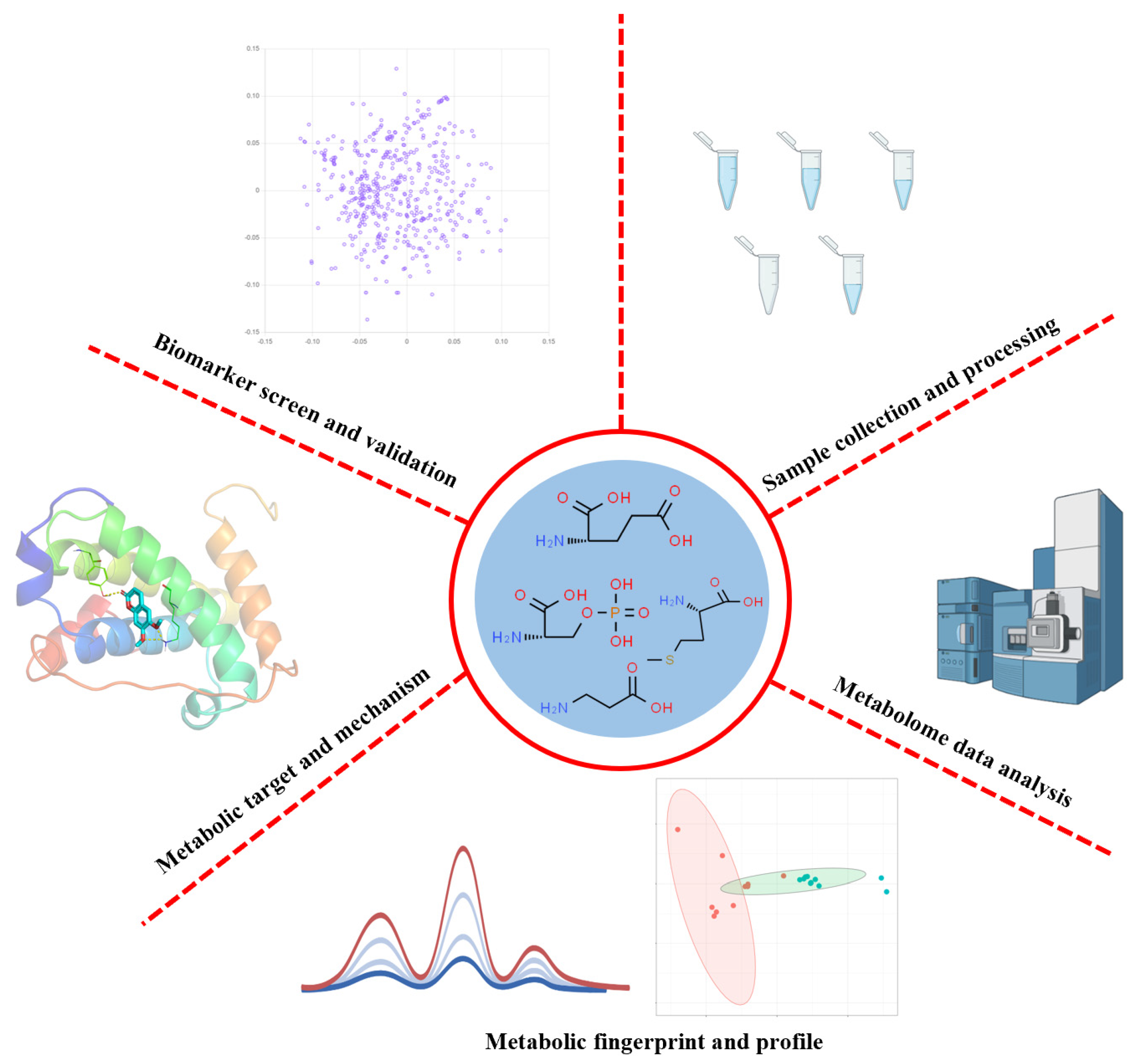
Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification
Abstract
:1. Introduction
2. Advanced Mass Spectrometry
3. Mass Spectrometry-Based Metabolomics for Metabolic Phenotype Signatures
4. Potential Biomarkers Discovery by Mass Spectrometry
5. For Disease Diagnosis and Classification
6. Disease Prognosis Analysis via Mass Spectrometry-Based Metabolomics
7. Mass Spectrometry-Based Metabolomics for Revealing Pathological Mechanism
8. Mass Spectrometry-Based Metabolomics for Monitoring Treatments
9. Modulating Metabolism Analysis Using Mass Spectrometry
10. Therapeutic Target Discovery via Mass Spectrometry-Based Metabolomics
11. Efficacy Evaluation of Herbal Medicine and Natural Products
12. Multi-Target and Effect Mechanism Exploration of Herbal Medicine
13. Limitations and Challenges of Mass Spectrometry-Based Metabolomics
14. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Fromentin, S.; Forslund, S.K.; Chechi, K.; Aron-Wisnewsky, J.; Chakaroun, R.; Nielsen, T.; Tremaroli, V.; Ji, B.; Prifti, E.; Myridakis, A.; et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 2022, 28, 303–314. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Keshari, K.R. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell 2022, 185, 2678–2689. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [PubMed]
- Liu, P.; Cai, Y.; Qiu, S.; Yang, Q.; Xie, Y.; Zhang, A. Critical roles of functional molecule metabolites. Front. Mol. Biosci. 2023, 10, 1119588. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Dickens, A.M.; Posti, J.P.; Czeiter, E.; Duberg, D.; Sinioja, T.; Kråkström, M.; Retel Helmrich, I.R.A.; Wang, K.K.W.; Maas, A.I.R.; et al. Serum metabolome associated with severity of acute traumatic brain injury. Nat. Commun. 2022, 13, 2545. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Z.; Guo, S.; Lin, C.; Yao, H.; Yang, Q.; Wang, Y.; Yu, X.; He, X.; Sun, W.; et al. Detection, mechanisms, and therapeutic implications of oncometabolites. Trends Endocrinol. Metab. 2023, 34, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Wang, Z.; Xie, Y.; Zhang, A. Decoding functional significance of small molecule metabolites. Biomed. Pharmacother. 2023, 158, 114188. [Google Scholar] [CrossRef]
- Anyfanti, P.; Nikolaidou, B.; Gkaliagkousi, E. Urine metabolomic phenotyping for detection of adrenocortical carcinoma: Still a long way to go. Lancet Diabetes Endocrinol. 2020, 8, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qiu, S.; Cai, Y.; Wang, Z.; Yang, Q.; Tang, S.; Xie, Y.; Zhang, A. Mass spectrometry-based metabolomics for discovering active ingredients and exploring action mechanism of herbal medicine. Front. Chem. 2023, 11, 1142287. [Google Scholar] [CrossRef]
- Li, Y.; Hou, G.; Zhou, H.; Wang, Y.; Tun, H.M.; Zhu, A.; Zhao, J.; Xiao, F.; Lin, S.; Liu, D.; et al. Multi-platform omics analysis reveals molecular signature for COVID-19 pathogenesis, prognosis and drug target discovery. Signal Transduct. Target. Ther. 2021, 6, 155. [Google Scholar] [CrossRef]
- Bao, X.H.; Bao, L.M.; Xiang, C.; Gerile, S.; Qiqige, S.; Xie, Y.L. Metabolic characterization of the badagan constitution in mongolian medicine by ultrahigh-performance liquid chromatography/quadrupole time-of-flight mass spectrometry/MS. World J. Tradit. Chin. Med. 2022, 8, 539–547. [Google Scholar] [CrossRef]
- Liu, F.; Yao, Y.; Lu, Z.; Zhang, Q.; Liu, C.; Zhu, C.; Lin, C. 5-Hydroxy-4-methoxycanthin-6-one alleviates dextran sodium sulfate-induced colitis in rats via regulation of metabolic profiling and suppression of NF-κB/p65 signaling pathway. Phytomedicine 2021, 82, 153438. [Google Scholar] [CrossRef]
- Zhang, J.; Fuhrer, T.; Ye, H.; Kwan, B.; Montemayor, D.; Tumova, J.; Darshi, M.; Afshinnia, F.; Scialla, J.J.; Anderson, A.; et al. High-Throughput Metabolomics and Diabetic Kidney Disease Progression: Evidence from the Chronic Renal Insufficiency (CRIC) Study. Am. J. Nephrol. 2022, 53, 215–225. [Google Scholar] [CrossRef]
- Hao, M.; Zhao, M.T.; Tong, H.J.; Ji, D.; Li, L.; Su, L.L. Ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry based bile and urine metabonomics study on the ameliorative effects of Curcuma wenyujin rhizoma on acute blood stasis in rats. World J. Tradit. Chin. Med. 2022, 8, 141–152. [Google Scholar]
- Dreyfuss, J.M.; Yuchi, Y.; Dong, X.; Efthymiou, V.; Pan, H.; Simonson, D.C.; Vernon, A.; Halperin, F.; Aryal, P.; Konkar, A.; et al. High-throughput mediation analysis of human proteome and metabolome identifies mediators of post-bariatric surgical diabetes control. Nat. Commun. 2021, 12, 6951. [Google Scholar] [CrossRef]
- Qiu, S.; Guo, S.; Yang, Q.; Xie, Y.; Tang, S.; Zhang, A. Innovation in identifying metabolites from complex metabolome-Highlights of recent analytical platforms and protocols. Front. Chem. 2023, 11, 1129717. [Google Scholar] [CrossRef]
- Rischke, S.; Hahnefeld, L.; Burla, B.; Behrens, F.; Gurke, R.; Garrett, T.J. Small molecule biomarker discovery: Proposed workflow for LC-MS-based clinical research projects. J. Mass. Spectrom. Adv. Clin. Lab. 2023, 28, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.X.; Xie, H.M.; Zuo, T.T. Ultra-high performance liquid chromatography/ion mobility-quadrupole time-of-flight massspectrometry and database-driven automatic peak annotation for the rapid profiling and characterization of the multicomponents from Stephaniae Tetrandrae radix (Fang-Ji). World J. Tradit. Chin. Med. 2021, 7, 120–129. [Google Scholar]
