyczkowska, J.; Stolarzewicz, I.; Kapturowska, A. Synthesis of 2-Phenylethyl Acetate in the Presence of Yarrowia lipolytica KKP 379 Biomass. J. Mol. Catal. B Enzym. 2012, 74, 241–245. [Google Scholar] [CrossRef]
- de Souza, C.E.C.; Ribeiro, B.D.; Coelho, M.A.Z. Characterization and Application of Yarrowia lipolytica Lipase Obtained by Solid-State Fermentation in the Synthesis of Different Esters Used in the Food Industry. Appl. Biochem. Biotechnol. 2019, 189, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Wołoszynowska, M.; Białecka-Florjańczyk, E.; Fabiszewska, A. Synthesis of Industrially Useful Phenolic Compounds Esters by Means of Biocatalysts Obtained along with Waste Fish Oil Utilization. Sustainability 2020, 12, 5804. [Google Scholar] [CrossRef]
- Zieniuk, B.; Wołoszynowska, M.; Białecka-Florjańczyk, E.; Fabiszewska, A. Application of Freeze-Dried Yarrowia lipolytica Biomass in the Synthesis of Lipophilic Antioxidants. Biotechnol. Lett. 2021, 43, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, K.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Studies on the Catalytic Properties of Crude Freeze-Dried Preparations of Yarrowia lipolytica Extracellular Lipases for Geranyl Ester Derivative Synthesis. Biomolecules 2021, 11, 839. [Google Scholar] [CrossRef]
- Sales, J.C.S.; de Castro, A.M.; Ribeiro, B.D.; Coelho, M.A.Z. Improved Production of Biocatalysts by Yarrowia lipolytica Using Natural Sources of the Biopolyesters Cutin and Suberin, and Their Application in Hydrolysis of Poly (Ethylene Terephthalate) (PET). Bioprocess Biosyst. Eng. 2021, 44, 2277–2287. [Google Scholar] [CrossRef]
- Sales, J.C.S.; de Castro, A.M.; Ribeiro, B.D.; Coelho, M.A.Z. Post-Consumer Poly(ethylene terephthalate) (PET) Depolymerization by Yarrowia lipolytica: A Comparison between Hydrolysis Using Cell-Free Enzymatic Extracts and Microbial Submerged Cultivation. Molecules 2022, 27, 7502. [Google Scholar] [CrossRef] [PubMed]
- Koczoń, P.; Bartyzel, B.; Iuliano, A.; Klensporf-Pawlik, D.; Kowalska, D.; Majewska, E.; Tarnowska, K.; Zieniuk, B.; Gruczyńska-Sękowska, E. Chemical Structures, Properties, and Applications of Selected Crude Oil-Based and Bio-Based Polymers. Polymers 2022, 14, 5551. [Google Scholar] [CrossRef]
- Pignede, G.; Wang, H.; Fudalej, F.; Gaillardin, C.; Seman, M.; Nicaud, J.M. Characterization of an extracellular lipase encoded by LIP2 in Yarrowia lipolytica. J. Bacteriol. 2000, 182, 2802–2810. [Google Scholar] [CrossRef]
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of Lipase-catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
- Li, W.; Li, R.; Yu, X.; Xu, X.; Guo, Z.; Tan, T.; Fedosov, S.N. Lipase-Catalyzed Knoevenagel Condensation in Water–Ethanol Solvent System. Does the Enzyme Possess the Substrate Promiscuity? Biochem. Eng. J. 2015, 101, 99–107. [Google Scholar] [CrossRef]
- Meng, Y.-H.; Chen, B.-Q.; Yang, N.; Wang, G.-L.; Li, Y.; Tan, T.-W. Oleic Acid Esterification in Solvent-Free Medium by Yarrowia lipolytica Lipase Immobilized on Fabric Membranes. J. Biobased Mater. Bioenergy 2010, 4, 73–78. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, G.; Yang, N.; Zhou, Z.; Li, Y.; Liang, X.; Chen, J.; Li, Y.; Li, J. Two-Step Synthesis of Fatty Acid Ethyl Ester from Soybean Oil Catalyzed by Yarrowia lipolytica Lipase. Biotechnol. Biofuels 2011, 4, 6. [Google Scholar] [CrossRef]
