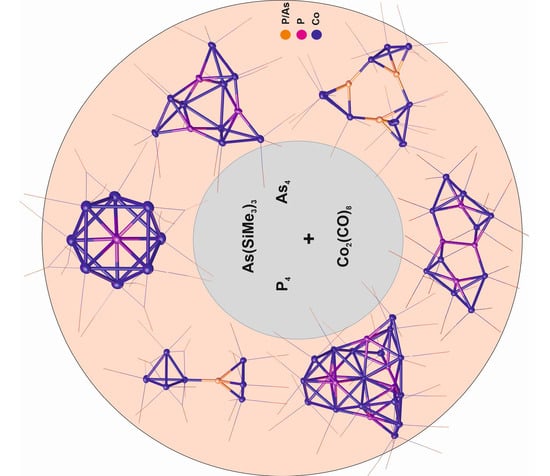
Synthesis and Characterization of Novel Cobalt Carbonyl Phosphorus and Arsenic Clusters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and X-ray Structures of Compound 1 and the Cobalt Clusters [{M(CO)n}{Co(CO)3}3E] (n = 5, M = Cr, E = P (2), M = W, E = As (3); n = 4, M = Fe, E = P (4))
2.2. Synthesis and X-ray Structures of the Cobalt Clusters [Co8(CO)16(µ-CO)4P] (5) and [{Co4(CO)11}{Co(CO)3}3P] (6)
2.3. Synthesis and X-ray Structures of the Cobalt Clusters [Co9(CO)24(µ4-P)3] (7) and [Co9(CO)21(µ5-P)3] (8)
2.4. Synthesis and X-ray Structures of the Cobalt Clusters [Co10(CO)24(µ3-P)2(µ6-P2)(µ-CO)2] (9) and [Co15(µ6-P)6(µ12-Co)(CO)30] (10)
2.5. New Synthetic Protocol for the Synthesis of the Cobalt Clusters [Co9(CO)24(µ4-As)3] (11) and [{Co4(CO)11}{Co(CO)3}3As] (12)
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization of Clusters 2–12
3.2.1. Synthesis and Characterization of Clusters 2 and 3
3.2.2. Synthesis and Characterization of Cluster 4
3.2.3. Synthesis and Characterization of Cluster 5
3.2.4. Synthesis and Characterization of Cluster 6
3.2.5. Synthesis and Characterization of Cluster 7
3.2.6. Synthesis and Characterization of Cluster 8
3.2.7. Synthesis and Characterization of Cluster 9
3.2.8. Synthesis and Characterization of Cluster 10
3.2.9. Synthesis and Characterization of Cluster 11
3.2.10. Synthesis and Characterization of Cluster 12
3.2.11. Synthesis and Characterization of Cluster A
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitmire, K.H. Transition metal complexes of the naked pnictide elements. Coord. Chem. Rev. 2018, 376, 114–195. [Google Scholar] [CrossRef]
- Shelyganov, P.A.; Elsayed Moussa, M.; Seidl, M.; Scheer, M. Diantimony Complexes [CpR2Mo2(CO)4(µ,η2-Sb2)] (CpR = C5H5, C5H4tBu) as Unexpected Ligands Stabilizing Silver(I)n (n = 1–4) Monomers, Dimers and Chains. Angew. Chem. Int. Ed. 2023, 62, e202215650. [Google Scholar] [CrossRef] [PubMed]
- Peresypkina, E.; Virovets, A.; Scheer, M. Organometallic polyphosphorus complexes as diversified building blocks in coordination chemistry. Coord. Chem. Rev. 2021, 446, 213995–214038. [Google Scholar] [CrossRef]
- Bai, J.; Virovets, A.V.; Scheer, M. Synthesis of Inorganic Fullerene-Like Molecules. Science. 2003, 300, 781–782. [Google Scholar] [CrossRef]
- Cesari, C.; Shon, J.-H.; Zacchini, S.; Berben, L.A. Metal carbonyl clusters of groups 8–10: Synthesis and catalysis. Chem. Soc. Rev. 2021, 50, 9503–9539. [Google Scholar] [CrossRef]
- Gauthier, J.A.; King, L.A.; Stults, F.T.; Flores, R.A.; Kibsgaard, J.; Regmi, Y.N.; Chan, K.; Jaramillo, T.F. Transition Metal Arsenide Catalysts for the Hydrogen Evolution Reaction. J. Phys. Chem. C 2019, 123, 24007–24012. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wang, X.-L.; Qin, C.; Shao, K.-Z.; Su, Z.-M.; Wang, E.-B. A carbon-free polyoxometalate molecular catalyst with a cobalt-arsenic core for visible light-driven water oxidation. Chem. Commun. 2016, 52, 9514–9517. [Google Scholar] [CrossRef]