- Lin, L.; Su, L.L.; Li, H.H.; Mao, C.Q.; Ji, D.; Xie, H. Study on quality markers and action mechanisms of inulae flos on anti-hepatitis through network pharmacology and high-performance liquid chromatography fingerprints. World J. Tradit. Chin. Med. 2022, 8, 426–435. [Google Scholar]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Lotta, L.A.; Pietzner, M.; Stewart, I.D.; Wittemans, L.B.L.; Li, C.; Bonelli, R.; Raffler, J.; Biggs, E.K.; Oliver-Williams, C.; Auyeung, V.P.W.; et al. A cross-platform approach identifies genetic regulators of human metabolism and health. Nat. Genet. 2021, 53, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, Y.; Li, P.; Li, B. Evaluation of Intestinal Drug Absorption and Interaction Using Quadruple Single-Pass Intestinal Perfusion Coupled with Mass Spectrometry Imaging. Anal. Chem. 2023, 95, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, Z.; Zou, Y.; Liao, J.; Li, B. A multimodal pipeline for image correction and registration of mass spectrometry imaging with microscopy. Anal. Chim. Acta 2023, 1283, 341969. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cha, X.; Zhang, Z.; Qian, J. Discrimination of serum metabolomics profiles in infants with sepsis, based on liquid chromatography-mass spectrometer. BMC Infect. Dis. 2023, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Paris, D.; Cuomo, P.; Fuschillo, S.; Ambrosino, P.; Tramice, A.; Palomba, L.; Motta, A. Metabolomics of COPD Pulmonary Rehabilitation Outcomes via Exhaled Breath Condensate. Cells 2022, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, Z.; Ferrari, M.W.; Liu, Y.; Li, C.; Zhang, T.; Lyu, G. The Correlation between Gut Microbiota and Serum Metabolomic in Elderly Patients with Chronic Heart Failure. Mediat. Inflamm. 2021, 2021, 5587428. [Google Scholar] [CrossRef] [PubMed]
- Swank, K.R.; Furness, J.E.; Baker, E.A.; Gehrke, C.K.; Biebelhausen, S.P.; Baker, K.C. Metabolomic Profiling in the Characterization of Degenerative Bone and Joint Diseases. Metabolites 2020, 10, 223. [Google Scholar] [CrossRef]
- Mehta, A.; Liu, C.; Nayak, A.; Tahhan, A.S.; Ko, Y.A.; Dhindsa, D.S.; Kim, J.H.; Hayek, S.S.; Sperling, L.S.; Mehta, P.K.; et al. Untargeted high-resolution plasma metabolomic profiling predicts outcomes in patients with coronary artery disease. PLoS ONE 2020, 15, e0237579. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Michelucci, E.; Ndreu, R.; Rocchiccioli, S.; Chatzianagnostou, K.; Berti, S.; Vassalle, C. Lipidomic Analysis to Assess the Correlation between Ceramides, Stress Hyperglycemia, and HbA1c in Acute Myocardial Infarction. Molecules 2023, 28, 716. [Google Scholar] [CrossRef]
- Gumus Balikcioglu, P.; Jachthuber Trub, C.; Balikcioglu, M.; Ilkayeva, O.; White, P.J.; Muehlbauer, M.; Bain, J.R.; Armstrong, S.; Freemark, M. Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity. Endocrinol. Diabetes Metab. 2023, 6, e388. [Google Scholar] [CrossRef]
- Baiges-Gaya, G.; Iftimie, S.; Castañé, H.; Rodríguez-Tomàs, E.; Jiménez-Franco, A.; López-Azcona, A.F.; Castro, A.; Camps, J.; Joven, J. Combining Semi-Targeted Metabolomics and Machine Learning to Identify Metabolic Alterations in the Serum and Urine of Hospitalized Patients with COVID-19. Biomolecules 2023, 13, 163. [Google Scholar] [CrossRef]
- Razquin, C.; Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Guasch-Ferré, M.; García-Gavilán, J.F.; Wittenbecher, C.; Alonso-Gómez, A.; Fitó, M.; Liang, L.; et al. Circulating Amino Acids and Risk of Peripheral Artery Disease in the PREDIMED Trial. Int. J. Mol. Sci. 2022, 24, 270. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Wang, C.; Zhao, S.; Han, S.; Jiang, P.; Cui, C. Targeted metabolomics analysis of serum amino acid profiles in patients with Moyamoya disease. Amino Acids 2022, 54, 137–146. [Google Scholar] [CrossRef]
- Abdelsattar, S.; Kasemy, Z.A.; Elsayed, M.; Elrahem, T.A.; Zewain, S.K. Targeted metabolomics as a tool for the diagnosis of kidney disease in Type II diabetes mellitus. Br. J. Biomed. Sci. 2021, 78, 184–190. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; García-Flores, M.; Rubio-Briones, J.; López-Guerrero, J.A.; Pineda-Lucena, A.; Puchades-Carrasco, L. Targeted Metabolomics Analyses Reveal Specific Metabolic Alterations in High-Grade Prostate Cancer Patients. J. Proteome Res. 2020, 19, 4082–4092. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Chen, R.; Han, S.; Liu, Y.; Liu, X.; Gao, M.; Yang, C.; Lu, D.; Sun, B.; et al. Serum amino acid metabolic profiles of ankylosing spondylitis by targeted metabolomics analysis. Clin. Rheumatol. 2020, 39, 2325–2336. [Google Scholar] [CrossRef]
- Kasakin, M.F.; Rogachev, A.D.; Predtechenskaya, E.V.; Zaigraev, V.J.; Koval, V.V.; Pokrovsky, A.G. Targeted metabolomics approach for identification of relapsing-remitting multiple sclerosis markers and evaluation of diagnostic models. Medchemcomm 2019, 10, 1803–1809. [Google Scholar] [CrossRef]
- Huo, Z.; Yu, L.; Yang, J.; Zhu, Y.; Bennett, D.A.; Zhao, J. Brain and blood metabolome for Alzheimer’s dementia: Findings from a targeted metabolomics analysis. Neurobiol. Aging 2020, 86, 123–133. [Google Scholar] [CrossRef]
- Peng, S.; Shen, Y.; Wang, M.; Zhang, J. Serum and CSF Metabolites in Stroke-Free Patients Are Associated with Vascular Risk Factors and Cognitive Performance. Front. Aging Neurosci. 2020, 12, 193. [Google Scholar] [CrossRef]
- Tavares, G.; Venturini, G.; Padilha, K.; Zatz, R.; Pereira, A.C.; Thadhani, R.I.; Rhee, E.P.; Titan, S.M.O. 1,5-Anhydroglucitol predicts CKD progression in macroalbuminuric diabetic kidney disease: Results from non-targeted metabolomics. Metabolomics 2018, 14, 39. [Google Scholar] [CrossRef]