- He, Y.; Li, K.; Bo, G.; Wang, J.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Enhancing Biodiesel Production via Liquid Yarrowia lipolytica Lipase 2 in Deep Eutectic Solvents. Fuel 2022, 316, 123342. [Google Scholar] [CrossRef]
- He, Y.; Li, K.; Wang, J.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. A Novel Strategy for Biodiesel Production by Combination of Liquid Lipase, Deep Eutectic Solvent and Ultrasonic-Assistance in Scaled-up Reactor: Optimization and Kinetics. J. Clean. Prod. 2022, 372, 133740. [Google Scholar] [CrossRef]
- Cao, H.; Wang, M.; Deng, L.; Liu, L.; Schwaneberg, U.; Tan, T.; Wang, F.; Nie, K. Sugar-Improved Enzymatic Synthesis of Biodiesel with Yarrowia lipolytica Lipase 2. Energy Fuels 2017, 31, 6248–6256. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Betancor, L.; Carrascosa, A.V.; Guisán, J.M. Release of Omega-3 Fatty Acids by the Hydrolysis of Fish Oil Catalyzed by Lipases Immobilized on Hydrophobic Supports. J. Am. Oil Chem. Soc. 2011, 88, 1173–1178. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Meunchan, M.; Cot, M.; Duquesne, S.; Bordes, F.; Marty, A. Yarrowia lipolytica Lipase Lip2: An Efficient Enzyme for the Production of Concentrates of Docosahexaenoic Acid Ethyl Ester. J. Biotechnol. 2014, 180, 30–36. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Marty, A.; Sandoval, G.; Ferreira-Dias, S. Optimization of Medium Chain Length Fatty Acid Incorporation into Olive Oil Catalyzed by Immobilized Lip2 from Yarrowia lipolytica. Biochem. Eng. J. 2013, 77, 20–27. [Google Scholar] [CrossRef]
- Akil, E.; Pereira, A.d.S.; El-Bacha, T.; Amaral, P.F.F.; Torres, A.G. Efficient Production of Bioactive Structured Lipids by Fast Acidolysis Catalyzed by Yarrowia lipolytica Lipase, Free and Immobilized in Chitosan-Alginate Beads, in Solvent-Free Medium. Int. J. Biol. Macromol. 2020, 163, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, S.; Xiang, X.; Shi, J.; Huang, J.; Deng, Q.; Huang, F.; Xiao, J. Facile Preparation of Magnetic Carbon Nanotubes-Immobilized Lipase for Highly Efficient Synthesis of 1,3-Dioleoyl-2-Palmitoylglycerol-Rich Human Milk Fat Substitutes. Food Chem. 2017, 228, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gao, Q.; Feng, W.; Pan, K. Immobilization of Yarrowia lipolytica Lipase on Bamboo Charcoal to Resolve (R, S)-phenylethanol in Organic Medium. Chem. Eng. Technol. 2013, 36, 1249–1254. [Google Scholar] [CrossRef]
- Cui, C.; Xie, R.; Tao, Y.; Zeng, Q.; Chen, B. Improving Performance of Yarrowia lipolytica Lipase Lip2-Catalyzed Kinetic Resolution of (R, S)-1-Phenylethanol by Solvent Engineering. Biocatal. Biotransform. 2015, 33, 38–43. [Google Scholar] [CrossRef]
- Syal, P.; Gupta, R. Heterologous Expression of Lipases YLIP4, YLIP5, YLIP7, YLIP13, and YLIP15 from Yarrowia lipolytica MSR80 in Escherichia coli: Substrate Specificity, Kinetic Comparison, and Enantioselectivity. Biotechnol. Appl. Biochem. 2017, 64, 851–861. [Google Scholar] [CrossRef]
- Wen, S.; Tan, T.; Yu, M. Immobilized Lipase YlLip2-Catalyzed Resolution of (±)α-Phenylethyl Amine in a Medium with Organic Cosolvent. Process Biochem. 2008, 43, 1259–1264. [Google Scholar] [CrossRef]
- Guieysse, D.; Sandoval, G.; Faure, L.; Nicaud, J.-M.; Monsan, P.; Marty, A. New Efficient Lipase from Yarrowia lipolytica for the Resolution of 2-Bromo-Arylacetic Acid Esters. Tetrahedron Asymmetry 2004, 15, 3539–3543. [Google Scholar] [CrossRef]