- Hong, C.S.; Berben, L.A.; Long, J.R. Synthesis and characterization of a decacobalt carbonyl cluster with two semi-intertitial phosphorus atoms. Dalton Trans. 2003, 2119–2120. [Google Scholar] [CrossRef]
- Buchwalter, P.; Rosé, J.; Lebeau, B.; Ersen, O.; Girleanu, M.; Rabu, P.; Braunstein, P.; Paillaud, J.-L. Characterization of cobalt phosphide nanoparticles derived from molecular clusters in mesoporous silica. J. Nanopart. Res. 2013, 15, 2132–2152. [Google Scholar] [CrossRef]
- Foust, A.S.; Foster, M.S.; Dahl, L.F. Organometallic pnictogen complexes. III. Preparation and structural characterization of the triarsenic-cobalt Atom cluster system As3Co(CO)3, The first known X3-transition metal analog of group VA tetrahedral X4 molecules. J. Am. Chem. Soc. 1969, 91, 5631–5633. [Google Scholar] [CrossRef]
- Foust, A.S.; Foster, M.S.; Dahl, L.F. Organometallic pnictogen complexes. IV. Synthesis, structure, and bonding of new organometallic arsenic-metal atom clusters containing a metal-bridged multiply bonded As2 ligand: Co2(CO)6As2 and Co2{(CO)5P(C6H5)3}As2. J. Am. Chem. Soc. 1969, 91, 5633–5635. [Google Scholar] [CrossRef]
- Arnold, L.J.; Mackay, K.M.; Nicholson, B.K. Reaction of arsane with cobalt or iron carbonyls, and the X-ray crystal structures of [Fe2(CO)8(µ4-As)]2[Fe2(CO)6] and [µ4-AsCo3(CO)8]3. J. Organomet. Chem. 1990, 387, 197–207. [Google Scholar] [CrossRef]
- Vizi-Orosz, A.; Pályi, G.; Markó, L. Phosphido cobalt carbonyl clusters: Co2(CO)6P2 and Co3(CO)9PS. J. Organomet. Chem. 1973, 60, C25–C26. [Google Scholar] [CrossRef]
- Vizi-Orosz, A. Phosphido cobalt carbonyl clusters Pn[Co(CO)3]4−n (n = 1, 2, 3). J. Organomet. Chem. 1976, 111, 61–64. [Google Scholar] [CrossRef]
- Seyferth, D.; Henderson, R.S. phosphaacetylenehexacarbonyldicobalt complexes: New cluster lewis bases. J. Organomet. Chem. 1978, 162, C35–C38. [Google Scholar] [CrossRef]
- Burckett-St. Laurent, J.C.T.R.; Hitchcock, P.B.; Kroto, H.W.; Nixon, J.F. Novel transition metal phospha-alkyne complexes. X-Ray crystal and molecular structure of a side-bonded tBuC≡P complex of zerovalent platinum, Pt(PPh3)2(tBuCP). J. Chem. Soc. Chem. Commun. 1981, 21, 1141–1143. [Google Scholar] [CrossRef]
- Ciani, G.; Sironi, A.; Martinengo, S.; Garlaschelli, L.; Pergola, R.D.; Zanello, P.; Laschi, F.; Masciocchi, N. Synthesis and X-ray characterization of the phosphide-carbonyl cluster anions [Co9(µ8,P)(CO)21]2− and [Co10(µ8,P)(CO)22]3−. Inorg. Chem. 2001, 40, 3905–3911. [Google Scholar] [CrossRef]