- Huang, H.; Shi, L.Y.; Wei, L.L.; Han, Y.S.; Yi, W.J.; Pan, Z.W.; Jiang, T.T.; Chen, J.; Tu, H.H.; Li, Z.B.; et al. Plasma metabolites Xanthine, 4-Pyridoxate, and d-glutamic acid as novel potential biomarkers for pulmonary tuberculosis. Clin. Chim. Acta 2019, 498, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Z.; Zhou, Y.; Yu, S.; Yang, H.; Zheng, L.; Liu, Y.; Sun, Y. Metabolic Profile for Prediction of Ischemic Stroke in Chinese Hypertensive Population. J. Stroke Cerebrovasc. Dis. 2019, 28, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Tomofuji, Y.; Suzuki, K.; Kishikawa, T.; Shojima, N.; Hosoe, J.; Inagaki, K.; Matsubayashi, S.; Ishihara, H.; Watada, H.; Ishigaki, Y.; et al. Identification of serum metabolome signatures associated with retinal and renal complications of type 2 diabetes. Commun. Med. 2023, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, S.; Arjmand, B.; Dilmaghani-Marand, A.; Mohammadi Fateh, S.; Dehghanbanadaki, H.; Najjar, N.; Alavi-Moghadam, S.; Ghodssi-Ghassemabadi, R.; Nasli-Esfahani, E.; Farzadfar, F.; et al. Targeted metabolomics analysis of amino acids and acylcarnitines as risk markers for diabetes by LC-MS/MS technique. Sci. Rep. 2022, 12, 8418. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Zhao, J.; Yang, T.; Zhang, F.; Liu, L. Serum metabolite signatures of epithelial ovarian cancer based on targeted metabolomics. Clin. Chim. Acta 2021, 518, 59–69. [Google Scholar] [CrossRef]
- Cho, Y.; Park, Y.; Sim, B.; Kim, J.; Lee, H.; Cho, S.N.; Kang, Y.A.; Lee, S.G. Identification of serum biomarkers for active pulmonary tuberculosis using a targeted metabolomics approach. Sci. Rep. 2020, 10, 3825. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, H.; Gao, S.; Liu, Y.; Liu, Y.; Zhang, T.; Cai, X.; Li, Z.; Li, L.; Li, Y.; et al. Identification of coronary heart disease biomarkers with different severities of coronary stenosis in human urine using non-targeted metabolomics based on UPLC-Q-TOF/MS. Clin. Chim. Acta 2019, 497, 95–103. [Google Scholar] [CrossRef]
- Li, R.; Zeng, L.; Xie, S.; Chen, J.; Yu, Y.; Zhong, L. Targeted metabolomics study of serum bile acid profile in patients with end-stage renal disease undergoing hemodialysis. Peer J. 2019, 7, e7145. [Google Scholar] [CrossRef]
- Li, X.; Gong, Y.; Zhang, Q.; Ni, X.; Pang, Q.; Chi, Y.; Jiajue, R.; Cui, L.; Jiang, X.; Wang, O.; et al. UPLC-MS based serum metabolomics for early diagnosis of refractory tumor-induced osteomalacia: A case-control study. J. Clin. Endocrinol. Metab. 2023, 262, 113196. [Google Scholar]
- Wang, X.N.; Song, Y.; Tang, W.; Li, P.; Li, B. Integration of fluorescence and MALDI imaging for microfluidic chip-based screening of potential thrombin inhibitors from natural products. Biosens. Bioelectron. 2023, 237, 115527. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Xie, D.; Ma, T.; Cui, H.; Li, J.; Zhang, A.; Sheng, Z.; Xie, Y. Global burden and influencing factors of chronic kidney disease due to type 2 diabetes in adults aged 20-59 years, 1990-2019. Sci. Rep. 2023, 13, 20234. [Google Scholar] [CrossRef] [PubMed]
- Hur, B.; Gupta, V.K.; Huang, H.; Wright, K.A.; Warrington, K.J.; Taneja, V.; Davis, J.M., 3rd; Sung, J. Plasma metabolomic profiling in patients with rheumatoid arthritis identifies biochemical features predictive of quantitative disease activity. Arthritis Res. Ther. 2021, 23, 164. [Google Scholar] [CrossRef]
- Yang, X.H.; Jing, Y.; Wang, S.; Ding, F.; Zhang, X.X.; Chen, S.; Zhang, L.; Hu, Q.G.; Ni, Y.H. Integrated Non-targeted and Targeted Metabolomics Uncovers Amino Acid Markers of Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Nam, M.; Kwon, M.; Seo, S.S.; Jung, S.; Han, J.S.; Hwang, G.S.; Kim, M.K. LC/MS-Based Polar Metabolite Profiling Identified Unique Biomarker Signatures for Cervical Cancer and Cervical Intraepithelial Neoplasia Using Global and Targeted Metabolomics. Cancers 2019, 11, 511. [Google Scholar] [CrossRef]
- Ahonen, L.; Jäntti, S.; Suvitaival, T.; Theilade, S.; Risz, C.; Kostiainen, R.; Rossing, P.; Orešič, M.; Hyötyläinen, T. Targeted Clinical Metabolite Profiling Platform for the Stratification of Diabetic Patients. Metabolites 2019, 9, 184. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Liang, Y.; Hu, R.; Xu, W.; Liu, Y. Plasma Targeted Metabolomics Analysis for Amino Acids and Acylcarnitines in Patients with Prediabetes, Type 2 Diabetes Mellitus, and Diabetic Vascular Complications. Diabetes Metab. J. 2021, 45, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zuo, J.J.; Dong, S.S.; Lan, Y.; Wu, C.W.; Mao, G.Y.; Zheng, C. Identification of Potential Serum Metabolic Biomarkers of Diabetic Kidney Disease: A Widely Targeted Metabolomics Study. J. Diabetes Res. 2020, 2020, 3049098. [Google Scholar] [CrossRef]
- Zhou, W.; Hong, Y.; Yin, A.; Liu, S.; Chen, M.; Lv, X.; Nie, X.; Tan, N.; Zhang, Z. Non-invasive urinary metabolomics reveals metabolic profiling of polycystic ovary syndrome and its subtypes. J. Pharm. Biomed. Anal. 2020, 185, 113262. [Google Scholar] [CrossRef]