- Cancino, M.; Bauchart, P.; Sandoval, G.; Nicaud, J.-M.; André, I.; Dossat, V.; Marty, A. A Variant of Yarrowia lipolytica Lipase with Improved Activity and Enantioselectivity for Resolution of 2-Bromo-Arylacetic Acid Esters. Tetrahedron Asymmetry 2008, 19, 1608–1612. [Google Scholar] [CrossRef]
- Bordes, F.; Cambon, E.; Dossat-Létisse, V.; André, I.; Croux, C.; Nicaud, J.M.; Marty, A. Improvement of Yarrowia lipolytica Lipase Enantioselectivity by Using Mutagenesis Targeted to the Substrate Binding Site. Chembiochem 2009, 10, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Cambon, E.; Piamtongkam, R.; Bordes, F.; Duquesne, S.; André, I.; Marty, A. Rationally Engineered Double Substituted Variants of Yarrowia lipolytica Lipase with Enhanced Activity Coupled with Highly Inverted Enantioselectivity towards 2-bromo Phenyl Acetic Acid Esters. Biotechnol. Bioeng. 2010, 106, 852–859. [Google Scholar] [CrossRef]
- Gérard, D.; Currie, F.; Medina Gonzalez, Y.; Camy, S.; Marty, A.; Condoret, J.-S. Resolution of 2-Bromo-Arylacetic Acid Ester by Yarrowia lipolytica Lipase in Water/Supercritical CO2 Two-Phase Systems. J. Supercrit. Fluids 2017, 121, 96–104. [Google Scholar] [CrossRef]
- Skouridou, V.; Chrysina, E.D.; Stamatis, H.; Oikonomakos, N.G.; Kolisis, F.N. Kinetic and Modelling Studies on the Lipase Catalysed Enantioselective Esterification of (±)-Perillyl Alcohol. J. Mol. Catal. B Enzym. 2004, 29, 9–12. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Sun, X.-T.; Liu, C.-Z. Improved Performance of Yarrowia lipolytica Lipase-Catalyzed Kinetic Resolution of (R, S)-2-Octanol by an Integrated Strategy of Interfacial Activation, Bioimprinting and Immobilization. Bioresour. Technol. 2013, 142, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, C.; Liu, C.-Z. Development of a Mixed Solvent System for the Efficient Resolution of (R, S)-2-Octanol Catalyzed by Magnetite-Immobilized Lipase. J. Mol. Catal. B Enzym. 2014, 101, 23–27. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, C.-Z. Novel Magnetic Cross-linked Lipase Aggregates for Improving the Resolution of (R, S)-2-octanol. Chirality 2015, 27, 199–204. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, C.-Z. Efficient Kinetic Resolution of (R, S)-2-Octanol Catalyzed by Magnetite-Immobilized Yarrowia lipolytica Lipase in Mixed Ionic Liquids. Catal. Lett. 2014, 144, 1552–1556. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Tan, T. Cyclic Resolution of Racemic Ibuprofen via Coupled Efficient Lipase and Acid-base Catalysis. Chirality 2009, 21, 349–353. [Google Scholar] [CrossRef]
- Gérard, D.; Guéroult, M.; Casas-Godoy, L.; Condoret, J.-S.; André, I.; Marty, A.; Duquesne, S. Efficient Resolution of Profen Ethyl Ester Racemates by Engineered Yarrowia lipolytica Lip2p Lipase. Tetrahedron Asymmetry 2017, 28, 433–441. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, Z.; Zeng, Q.; Chen, B. Insight into the Synthesis of Isosorbide Diester Plasticizer Using Immobilized Lipases. RSC Adv. 2016, 6, 108180–108186. [Google Scholar] [CrossRef]
- Tang, J.; Chen, G.; Wang, L.; Miao, M.; Jiang, B.; Feng, B. Immobilization of Y. lipolytica Lipase and the Continuous Synthesis of Geranyl Propionate. J. Mol. Catal. B Enzym. 2016, 133, S311–S316. [Google Scholar] [CrossRef]
