Possible Roles of Carbohydrate Management and Cytokinin in the Process of Defoliation–Regrowth Cycles in Rice
Abstract
:1. Introduction
2. Results
2.1. Vegetative Regrowth after Periodic Defoliation in Rice
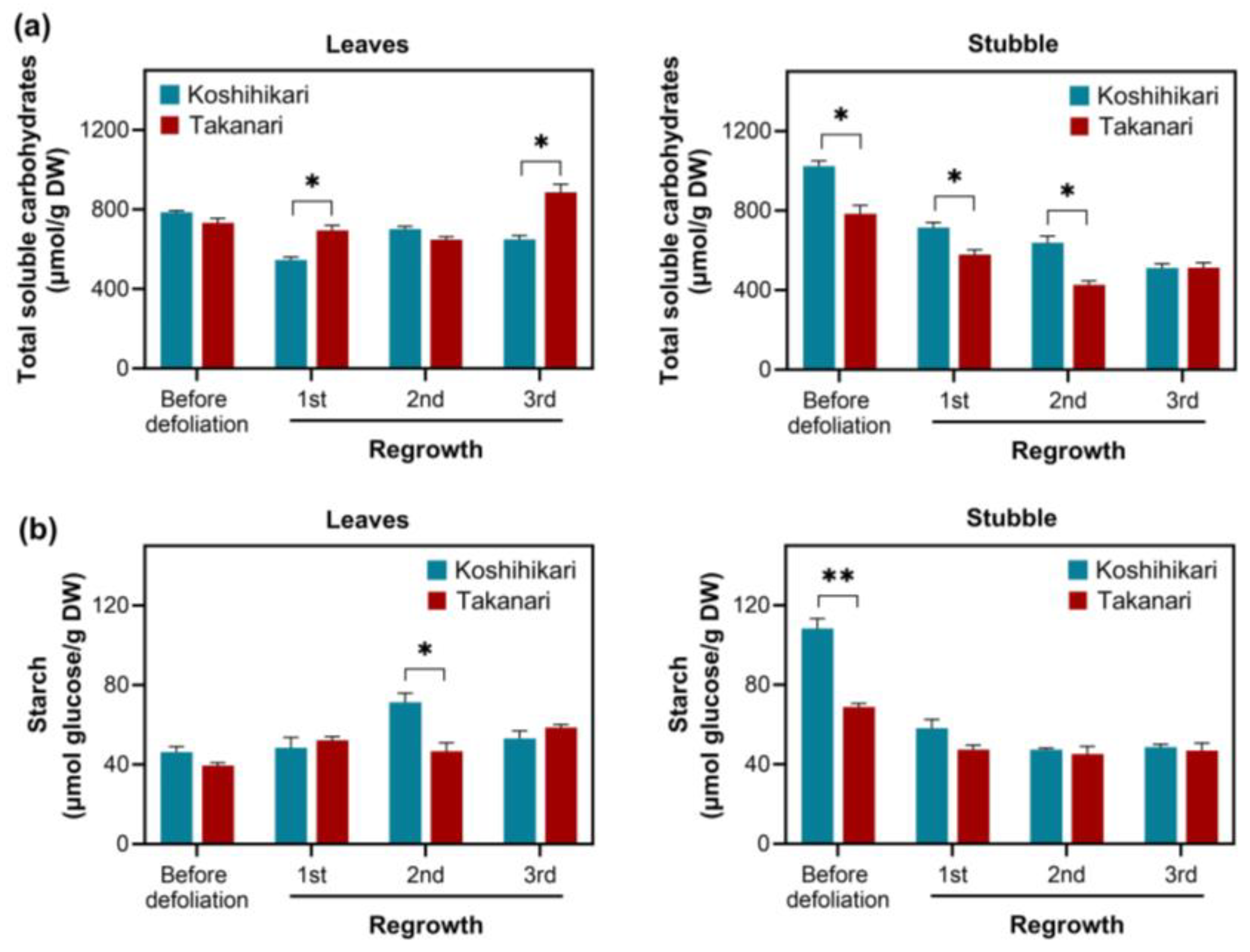
2.2. Carbohydrate Management in Rice Exposed to Periodic Defoliation
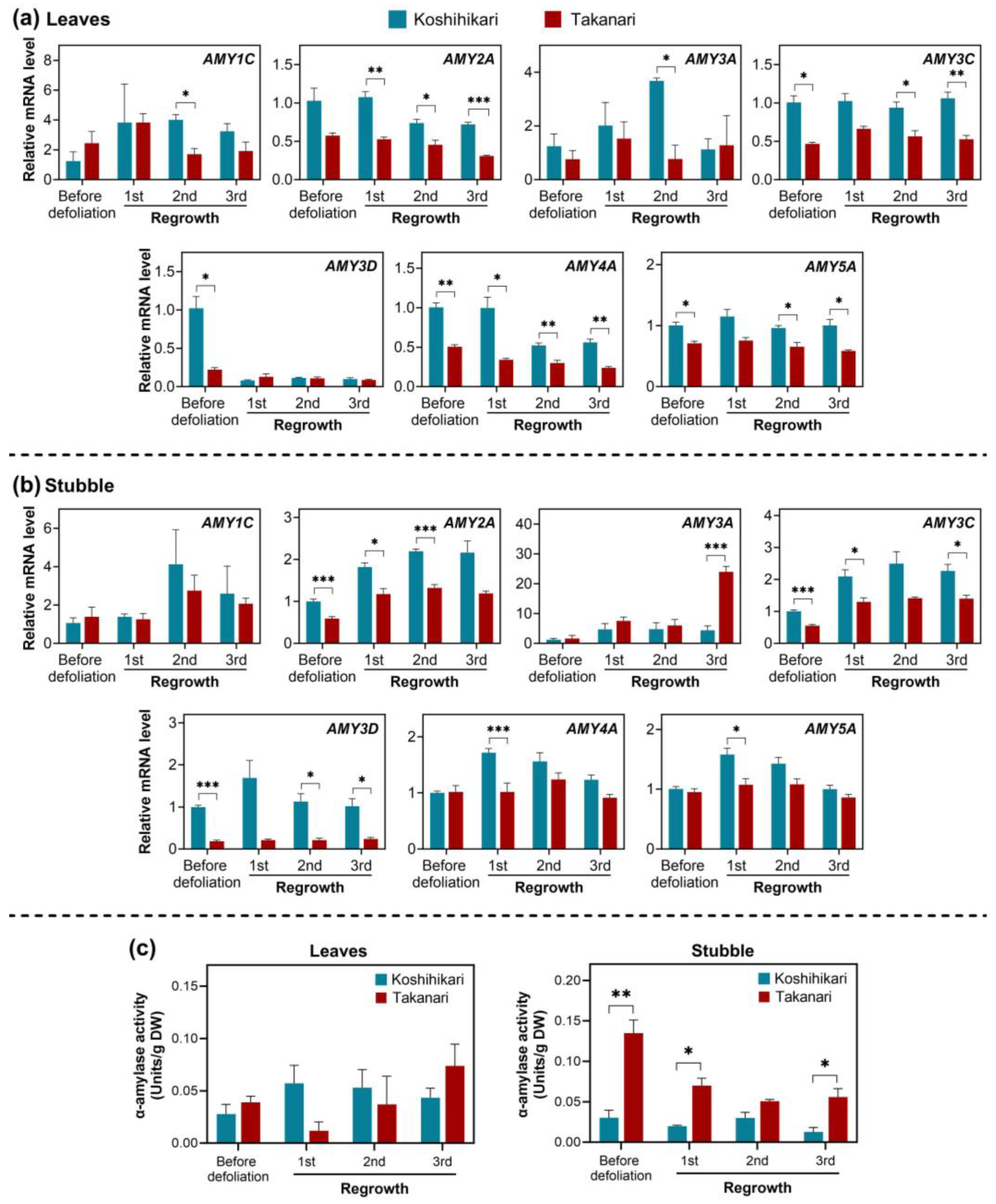
2.3. Gene Expression and Activities of Enzymes