- Morgell, A.; Reisz, J.A.; Ateeb, Z.; Davanian, H.; Reinsbach, S.E.; Halimi, A.; Gaiser, R.; Valente, R.; Arnelo, U.; Del Chiaro, M.; et al. Metabolic Characterization of Plasma and Cyst Fluid from Cystic Precursors to Pancreatic Cancer Patients Reveal Metabolic Signatures of Bacterial Infection. J. Proteome Res. 2021, 20, 2725–2738. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, H.; Tian, Y.; Wang, Y.; He, Y.; Lan, T.; Li, Y.; Bai, M.; Chen, X.; Chen, Z.; et al. Non-targeted Metabolomics Profiling of Plasma Samples from Patients with Major Depressive Disorder. Front. Psychiatry 2022, 12, 810302. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Chang, C.W.; Hsu, J.Y.; Li, S.W.; Sung, P.S.; Wang, R.H.; Wu, C.H.; Liao, P.C. Discovering Hair Biomarkers of Alzheimer’s Disease Using High Resolution Mass Spectrometry-Based Untargeted Metabolomics. Molecules 2023, 28, 2166. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lin, L.; Huang, G.; Xie, Y.; Peng, Z.; Liu, F.; Bai, G.; Li, W.; Gao, L.; Wang, Y.; et al. Metabolomic profiles in serum and urine uncover novel biomarkers in children with nephrotic syndrome. Eur. J. Clin. Investig. 2023, 53, 13978. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Chekmeneva, E.; Jackson, H.; Sands, C.; Mills, E.; Arancon, D.; Li, H.K.; Arkell, P.; Rawson, T.M.; Hammond, R.; et al. Antiviral metabolite 3′-deoxy-3′,4′-didehydro-cytidine is detectable in serum and identifies acute viral infections including COVID-19. Med 2022, 3, 204–215.e6. [Google Scholar] [CrossRef]
- Shoji, S.; Maekawa, M.; Ogura, J.; Sato, T.; Mano, N. Identification cholesterol metabolites altered before the onset of nonalcoholic steatohepatitis by targeted metabolomics. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159135. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Cai, M.; Cao, Y.; Piao, X. Discovery of a New Biomarker Pattern for Differential Diagnosis of Acute Ischemic Stroke Using Targeted Metabolomics. Front. Neurol. 2019, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Xu, B.; Cui, Y.; He, Y.; Yang, T.; Shao, Y.; Ding, M. The urinary bile acid profiling analysis of asymptomatic hypercholanemia of pregnancy: A pseudo-targeted metabolomics study. Clin. Chim. Acta 2019, 49, 67–75. [Google Scholar] [CrossRef]
- Wang, J.; Kunzke, T.; Prade, V.M.; Shen, J.; Buck, A.; Feuchtinger, A.; Haffner, I.; Luber, B.; Liu, D.H.W.; Langer, R.; et al. Spatial Metabolomics Identifies Distinct Tumor-Specific Subtypes in Gastric Cancer Patients. Clin. Cancer Res. 2022, 28, 2865–2877. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, W.; Ji, Z.; Liu, X.; Qiao, Y. Serum metabolites as early detection markers of non-muscle invasive bladder cancer in Chinese patients. Front. Oncol. 2023, 13, 1061083. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Gao, S.; Xue, Q.; Tan, F.; Li, Z.; Gao, Y. Plasma metabolomics study in screening and differential diagnosis of multiple primary lung cancer. Int. J. Surg. 2023, 109, 297–312. [Google Scholar] [CrossRef]
- Evangelista, E.B.; Kwee, S.A.; Sato, M.M.; Wang, L.; Rettenmeier, C.; Xie, G.; Jia, W.; Wong, L.L. Phospholipids are A Potentially Important Source of Tissue Biomarkers for Hepatocellular Carcinoma: Results of a Pilot Study Involving Targeted Metabolomics. Diagnostics 2019, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, D.; Liu, R.; Shi, Y.; Wang, R.; Zhu, Z.; Yuan, Y. Non-target metabolomic analysis reveals the therapeutic effect of Saposhnikovia divaricata decoction on collagen-induced arthritis rats. J. Ethnopharmacol. 2021, 71, 113837. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wang, J.; Zhang, H.; Yang, K.; Li, J.; Zhao, T.; Liu, J.; Wu, J.; Chen, Y. Alterations of Urinary Microbial Metabolites and Immune Indexes Linked With COVID-19 Infection and Prognosis. Front. Immunol. 2022, 13, 841739. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, J.; Chen, Q.; Xu, X.; Gao, J.; Li, X.; Shao, Q.; Zhou, B.; Zhou, H.; Wei, S.; et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 2022, 39, 110986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, J.; Jin, E.; Zhong, Y.; Zhang, L.; Han, X.; Liu, J.; Cheng, Y.; Hou, J.; Shi, X.; et al. Serum Untargeted Metabolomics Reveal Potential Biomarkers of Progression of Diabetic Retinopathy in Asians. Front. Mol. Biosci. 2022, 9, 871291. [Google Scholar] [CrossRef]
- Peng, X.; Wang, X.; Shao, X.; Wang, Y.; Feng, S.; Wang, C.; Ye, C.; Chen, J.; Jiang, H. Serum Metabolomics Benefits Discrimination Kidney Disease Development in Type 2 Diabetes Patients. Front. Med. 2022, 9, 819311. [Google Scholar] [CrossRef]
- Xu, H.; Sun, J.; Zhou, L.; Du, Q.C.; Zhu, H.Y.; Chen, Y.; Wang, X.Y. Development of a lipid metabolism-related gene model to predict prognosis in patients with pancreatic cancer. World J. Clin. Cases 2021, 9, 10884–10898. [Google Scholar] [CrossRef]
- Nenu, I.; Stefanescu, H.; Procopet, B.; Sparchez, Z.; Minciuna, I.; Mocan, T.; Leucuta, D.; Morar, C.; Grigorescu, M.; Filip, G.A.; et al. Navigating through the Lipid Metabolism Maze: Diagnosis and Prognosis Metabolites of Hepatocellular Carcinoma versus Compensated Cirrhosis. J. Clin. Med. 2022, 11, 1292. [Google Scholar] [CrossRef]
- Gu, M.; Pan, H.; Yuan, Y.; Zhou, X.; Chen, L.; Wang, X.; Fang, F.; Hu, L.; Xie, Y.; Shen, C. Sera Metabolomics Characterization of Patients at Different Stages in Wuhan Identifies Critical Biomarkers of COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 882661. [Google Scholar] [CrossRef]