- Ping, L.; Yuan, X.; Zhang, M.; Chai, Y.; Shan, S. Improvement of Extracellular Lipase Production by a Newly Isolated Yarrowia lipolytica Mutant and Its Application in the Biosynthesis of L-Ascorbyl Palmitate. Int. J. Biol. Macromol. 2018, 106, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Guan, N.; Xing, C.; Chen, B.; Tan, T. Immobilization of Yarrowia lipolytica Lipase Ylip2 for the Biocatalytic Synthesis of Phytosterol Ester in a Water Activity Controlled Reactor. Colloids Surf. B Biointerfaces 2016, 146, 490–497. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Flores-Carreón, A.; Martínez-Richa, A. Enzymatic Ring-opening Polymerization of ε-caprolactone by a New Lipase from Yarrowia lipolytica. J. Appl. Polym. Sci. 2008, 109, 708–719. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Marcos-Fernández, Á.; Vera-Graziano, R.; Martínez-Richa, A. Enzymatic Ring-opening Polymerization of ε-caprolactone by Yarrowia lipolytica Lipase in Ionic Liquids. J. Polym. Sci. A Polym. Chem. 2009, 47, 5792–5805. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Martínez-Richa, A. One-pot Biocatalytic Synthesis of Sugar Based Poly (ε-caprolactone). Macromol. Symp. 2009, 283–284, 144–151. [Google Scholar] [CrossRef]
- Sandoval, G.; Rivera, I.; Barrera-Rivera, K.A.; Martínez-Richa, A. Biopolymer Synthesis Catalyzed by Tailored Lipases. Macromol. Symp. 2010, 289, 135–139. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Marcos-Fernández, Á.; Martínez-Richa, A. Chemo-Enzymatic Syntheses of Polyester-Urethanes. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 227–235. ISBN 9780841225817. [Google Scholar]
- Barrera-Rivera, K.A.; Martínez-Richa, A. Syntheses and Characterization of Aliphatic Polyesters via Yarrowia lipolytica Lipase Biocatalysis. In Green Polymer Chemistry: Biocatalysis and Materials II; American Chemical Society: Washington, DC, USA, 2013; pp. 59–68. ISBN 9780841228955. [Google Scholar]
- Barrera-Rivera, K.A.; Peponi, L.; Marcos-Fernández, Á.; Kenny, J.M.; Martínez-Richa, A. Synthesis, Characterization and Hydrolytic Degradation of Polyester-Urethanes Obtained by Lipase Biocatalysis. Polym. Degrad. Stab. 2014, 108, 188–194. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.; Martínez-Richa, A. Yarrowia lipolytica Extracellular Lipase Lip2 as Biocatalyst for the Ring-Opening Polymerization of ε-Caprolactone. Molecules 2017, 22, 1917. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Huang, H.; Li, S. Efficient Synthesis of (R)-2-Chloro-1-Phenylethol Using a Yeast Carbonyl Reductase with Broad Substrate Spectrum and 2-Propanol as Cosubstrate. Biochem. Eng. J. 2015, 103, 277–285. [Google Scholar] [CrossRef]
- Xu, Q.; Tao, W.-Y.; Huang, H.; Li, S. Highly Efficient Synthesis of Ethyl (S)-4-Chloro-3-Hydroxybutanoate by a Novel Carbonyl Reductase from Yarrowia lipolytica and Using Mannitol or Sorbitol as Cosubstrate. Biochem. Eng. J. 2016, 106, 61–67. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Zhang, C.; Pei, C.-H.; Han, M.-N.; Li, W. Enantioselective Synthesis of Enantiopure Chiral Alcohols Using Carbonyl Reductases Screened from Yarrowia lipolytica. J. Appl. Microbiol. 2019, 126, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Napora-Wijata, K.; Strohmeier, G.; Sonavane, M.; Avi, M.; Robins, K.; Winkler, M. Enantiocomplementary Yarrowia lipolytica Oxidoreductases: Alcohol Dehydrogenase 2 and Short Chain Dehydrogenase/Reductase. Biomolecules 2013, 3, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Choi, K.-Y. Whole Cell Biotransformation of 1-Dodecanol by Escherichia coli by Soluble Expression of ADH Enzyme from Yarrowia lipolytica. Biotechnol. Bioprocess Eng. 2021, 26, 247–255. [Google Scholar] [CrossRef]