- Della Pergola, R.; Sironi, A.; Colombo, V.; Garlaschelli, L.; Racioppi, S.; Sironi, A.; Macchi, P. Periodical trends in [Co6E(CO)16]− clusters: Structural, synthetic and energy changes produced by substitution of P with As. J. Organomet. Chem. 2017, 849–850, 130–136. [Google Scholar] [CrossRef]
- Della Pergola, R.; Garlaschelli, L.; Macchi, P.; Facchinetti, I.; Ruffo, R.; Racioppi, S.; Sironi, A. From small metal clusters to molecular nanoarchitectures with a core-shell structure: The synthesis, redox fingerprint, theoretical analysis, and solid-state structure of [Co38As12(CO)50]4−. Inorg. Chem. 2022, 61, 26, 9886–9896. [Google Scholar] [CrossRef] [PubMed]
- Dielmann, F.; Sierka, M.; Virovets, A.V.; Scheer, M. Access to extended polyphosphorus frameworks. Angew. Chem. Int. Ed. 2010, 49, 6860–6864. [Google Scholar] [CrossRef] [PubMed]
- Graßl, C.; Bodensteiner, M.; Zabel, M.; Scheer, M. Synthesis of arsenic-rich Asn ligand complexes from yellow arsenic. Chem. Sci. 2015, 6, 1379–1382. [Google Scholar] [CrossRef]
- Dielmann, F.; Timoshkin, A.; Piesch, M.; Balázs, G.; Scheer, M. The cobalt cyclo-P4 sandwich complex and its role in the formation of polyphosphorus compounds. Angew. Chem. Int. Ed. 2017, 56, 1671–1675. [Google Scholar] [CrossRef]
- Del Mar Conejo, M.; Pastor, A.; Montilla, F.; Galindo, A. P atom as ligand in transition metal chemistry: Structural aspects. Coord. Chem. Rev. 2021, 434, 213730–213773. [Google Scholar] [CrossRef]
- Fischer, E.O.; Bathelt, W.; Müller, J. Arsine pentacarbonyl complexes of chromium(0), Molybdenum(0#9 and Tungsten(0). Chem. Ber. 1970, 103, 1815–1821. [Google Scholar] [CrossRef]
- Lal De, R.; Vahrenkamp, H. Polynuclear complexes from PH3 and RPH2 complexes with Co2(CO)8. Z. Naturforsch. 1985, 40b, 1250–1257. [Google Scholar] [CrossRef]
- Coleman, J.M.; Dahl, L.F. Molecular structures of [(C6H5)2PCoC5H5]2 and [(C6H5)2PNiC5H5]2. An assessment of the influence of a metal-metal bond on the molecular geometry of an organometallic ligand-bridged complex. J. Am. Chem. Soc. 1967, 89, 542–552. [Google Scholar] [CrossRef]
- Dreher, C.; Zabel, M.; Bodensteiner, M.; Scheer, M. [(CO)4W(PH3)2] as a source of semi-interstitial phosphorus ligands in cobalt carbonyl clusters. Organometallics 2010, 29, 5187–5191. [Google Scholar] [CrossRef]
- Elsayed Moussa, M.; Rummel, E.-M.; Eckhardt, M.; Riesinger, C.; Scheer, M. Unusual cleavage of phosphaalkynes triple bond in the coordination sphere of transition metals. Dalton Trans. 2023, 52, 15656–15659. [Google Scholar] [CrossRef]
- Vizi-Orosz, A.; Galamb, V.; Pályi, G.; Markó, L.; Bor, G.; Natile, G. AsCo3(CO)9, its cyclic trimer, As3Co9(CO)24 and the phosphorus-containing analog As3Co9(CO)24. J. Organomet. Chem. 1976, 107, 235–240. [Google Scholar] [CrossRef]