- Xie, G.; Yan, A.; Lin, P.; Wang, Y.; Guo, L. Trimethylamine N-oxide-a marker for atherosclerotic vascular disease. Rev. Cardiovasc. Med. 2021, 22, 787–797. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Chen, F.; Li, X.; Zhang, J.; Shen, M.; Tang, R.; Huang, Z. Serum Abnormal Metabolites for Evaluating Therapeutic Response and Prognosis of Patients with Multiple Myeloma. Front. Oncol. 2022, 12, 808290. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.; Pérez-Fragoso, A.; Maravillas-Montero, J.L.; Llorente, L.; Mejía-Domínguez, N.R.; Páez-Franco, J.C.; Romero-Ramírez, S.; Sosa-Hernández, V.A.; Cervantes-Díaz, R.; Absalón-Aguilar, A.; et al. Redefining COVID-19 Severity and Prognosis: The Role of Clinical and Immunobiotypes. Front. Immunol. 2021, 12, 689966. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Z.; Ma, W.; Fang, C.; Pei, Y.; Jing, Y.; Huang, J.; Han, X.; Xiao, W. Untargeted metabolomic profiling of sepsis-induced cardiac dysfunction. Front. Endocrinol. 2023, 14, 1060470. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, A.; McFall, G.P.; Sapkota, S.; Black, S.E.; Chertkow, H.; Duchesne, S.; Masellis, M.; Li, L.; Dixon, R.A.; Bellec, P. A multiomics approach to heterogeneity in Alzheimer’s disease: Focused review and roadmap. Brain 2020, 143, 1315–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Lv, B.; Hou, X.; Liu, Q.; Liao, C.; Xu, R.; Zhang, Y.; Xu, F.; Zhang, P. Oleic Acid and Insulin as Key Characteristics of T2D Promote Colorectal Cancer Deterioration in Xenograft Mice Revealed by Functional Metabolomics. Front. Oncol. 2021, 11, 685059. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, S.; He, Y.; Xu, M.; Qiao, X.; Zhu, Y.; Wu, W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Markers 2022, 2022, 9484217. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Qin, N.; Jiang, Y.; Shi, W.; Wang, L.; Kong, L.; Wang, C.; Guo, Y.; Zhang, J.; Ma, Q. High-Throughput Untargeted Serum Metabolomics Analysis of Hyperuricemia Patients by UPLC-Q-TOF/MS. Evid. Based Complement. Altern. Med. 2021, 2021, 5524772. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Weng, Z.; Zhang, S.; Li, X.; Zhou, S.; Liang, J. LC-MS/MS-based metabolomic profiling identifies candidate biomarkers in follicular fluid of infertile women with chronic pelvic inflammatory disease. Int. J. Clin. Exp. Pathol. 2023, 16, 20–31. [Google Scholar]
- Ping, F.; Li, Y.; Cao, Y.; Shang, J.; Zhang, Z.; Yuan, Z.; Wang, W.; Guo, Y. Metabolomics Analysis of the Development of Sepsis and Potential Biomarkers of Sepsis-Induced Acute Kidney Injury. Oxid. Med. Cell. Longev. 2021, 2021, 6628847. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Scott, J.W.; Desjardins, E.M.; Smith, B.K.; Day, E.A.; Ford, R.J.; Langendorf, C.G.; Ling, N.X.Y.; Nero, T.L.; Loh, K.; et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nat. Metab. 2020, 2, 873–881. [Google Scholar] [CrossRef]
- Babu, A.F.; Csader, S.; Männistö, V.; Tauriainen, M.M.; Pentikäinen, H.; Savonen, K.; Klåvus, A.; Koistinen, V.; Hanhineva, K.; Schwab, U. Effects of exercise on NAFLD using non-targeted metabolomics in adipose tissue, plasma, urine, and stool. Sci. Rep. 2022, 12, 6485. [Google Scholar]
- Xie, F.; Xu, L.; Zhu, H.; Li, Y.; Nong, L.; Chen, Y.; Zeng, Y.; Cen, S. Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model. Metabolites 2022, 12, 479. [Google Scholar] [CrossRef]
- Tang, R.; Li, L. Modulation of Short-Chain Fatty Acids as Potential Therapy Method for Type 2 Diabetes Mellitus. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6632266. [Google Scholar]
- Pathak, P.; Helsley, R.N.; Brown, A.L.; Buffa, J.A.; Choucair, I.; Nemet, I.; Gogonea, C.B.; Gogonea, V.; Wang, Z.; Garcia-Garcia, J.C.; et al. Small-molecule inhibition of gut microbial choline trimethylamine lyase activity alters host cholesterol and bile acid metabolism. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1474–H1486. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, Z.; Li, X.; Yang, Y.; Qi, R. Metabolomics profiling in prediction of chemo-immunotherapy efficiency in advanced non-small cell lung cancer. Front. Oncol. 2023, 12, 1025046. [Google Scholar] [CrossRef]
- Yao, H.; Yu, P.C.; Jiang, C.M. Metabolomics-driven identification of perturbations in amino acid and sphingolipid metabolism as therapeutic targets in a rat model of anorexia nervosa disease using chemometric analysis and a multivariate analysis platform. RSC Adv. 2020, 10, 4928–4941. [Google Scholar] [CrossRef]
- Cheema, A.K.; Grindrod, S.; Zhong, X.; Jain, S.; Menon, S.S.; Mehta, K.Y.; Suy, S.; Collins, S.; Wang, Y.; Timofeeva, O.; et al. Discovery of Metabolic Biomarkers Predicting Radiation Therapy Late Effects in Prostate Cancer Patients. Adv. Exp. Med. Biol. 2019, 1164, 141–150. [Google Scholar]
- Ghergurovich, J.M.; García-Cañaveras, J.C.; Wang, J.; Schmidt, E.; Zhang, Z.; TeSlaa, T.; Patel, H.; Chen, L.; Britt, E.C.; Piqueras-Nebot, M.; et al. A small molecule G6PD inhibitor reveals immune dependence on pentose phosphate pathway. Nat. Chem. Biol. 2020, 16, 731–739. [Google Scholar] [CrossRef]
- Tong, L.; Xiao, X.; Li, M.; Fang, S.; Ma, E.; Yu, X.; Zhu, Y.; Wu, C.; Tian, D.; Yang, F.; et al. A glucose-like metabolite deficient in diabetes inhibits cellular entry of SARS-CoV-2. Nat. Metab. 2022, 4, 547–558. [Google Scholar] [CrossRef]
- Yue, L.; Zeng, P.; Li, Y.; Chai, Y.; Wu, C.; Gao, B. Nontargeted and targeted metabolomics approaches reveal the key amino acid alterations involved in multiple myeloma. Peer J. 2022, 10, e12918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fu, H.; Zhou, T.; Cai, M.; Huang, Y.; Gan, Q.; Zhang, C.; Qian, C.; Wang, J.; Zhang, Z.; et al. Alteration of Bile Acids and Omega-6 PUFAs Are Correlated with the Progression and Prognosis of Drug-Induced Liver Injury. Front. Immunol. 2022, 13, 772368. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Suhail, H.; Datta, I.; Ahmed, M.E.; Poisson, L.M.; Waters, J.; Rashid, F.; Bin, R.; Singh, J.; Cerghet, M.; et al. Blood-based untargeted metabolomics in relapsing-remitting multiple sclerosis revealed the testable therapeutic target. Proc. Natl. Acad. Sci. USA 2022, 119, e2123265119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Miyata, N.; Winter, M.G.; Arenales, A.; Hughes, E.R.; Spiga, L.; Kim, J.; Sifuentes-Dominguez, L.; Starokadomskyy, P.; Gopal, P.; et al. Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J. Exp. Med. 2019, 216, 2378–2393. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; de Lima Romão, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 55, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Somarribas Patterson, L.F.; Mohapatra, S.R.; Dewi, D.L.; Sadik, A.; Platten, M.; Trump, S. The therapeutic potential of targeting tryptophan catabolism in cancer. Br. J. Cancer 2020, 122, 30–44. [Google Scholar] [CrossRef]