- Bordewick, S.; Beier, A.; Balke, K.; Bornscheuer, U.T. Baeyer-Villiger Monooxygenases from Yarrowia lipolytica Catalyze Preferentially Sulfoxidations. Enzyme Microb. Technol. 2018, 109, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bendigiri, C.; Harini, K.; Yenkar, S.; Zinjarde, S.; Sowdhamini, R.; RaviKumar, A. Evaluating Ylehd, a Recombinant Epoxide Hydrolase from Yarrowia lipolytica as a Potential Biocatalyst for the Resolution of Benzyl Glycidyl Ether. RSC Adv. 2018, 8, 12918–12926. [Google Scholar] [CrossRef] [PubMed]
- Godase, V.P.; Kumar, V.R.; Kumar, A.R. Potential of Y. lipolytica Epoxide Hydrolase for Efficient Production of Enantiopure (R)-1,2-Octanediol. AMB Express 2023, 13, 77. [Google Scholar] [CrossRef]
- Madzak, C. Engineering Yarrowia lipolytica for Use in Biotechnological Applications: A Review of Major Achievements and Recent Innovations. Mol. Biotechnol. 2018, 60, 621–635. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica Strains and Their Biotechnological Applications: How Natural Biodiversity and Metabolic Engineering Could Contribute to Cell Factories Improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Wang, C.-M.; Men, X.; Yue, T.-Q.; Madzak, C.; Xiang, X.-H.; Xiang, H.-Y.; Zhang, H.-B. Construction of Arming Yarrowia lipolytica Surface-Displaying Soybean Seed Coat Peroxidase for Use as Whole-Cell Biocatalyst. Enzyme Microb. Technol. 2020, 135, 109498. [Google Scholar] [CrossRef] [PubMed]
- Theerachat, M.; Emond, S.; Cambon, E.; Bordes, F.; Marty, A.; Nicaud, J.-M.; Chulalaksananukul, W.; Guieysse, D.; Remaud-Siméon, M.; Morel, S. Engineering and Production of Laccase from Trametes versicolor in the Yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 125, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Shiningavamwe, A.; Obiero, G.; Albertyn, J.; Nicaud, J.-M.; Smit, M. Heterologous Expression of the Benzoate Para-Hydroxylase Encoding Gene (CYP53B1) from Rhodotorula minuta by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2006, 72, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Theron, C.W.; Labuschagné, M.; Albertyn, J.; Smit, M.S. Heterologous Coexpression of the Benzoate-para-hydroxylase CYP53B1 with Different Cytochrome P450 Reductases in Various Yeasts. Microb. Biotechnol. 2019, 12, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Geier, M.; Bühler, B.; Schmid, A.; Mauersberger, S.; Glieder, A. Steroid Biotransformations in Biphasic Systems with Yarrowia lipolytica Expressing Human Liver Cytochrome P450 Genes. Microb. Cell Fact. 2012, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Gómez, M.P.; Kermasha, S.; Nicaud, J.-M.; Belin, J.-M.; Husson, F. Secondary Structure Conformation of Hydroperoxide Lyase from Green Bell Pepper, Cloned in Yarrowia lipolytica, and Its Activity in Selected Media. J. Mol. Catal. B Enzym. 2008, 52–53, 128–132. [Google Scholar] [CrossRef]