- Maxwell, S.B.H.L.R.; Mosley, V.M. Electron diffraction by gases. J. Chem. Phys. 1935, 3, 699–709. [Google Scholar] [CrossRef]
- Lang, H.; Huttner, G.; Sigwarth, B.; Jibril, I.; Zsolnai, L.; Orama, O. µ3-P und µ3-As-verbrückte cluster als liganden. J. Organomet. Chem. 1986, 304, 137–155. [Google Scholar] [CrossRef]
- Vogel, U.; Scheer, M. Zur oxidativen addition von komplexierten phosphanen bzw. arsanen an platin(0)-komplexen. Anorg. Allg. Chem. 2001, 627, 1593–1598. [Google Scholar] [CrossRef]
- Hunger, C.; Ojo, W.-S.; Bauer, S.; Xu, S.; Zabel, M.; Chaudret, B.; Lacroix, L.-M.; Scheer, M.; Nayral, C.; Delpech, F. Stoichiometry-controlled FeP nanoparticles synthesized from a single source precursor. Chem. Commun. 2013, 49, 11788–11790. [Google Scholar] [CrossRef] [PubMed]
- Dreher, C. Darstellung und Reaktivität von P–H-funktionellen Übergangsmetallcarbonylkomplexen. PhD Thesis, Universität Regensburg, Regensburg, Germany, 2009. [Google Scholar]
- Seidl, M.; Balázs, G.; Scheer, M. The Chemistry of Yellow Arsenic. Chem. Rev. 2019, 119, 8406–8434. [Google Scholar] [CrossRef]
- Becker, G.; Gutekunst, G.; Wessely, H.J. Trimethylsilylverbindungen der Vb-Elemente. I Synthese und Eigenschaften von Trimethylsilylarsanen. Z. Anorg. Allg. Chem. 1980, 462, 113–129. [Google Scholar] [CrossRef]
- Kendall, R.A.; Früchtl, H.A. The impact of the Resolution of the Identity approximate integral method on modern ab initio algorithm development. Theor. Chim. Acta 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef]
- Kruse, H.; Grimme, S. A geometrical correction for the inter- and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems. J. Chem. Phys. 2012, 136, 154101. [Google Scholar] [CrossRef] [PubMed]
- Caldeweyher, E.; Bannwarth, C.; Grimme, S. Extension of the D3 dispersion coefficient model. J. Chem. Phys. 2017, 147, 034112. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies Ltd. CrysAlis PRO; Agilent Technologies Ltd.: Oxfordshire, UK, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed Moussa, M.; Bauer, S.; Graßl, C.; Riesinger, C.; Balázs, G.; Scheer, M. Synthesis and Characterization of Novel Cobalt Carbonyl Phosphorus and Arsenic Clusters. Molecules 2024, 29, 2025. https://doi.org/10.3390/molecules29092025
Elsayed Moussa M, Bauer S, Graßl C, Riesinger C, Balázs G, Scheer M. Synthesis and Characterization of Novel Cobalt Carbonyl Phosphorus and Arsenic Clusters. Molecules. 2024; 29(9):2025. https://doi.org/10.3390/molecules29092025
Chicago/Turabian StyleElsayed Moussa, Mehdi, Susanne Bauer, Christian Graßl, Christoph Riesinger, Gábor Balázs, and Manfred Scheer. 2024. "Synthesis and Characterization of Novel Cobalt Carbonyl Phosphorus and Arsenic Clusters" Molecules 29, no. 9: 2025. https://doi.org/10.3390/molecules29092025