- Meier-Stephenson, F.S.; Meier-Stephenson, V.C.; Carter, M.D.; Meek, A.R.; Wang, Y.; Pan, L.; Chen, Q.; Jacobo, S.; Wu, F.; Lu, E.; et al. Alzheimer’s disease as an autoimmune disorder of innate immunity endogenously modulated by tryptophan metabolites. Alzheimer’s Dement. 2022, 8, e12283. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Snowden, S.; Suvitaival, T.; Ali, A.; Merkler, D.J.; Ahmad, T.; Westwood, S.; Baird, A.; Proitsi, P.; Nevado-Holgado, A.; et al. Primary fatty amides in plasma associated with brain amyloid burden, hippocampal volume, and memory in the European Medical Information Framework for Alzheimer’s Disease biomarker discovery cohort. Alzheimer’s Dement. 2019, 15, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Hileman, C.O.; Kalayjian, R.C.; Azzam, S.; Schlatzer, D.; Wu, K.; Tassiopoulos, K.; Bedimo, R.; Ellis, R.J.; Erlandson, K.M.; Kallianpur, A.; et al. Plasma Citrate and Succinate Are Associated with Neurocognitive Impairment in Older People with HIV. Clin. Infect. Dis. 2021, 73, e765–e772. [Google Scholar] [CrossRef]
- Stewart Campbell, A.; Needham, B.D.; Meyer, C.R.; Tan, J.; Conrad, M.; Preston, G.M.; Bolognani, F.; Rao, S.G.; Heussler, H.; Griffith, R.; et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: An open-label phase 1b/2a trial. Nat. Med. 2022, 28, 528–534. [Google Scholar] [CrossRef]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef] [PubMed]
- Gatius, S.; Jove, M.; Megino-Luque, C.; Albertí-Valls, M.; Yeramian, A.; Bonifaci, N.; Piñol, M.; Santacana, M.; Pradas, I.; Llobet-Navas, D.; et al. Metabolomic Analysis Points to Bioactive Lipid Species and Acireductone Dioxygenase 1 (ADI1) as Potential Therapeutic Targets in Poor Prognosis Endometrial Cancer. Cancers 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hui, R.; Nouws, J.; Sauler, M.; Zeng, T.; Wu, Q. Untargeted metabolomics analysis of esophageal squamous cell cancer progression. J. Transl. Med. 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Nie, F.; Zheng, X.Y.; Zhao, S.J. L-tyrosine metabolic pathway in microorganisms and its application in the biosynthesis of plant-derived natural products. World J. Tradit. Chin. Med. 2022, 8, 386–394. [Google Scholar] [CrossRef]
- Sriwi, D.; Alabdaljabar, M.S.; Jacob, M.; Mujamammi, A.H.; Gu, X.; Sabi, E.M.; Li, L.; Hussein, M.H.; Dasouki, M.; Abdel Rahman, A.M. Metabolomics Profiling of Cystic Renal Disease towards Biomarker Discovery. Biology 2021, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Bian, X.; Zhai, Y.; Li, C.; Li, N.; Hao, C.; Huang, H.; Luo, W.; Huang, Z.; Liao, C.; et al. Metabolomics reveals a correlation between hydroxyeicosatetraenoic acids and allergic asthma: Evidence from three years’ immunotherapy. Pediatr. Allergy Immunol. 2021, 32, 1654–1662. [Google Scholar] [CrossRef]
- Di, F.; Gao, D.; Yao, L.; Zhang, R.; Qiu, J.; Song, L. Differences in metabonomic profiles of abdominal subcutaneous adipose tissue in women with polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1077604. [Google Scholar] [CrossRef]
- Mehta, O.; Vijay, A.; Gohir, S.A.; Kelly, T.; Zhang, W.; Doherty, M.; Walsh, D.A.; Aithal, G.; Valdes, A.M. Serum Metabolome Analysis identified amino-acid metabolism associated with pain in people with symptomatic knee Osteoarthritis—A cross-sectional study. J. Pain 2023, 24, 1251–1261. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, P.; Li, S.; Li, B.; Li, Y.; Ma, L. Lysophospholipids and branched chain amino acids are associated with aging: A metabolomics-based study of Chinese adults. Eur. J. Med. Res. 2023, 28, 58. [Google Scholar] [CrossRef]
- Zheng, Y.; He, Z.; Kong, Y.; Huang, X.; Zhu, W.; Liu, Z.; Gong, L. Combined Metabolomics with Transcriptomics Reveals Important Serum Biomarkers Correlated with Lung Cancer Proliferation through a Calcium Signaling Pathway. J. Proteome Res. 2021, 20, 3444–3454. [Google Scholar] [CrossRef]
- Wei, Y.; Jasbi, P.; Shi, X.; Turner, C.; Hrovat, J.; Liu, L.; Rabena, Y.; Porter, P.; Gu, H. Early Breast Cancer Detection Using Untargeted and Targeted Metabolomics. J. Proteome Res. 2021, 20, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, Z.; Gong, L. Biomarker identification and pathway analysis of rheumatoid arthritis based on metabolomics in combination with ingenuity pathway analysis. Proteomics 2021, 21, e2100037. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Yang, L.; Wan, Y.; Liu, C.; Jiang, S.; Shang, E.X.; Duan, J.A. Integrated gut microbiota and fecal metabolomics reveal the renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced CKD rats. J. Chromatogr. B 2021, 1174, 122728. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Chen, H.; Zhou, X. Untargeted lipidomics analysis of Mori Fructus polysaccharide on acute alcoholic liver injury in mice using ultra performance liquid chromatography-quadrupole- orbitrap-high resolution mass spectrometry. Int. Immunopharmacol. 2021, 97, 107521. [Google Scholar] [CrossRef]
- Ansone, L.; Briviba, M.; Silamikelis, I.; Terentjeva, A.; Perkons, I.; Birzniece, L.; Rovite, V.; Rozentale, B.; Viksna, L.; Kolesova, O.; et al. Amino Acid Metabolism is Significantly Altered at the Time of Admission in Hospital for Severe COVID-19 Patients: Findings from Longitudinal Targeted Metabolomics Analysis. Microbiol. Spectr. 2021, 9, e0033821. [Google Scholar] [CrossRef]