- Zhang, B.; Song, Y.; Chen, H.; Zhang, H.; Chen, W. Production of Trans-10, Cis-12-Conjugated Linoleic Acid Using Permeabilized Whole-Cell Biocatalyst of Yarrowia lipolytica. Biotechnol. Lett. 2016, 38, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Yuzbasheva, E.Y.; Yuzbashev, T.V.; Perkovskaya, N.I.; Mostova, E.B.; Vybornaya, T.V.; Sukhozhenko, A.V.; Toropygin, I.Y.; Sineoky, S.P. Cell Surface Display of Yarrowia lipolytica Lipase Lip2p Using the Cell Wall Protein YlPir1p, Its Characterization, and Application as a Whole-Cell Biocatalyst. Appl. Biochem. Biotechnol. 2015, 175, 3888–3900. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, K.; Zhou, Q.; Xu, Z.; Yan, Y.; Xu, L.; Madzak, C.; Yan, J. Engineering Yarrowia lipolytica for Sustainable Production of Fatty Acid Methyl Esters Using in Situ Self-Cycled Glycerol as a Carbon Source. ACS Sustain. Chem. Eng. 2018, 6, 7645–7651. [Google Scholar] [CrossRef]
- Wei, L.-J.; Ma, Y.-Y.; Cheng, B.-Q.; Gao, Q.; Hua, Q. Metabolic Engineering Yarrowia lipolytica for a Dual Biocatalytic System to Produce Fatty Acid Ethyl Esters from Renewable Feedstock in Situ and in One Pot. Appl. Microbiol. Biotechnol. 2021, 105, 8561–8573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Z.-P.; Liu, S.; Wang, Y.-L.; Zhang, Z.-F.; Liu, X.-M.; Du, Y.-M.; Yuan, X.-L. Overexpression of Secreted Sucrose Isomerase in Yarrowia lipolytica and Its Application in Isomaltulose Production after Immobilization. Int. J. Biol. Macromol. 2019, 121, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Cheng, H.; Deng, Z. Isomaltulose Production by Yeast Surface Display of Sucrose Isomerase from Pantoea dispersa on Yarrowia lipolytica. J. Funct. Foods 2017, 32, 208–217. [Google Scholar] [CrossRef]







| Substrate | Product | Conversion/Yield [%] | Enantiomeric Excess and Absolute Configuration | Reference |
|---|---|---|---|---|
| Reaction type—Hydrogenation (Bioreduction of C=C bond) | ||||
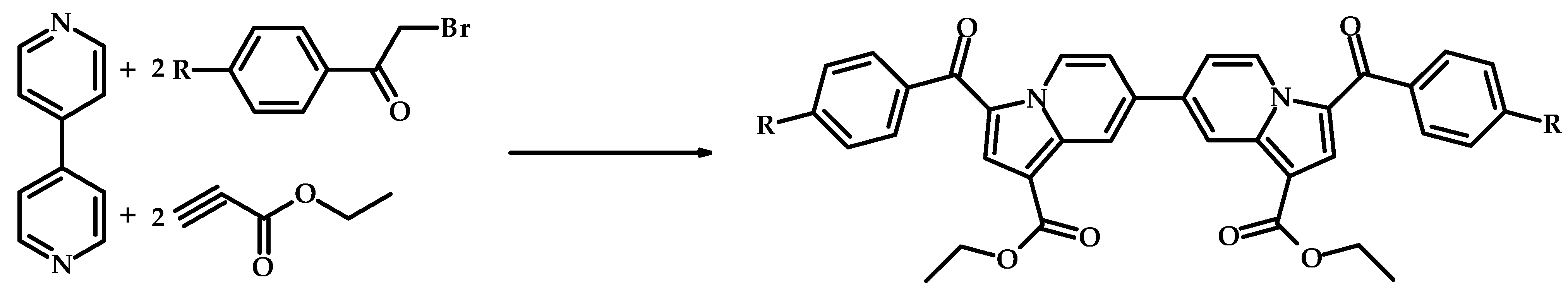
 |  | 88 (after 3 h); >99 (after 6 h) | - | [15] |
| 3-(2″-furyl)-1-(2′-hydroxyphenyl)-prop-2-en-1-one | 3-(2″-furyl)-1-(2′-hydroxyphenyl)-propan-1-one | |||
 |  | 67 (after 1 h); >99 (after 3 h) | - | |
| 3-(2″-thienyl)-1-(2′-hydroxyphenyl)-prop-2-en-1-one | 3-(2″-thienyl)-1-(2′-hydroxyphenyl)-propan-1-one | |||
 |  | 88–99 (after 1 h) | - | [16] |
| 2′-hydroxy-(di)methoxychalcones and corresponding dihydrochalcones; R1, R2, R3, and R4 = H or OCH3 | ||||
 |  | 7 (after 1 h); 20 (after 7 h) | - | |
| 2′-hydroxy-3″,4″,5″-trimethoxychalcone | 2′-hydroxy-3″,4″,5″-trimethoxy-α,β-dihydrochalcone | |||
| Reaction type—Reduction | ||||
 |  | 40 (after 24 h) | 93%; R | [17] |
| 2-benzylidenecyclohexanone | (R)-2-benzylidenecyclohexanol | |||
 |  | 25–88 (after 48 h) | 99%; S | [18,19,20,21] |
| 1-chloro-3-(1-naphthyloxy)propan-2-one | (S)-1-chloro-3-(1-naphthyloxy)propan-2-ol | |||
 |  | 90 (after 48 h) | 90%; S | [20] |
| N-[4-(3-chloro-2-oxo-propoxy)phenyl]acetamide | N-[4-[(S)-3-chloro-2-hydroxy-propoxy]phenyl]acetamide | |||
 |  | 90 (after 48 h) | 99%; S | |
| 1-chloro-3-(2,5-dimethylphenoxy)propan-2-one | (S)-1-chloro-3-(2,5-dimethylphenoxy)propan-2-ol | |||
 |  | 90 (after 48 h) | 5%; S | |
| 1-chloro-3-phthalimidopropan-2-one | (S)-1-chloro-3-phthalimidopropan-2-ol | |||
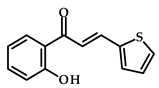
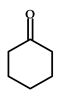 |  | 34–63 (after 48 h) | - | [21] |