- Xu, R.; Liang, J.; Cheng, M.; Wu, H.; Wu, H.; Cao, S.; Zhao, W.; Xu, R.; Zhou, A. Liver and urine metabolomics reveal the protective effect of Gandou decoction in copper-laden Hepatolenticular degeneration model rats. J. Chromatogr. B 2021, 1179, 122844. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.M.; Jung, J.; Lee, J.E.; Shin, S.M.; Sung, H.K.; Go, H.Y.; Jang, S. Metabolomic analysis of Gyejibongnyeong-Hwan for shoulder pain: A randomized, wait-list controlled pilot trial. Phytomedicine 2022, 104, 154248. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Fu, K.; Liu, Y.; Gong, L.; Peng, C.; Li, Y. Hepatoprotective effect of phillygenin on carbon tetrachloride-induced liver fibrosis and its effects on short chain fatty acid and bile acid metabolism. J. Ethnopharmacol. 2022, 296, 115478. [Google Scholar] [CrossRef]
- Yang, L.; Wan, Y.; Li, W.; Liu, C.; Li, H.F.; Dong, Z.; Zhu, K.; Jiang, S.; Shang, E.; Qian, D.; et al. Targeting intestinal flora and its metabolism to explore the laxative effects of rhubarb. Appl. Microbiol. Biotechnol. 2022, 106, 1615–1631. [Google Scholar] [CrossRef]
- Yang, Z.; Dan, W.; Li, Y.; Zhou, X.; Liu, T.; Shi, C.; Li, R.; Zhang, Y.; Zhang, J.; Yan, J.; et al. Untargeted metabolomics analysis of the anti-diabetic effect of Red ginseng extract in Type 2 diabetes Mellitus rats based on UHPLC-MS/MS. Biomed. Pharmacother. 2022, 146, 12495. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, Y.Y.; Feng, S.S.; Meng, M.W.; Chen, S.Y.; Huang, H.M.; Ling, X.; Song, H.; Liang, Y.H.; Ou, S.F.; et al. Urinary metabonomics study of anti-depressive mechanisms of Millettia speciosa Champ on rats with chronic unpredictable mild stress-induced depression. J. Pharm. Biomed. Anal. 2021, 205, 114338. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, M.; Zhai, Y.; Li, X.; Wang, Y.; Xie, T.; Yao, W.; Shan, J.; Zhang, L.; Ding, A. A novel strategy based on targeted cellular metabolomics for quantitatively evaluating anti-aging effect and screening effective extracts of Erzhi Wan. J. Chromatogr. B 2021, 1178, 122857. [Google Scholar] [CrossRef]
- Zhao, W.; An, R.; Liu, F.; Gu, J.; Sun, Y.; Xu, S.; Pan, Y.; Gao, Z.; Ji, H.; Du, Z. Urinary metabolomics analysis of the protective effects of Daming capsule on hyperlipidemia rats using ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2021, 44, 3305–3318. [Google Scholar] [CrossRef]
- Wang, A.; Pi, Z.; Liu, S.; Zheng, Z.; Liu, Z.; Song, F. Mass spectrometry-based urinary metabolomics for exploring the treatment effects of Radix ginseng-Schisandra chinensis herb pair on Alzheimer’s disease in rats. J. Sep. Sci. 2021, 44, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shi, X.; Hou, J.; Gao, S.; Chao, Y.; Ding, J.; Chen, L.; Qian, Y.; Shao, G.; Si, Y.; et al. Integrating metabolomics and network pharmacology to explore Rhizoma Coptidis extracts against sepsis-associated acute kidney injury. J. Chromatogr. B 2021, 1164, 122525. [Google Scholar]
- Qiaolongbatu, X.; Zhao, W.; Huang, X.; Qian, F.; Yang, X.; Wu, J.; Ma, C.; Qu, H.; Wang, L.; Fan, G.; et al. The Therapeutic Mechanism of Schisandrol A and Its Metabolites on Pulmonary Fibrosis Based on Plasma Metabonomics and Network Analysis. Drug Des. Dev. Ther. 2023, 17, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.H.; Zhang, W.M.; Tang, J.Y.; Fei, Q.Q.; Hu, M.M.; Chen, X.W.; Su, L.L.; Fei, C.H.; Ji, D.; Mao, C.Q.; et al. Effective substance and mechanism of Ziziphi Spinosae Semen extract in treatment of insomnia based on serum metabolomics and network pharmacology]. Zhongguo Zhong Yao Za Zhi 2022, 47, 188–202. [Google Scholar]
- Dong, R.; Tian, Q.; Shi, Y.; Chen, S.; Zhang, Y.; Deng, Z.; Wang, X.; Yao, Q.; Han, L. An Integrated Strategy for Rapid Discovery and Identification of Quality Markers in Gardenia Fructus Using an Omics Discrimination-Grey Correlation-Biological Verification Method. Front. Pharmacol. 2021, 12, 705498. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Li, J.; Yang, L.; Wu, G.; Liu, S. Comparative Metabolomics Study of Chaenomeles speciosa (Sweet) Nakai from Different Geographical Regions. Foods 2022, 11, 1019. [Google Scholar] [CrossRef]
- Li, Z.T.; Zhang, F.X.; Fan, C.L.; Ye, M.N.; Chen, W.W.; Yao, Z.H.; Yao, X.S.; Dai, Y. Discovery of potential Q-marker of traditional Chinese medicine based on plant metabolomics and network pharmacology: Periplocae Cortex as an example. Phytomedicine 2021, 85, 153535. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhou, C.; Wang, Z.; Kuang, T.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Tang, C. Unveiling Dynamic Changes of Chemical Constituents in Raw and Processed Fuzi with Different Steaming Time Points Using Desorption Electrospray Ionization Mass Spectrometry Imaging Combined with Metabolomics. Front. Pharmacol. 2022, 13, 842890. [Google Scholar] [CrossRef]
- Cao, X.; Shang, Y.; Kong, W.; Jiang, S.; Liao, J.; Dai, R. Flavonoids derived from Anemarrhenae Rhizoma ameliorate inflammation of benign prostatic hyperplasia via modulating COX/LOX pathways. J. Ethnopharmacol. 2022, 284, 114740. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Yan, R.; Deng, S.; Li, M.; Zhang, J.; Ma, L.; Yu, H. Evaluation of Biological Mechanisms of Eucommiae Folium in Hypertensive Kidney Injury by Integration of Untargeted Metabolomics and Network Pharmacology. J. Proteome Res. 2021, 20, 102–3113. [Google Scholar] [CrossRef]
- Liu, S.; Xing, J.; Zheng, Z.; Liu, Z.; Song, F.; Liu, S. Effect of Qishen granules on isoproterenol-induced chronic heart failure in rats evaluated by comprehensive metabolomics. Phytother. Res. 2022, 36, 4573–4586. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.Z.; Yao, S.; Zhu, Z.W.; Wang, X.; Sun, H.H.; Ji, W.F. Hu’po Anshen Decoction Accelerated Fracture-Healing in a Rat Model of Traumatic Brain Injury Through Activation of PI3K/AKT Pathway. Front. Pharmacol. 2022, 13, 952696. [Google Scholar] [CrossRef]
- Hua, Y.L.; Ma, Q.; Zhang, X.S.; Ji, P.; Yuan, Z.W.; Wei, Y.M. Integrated strategy for mechanism of Baitouweng Decoction in treating dampness-heat diarrhea based on urine metabolomics coupled with network pharmacology. Zhongguo Zhong Yao Za Zhi 2022, 47, 3887–3897. [Google Scholar]
- Xie, F.; Xu, L.; Zhu, H.; Chen, Y.; Li, Y.; Nong, L.; Zeng, Y.; Cen, S. The Potential Antipyretic Mechanism of Ellagic Acid with Brain Metabolomics Using Rats with Yeast-Induced Fever. Molecules 2022, 27, 2465. [Google Scholar] [CrossRef]
- Dong, M.; Du, H.; Li, X.; Zhang, L.; Wang, X.; Wang, Z.; Jiang, H. Discovery of Biomarkers and Potential Mechanisms of Agarwood Incense Smoke Intervention by Untargeted Metabolomics and Network Pharmacology. Drug Des. Dev. Ther. 2022, 16, 65–278. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Deng, Y.; Zhao, C.; Xiao, W.; Wang, Z.; Xiong, Z.; Zhao, L. Integrative serum metabolomic analysis for preventive effects of Yaobitong capsule in adjuvant-induced rheumatoid arthritis rat based on RP/HILIC-UHPLC-Q-TOF MS. Anal. Biochem. 2022, 637, 114474. [Google Scholar]
- Ji, P.; Li, C.C.; Wei, Y.M.; Hua, Y.L.; Yao, W.L.; Wu, F.L.; Zhang, X.S.; Yuan, Z.W.; Zhao, N.S.; Zhang, Y.H.; et al. A new method providing complementary explanation of the blood-enriching function and mechanism of unprocessed Angelica sinensis and its four kinds of processed products based on tissue-integrated metabolomics and confirmatory analysis. Biomed. Chromatogr. 2022, 36, e5252. [Google Scholar] [CrossRef]
- Syabana, M.A.; Yuliana, N.D.; Batubara, I.; Fardiaz, D. α-glucosidase inhibitors from Syzygium polyanthum (Wight) Walp leaves as revealed by metabolomics and in silico approaches. J. Ethnopharmacol. 2022, 282, 114618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yao, Y.; Wang, M.; Liu, F.; Wang, Q.; Ma, H.; Xie, Y.; Ma, Y.; Dai, P.; Zhu, C.; et al. A UPLC-MS/MS-based metabolomics analysis of the pharmacological mechanisms of rabdosia serra against cholestasis. Phytomedicine 2021, 9, 153683. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Lyu, C.; Li, Q.; Kou, F.; Jiang, M.; Wei, H. Metabolomics study on the intervention effect of Radix Salviae Miltiorrhizae extract in exercise-induced exhaustion rat using gas chromatography coupled to mass spectrometry. J. Chromatogr. B 2021, 1178, 122805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Chen, W.; Fu, J.; Liu, Y.; Di, T.; Qi, C.; Chen, Z.; Li, P. Systems Pharmacology Approach and Experiment Evaluation Reveal Multidimensional Treatment Strategy of LiangXueJieDu Formula for Psoriasis. Front. Pharmacol. 2021, 12, 626267. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhu, L.L.; Yu, C.Y. Quantitative evaluation of the compatibility effects of aidi injection on the treatment of hepatocellular carcinoma using targeted metabolomics: A new strategy on the mechanism study of an anticancer compound in traditional chinese medicine. World J. Tradit. Chin. Med. 2021, 7, 111–119. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, L.; Xue, Q.; He, Q.; Chen, X.; Meng, J.; Wang, S.; Liang, S. LC-MS based plasma metabolomics study of the intervention effect of different polar parts of Hawthorn on hyperlipidemia rats. J. Sep. Sci. 2021, 44, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Song, F.; Liu, Z.; Liu, S. Therapeutic Effectiveness of Gardenia jasminoides on Type 2 Diabetic Rats: Mass Spectrometry-Based Metabolomics Approach. J. Agric. Food Chem. 2020, 68, 9673–9682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, S.J.; Man, Y.H.; Zhuang, G. Serum metabonomics coupled with HPLC-LTQ/orbitrap MS and multivariate data analysis on the ameliorative effects of Bidens bipinnata L. in hyperlipidemic rats. J. Ethnopharmacol. 2020, 262, 113196. [Google Scholar] [CrossRef]
- Sens, A.; Rischke, S.; Hahnefeld, L.; Dorochow, E.; Schäfer, S.M.G.; Thomas, D.; Köhm, M.; Geisslinger, G.; Behrens, F.; Gurke, R. Pre-analytical sample handling standardization for reliable measurement of metabolites and lipids in LC-MS-based clinical research. J. Mass. Spectrom. Adv. Clin. Lab. 2023, 28, 35–46. [Google Scholar] [CrossRef]
- Sarmad, S.; Viant, M.R.; Dunn, W.B.; Goodacre, R.; Wilson, I.D.; Chappell, K.E.; Griffin, J.L.; O’Donnell, V.B.; Naicker, B.; Lewis, M.R.; et al. A proposed framework to evaluate the quality and reliability of targeted metabolomics assays from the UK Consortium on Metabolic Phenotyping (MAP/UK). Nat. Protoc. 2023, 18, 1017–1027. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Tian, Q.; Guo, S.; Xie, D.; Cai, Y.; Wang, Z.; Chu, H.; Qiu, S.; Tang, S.; Zhang, A. Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification. Molecules 2024, 29, 2198. https://doi.org/10.3390/molecules29102198
Lin C, Tian Q, Guo S, Xie D, Cai Y, Wang Z, Chu H, Qiu S, Tang S, Zhang A. Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification. Molecules. 2024; 29(10):2198. https://doi.org/10.3390/molecules29102198
Chicago/Turabian StyleLin, Chunsheng, Qianqian Tian, Sifan Guo, Dandan Xie, Ying Cai, Zhibo Wang, Hang Chu, Shi Qiu, Songqi Tang, and Aihua Zhang. 2024. "Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification" Molecules 29, no. 10: 2198. https://doi.org/10.3390/molecules29102198