| Cyclohexanone | Cyclohexanol | |||
 |  | 36 (after 9 h); 83 (after 21 h) | 19% or 31%; 1R,3R | [22] |
| diethyl 2-(3-oxocyclohexyl)malonate | diethyl 2-[(1R,3R)-3-hydroxycyclohexyl]malonate | |||
 |  | 92 (after 48 h) | 79%; 2R,3R | [23] |
| (R)-1,2-(cyclohexylidenedioxy)hept-6-en-3-one | (2R,3R)-1,2-(cyclohexylidenedioxy)hept-6-en-3-ol | |||
 | 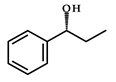 | 60–94 (after 9 days) | 35–76%; R | [24] |
| 1-phenylpropan-1-one | (R)-1-phenylpropan-1-ol | |||
 |  | 80–100 (after 24 h) | >99%; 3R,1′R | [25] |
| α-acetylbutyrolactone | (3R,1′R)-α’-1′-hydroxyethyl-γ-butyrolactone | |||
| Reaction type—Oxidation | ||||
 |  | 27.19–29.50 (after 12 days) | - | [26] |
| (+)-valencene | (+)-nootkatone | 38.8–77.9 (after 120 h) | - | [27] |
 |  | 52 (after 72 h) | 85%; R | [28] |
| (±)-trans-flavan-4-ol | (R)-flavanone | |||
 |  | 40–43 (after 32 h) | 82–100%; 1R,5S | [29] |
| (6S)-bicyclo[3.2.0]hept-2-en-6-ol | (1R,5S)-bicyclo[3.2.0]hept-2-en-6-one | |||
 | 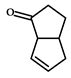 | 49 (after 48 h) | 67%; 1R,5R | |
| (1S)-bicyclo[3.3.0]oct-7-en-2-ol | (1R,5R)-bicyclo[3.3.0]oct-7-en-2-one | |||
 |  | 42 (after 72 h) | - | |
| 6-methylhept-5-en-2-ol | 6-methylhept-5-en-2-one | |||
 |  | 31–50 (after 48 h) | 55–92%; R | |
| cis-2-methylcyclohexanol | (R)-2-methylcyclohexanone | |||
 |  | 11 (after 48 h) | >95%; S | |
| trans-2-methylcyclohexanol | (S)-2-methylcyclohexanone | |||
| Synthesized Polymer | Immobilization Support | Reaction Conditions | Yield (%) | Molecular Weight (Da) | Degree of Crystallinity (%) | Reference | |
|---|---|---|---|---|---|---|---|
| Poly(ε-caprolactone) | - | 3 mmol of ε-caprolactone and 100 mg of lipase; heptane as a solvent | 360 h, 60 °C | 100 | 620/975 a | 78.6 | [86] |
| 120 h, 65 °C | 100 | 608/734 a | 76.0 | ||||
| 264 h, 70 °C | 100 | 540/660 a | 54.0 | ||||
| 17.52 mmol of ε-caprolactone and 100 mg of lipase; [BuPy][BF4] as a solvent | 24 h, 60 °C | 100 | 8000/8158 b | 74.0 | [87] | ||
| 13.14 mmol of ε-caprolactone and 100 mg of lipase; [BuPy][BF4] as a solvent | 16 h, 100 °C | 100 | 1808/2340 b | 64.0 | |||
| 35 mmol of ε-caprolactone and 100 mg of lipase; [EMIM][BF4] as a solvent | 16 h, 100 °C | 100 | 837/1823 b | 76.0 | |||
| 22 mmol of ε-caprolactone and 100 mg of lipase; [BMIM][BF4] as a solvent | 16 h, 100 °C | 100 | 1377/1758 b | 55.0 | |||
| 35 mmol of ε-caprolactone and 100 mg of lipase; [BuPy][CF3COO] as a solvent | 16 h, 100 °C | 100 | 1158/1734 b | 66.0 | |||
| 43.8 mmol of ε-caprolactone and 100 mg of lipase; [EMIM][NO3] as a solvent | 24 h, 90 °C | 100 | 1172/2843 b | 68.0 | |||
| 43.8 mmol of ε-caprolactone and 100 mg of lipase; [BuPy][CF3COO] as a solvent | 24 h, 90 °C | 100 | 1300/2603 b | 71.0 | |||
| 52.57 mmol of ε-caprolactone and 100 mg of lipase; [BuPy][BF4] as a solvent | 6 h, 150 °C | 100 | 3250/3788 b | 56.0 | |||
| 35.04 mmol of ε-caprolactone and 100 mg of lipase; [BMIM][BF4] as a solvent | 6 h, 150 °C | 100 | 2699/3092 b | 58.0 | |||
| 52.57 mmol of ε-caprolactone and 100 mg of lipase; [EMIM][BF4] as a solvent | 6 h, 150 °C | 100 | 2426/2787 b | 55.0 | |||
| 52.57 mmol of ε-caprolactone and 100 mg of lipase; [BuPy][CF3COO] as a solvent | 6 h, 150 °C | 100 | 2693/2953 b | 53.0 | |||
| Poly(ε-caprolactone) with an isosorbide headgroup | Lewatit 1026 | 1 mmol of ε-caprolactone, 0.125 mmol of isosorbide and 12 mg of lipase | 94 h, 80 °C | 100 | 1068 c | 60.8 | [88] |
| Lewatit K2629 | 24 h, 90 °C | 100 | ND | 30.1 | |||
| Poly(ε-caprolactone) | Lewatit | 1.08 mmol of ε-caprolactone and 10% (w/w) of lipase | 6 h, 150 °C | 74 | 1358 d | ND | [89] |
| Accurel | 3 | 653 d | ND | ||||
| Dendritic polymers composed of adipic acid and glycerol | Lewatit | 40 g/L of adipic acid and glycerol, 5 mL of tert-butanol as a solvent, 100 mg of lipase | 48 h, 50 °C | ND | ND | ND | |
| Poly(ε-caprolactone) diols with diethylene glycol | Lewatit 1026 | 10 mmol of ε-caprolactone, 1 mmol of diethylene glycol, and 12 mg of lipase | 6 h, 120 °C | ND | 4321/1363/836 e | ND | [90] |
| 10 mmol of ε-caprolactone, 0.5 mmol of diethylene glycol, and 12 mg of lipase | 5101/1978/1305 e | ND | |||||
| 10 mmol of ε-caprolactone, 0.25 mmol of diethylene glycol, and 12 mg of lipase | 7426/2429/1780 e | ND | |||||
| PEG-ε-caprolactone copolymers | 10 mmol of ε-caprolactone, 1 mmol of PEG200, and 12 mg of lipase | 6 h, 120 °C | ND | 3817/974/1066 e | ND | ||
| 10 mmol of ε-caprolactone, 1 mmol of PEG400, and 12 mg of lipase | 4083/1120/1211 e | ND | |||||
| 10 mmol of ε-caprolactone, 1 mmol of PEG1000, and 12 mg of lipase | 4481/971/2504 e | ND | |||||
| Poly(ε-caprolactone) diols with 1,3-propanediol | Lewatit 1026 | 10 mmol of ε-caprolactone, 0.5 mmol of 1,3-propanediol, 12 mg of lipase | 6 h, 120 °C | 100 | 5475 f | ND | [91] |
| 10 mmol of ε-caprolactone, 0.25 mmol of 1,3-propanediol, 12 mg of lipase | 100 | 5922 f | ND | ||||
| Lewatit K2629 | 10 mmol of ε-caprolactone, 1 mmol of 1,3-propanediol, 12 mg of lipase | 100 | 3755 f | ND | |||
| 10 mmol of ε-caprolactone, 0.5 mmol of 1,3-propanediol, 12 mg of lipase | 100 | 4099 f | ND | ||||
| PEG-ε-caprolactone copolymers | Lewatit VPOC K2629 | 10 mmol of ε-caprolactone, 1 mmol of PEG200, 12 mg of lipase | 12 h, 120 °C | ~100 | 845/2610 b | ND | [92] |
| Amberlyst15 | ~100 | 856/3892 b | ND | ||||
| Poly(ε-caprolactone) diols with diethylene glycol | Lewatit VPOC K2629 | 10 mmol of ε-caprolactone, 0.5 mmol of diethylene glycol, 12 mg of lipase | ~100 | 553/3602 b | ND | ||
| Amberlyst15 | 10 mmol of ε-caprolactone, 1 mmol of diethylene glycol, 12 mg of lipase | ~100 | 631/2478 b | ND | |||
| Poly(ε-caprolactone) diols with ethylene glycol | Lewatit VPOC K2629 | 10 mmol of ε-caprolactone, 1 mmol of ethylene glycol, 12 mg of lipase | ~100 | 617/2719 b | ND | ||
| Amberlyst15 | ~100 | 743/4005 b | ND | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieniuk, B.; Jasińska, K.; Wierzchowska, K.; Uğur, Ş.; Fabiszewska, A. Yarrowia lipolytica Yeast: A Treasure Trove of Enzymes for Biocatalytic Applications—A Review. Fermentation 2024, 10, 263. https://doi.org/10.3390/fermentation10050263
Zieniuk B, Jasińska K, Wierzchowska K, Uğur Ş, Fabiszewska A. Yarrowia lipolytica Yeast: A Treasure Trove of Enzymes for Biocatalytic Applications—A Review. Fermentation. 2024; 10(5):263. https://doi.org/10.3390/fermentation10050263
Chicago/Turabian StyleZieniuk, Bartłomiej, Karina Jasińska, Katarzyna Wierzchowska, Şuheda Uğur, and Agata Fabiszewska. 2024. "Yarrowia lipolytica Yeast: A Treasure Trove of Enzymes for Biocatalytic Applications—A Review" Fermentation 10, no. 5: 263. https://doi.org/10.3390/fermentation10050263