Associated with Carbohydrate Management in Rice Exposed to Periodic Defoliation
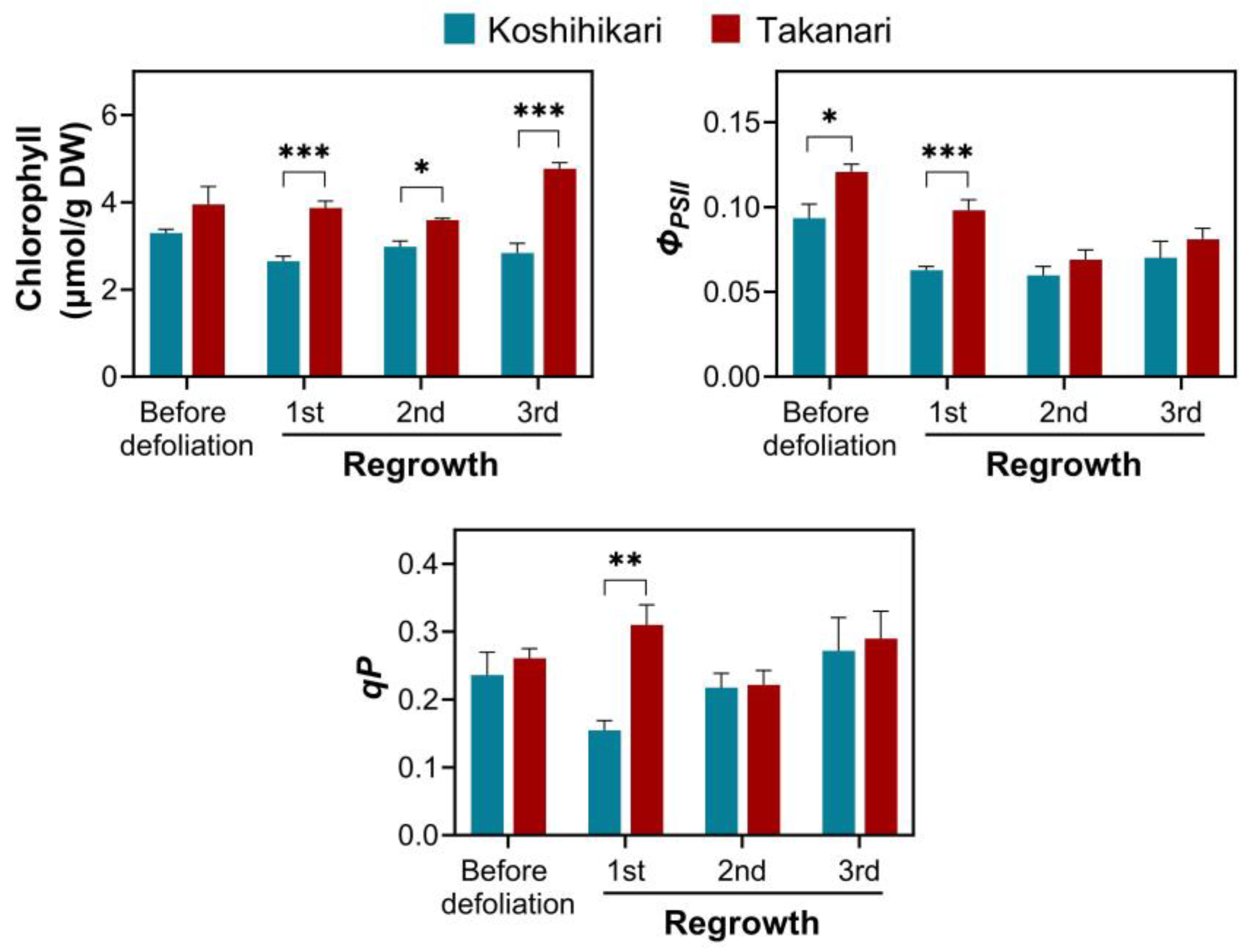
2.4. Effect of Photosynthesis on Vegetative Regrowth upon Defoliation in Rice
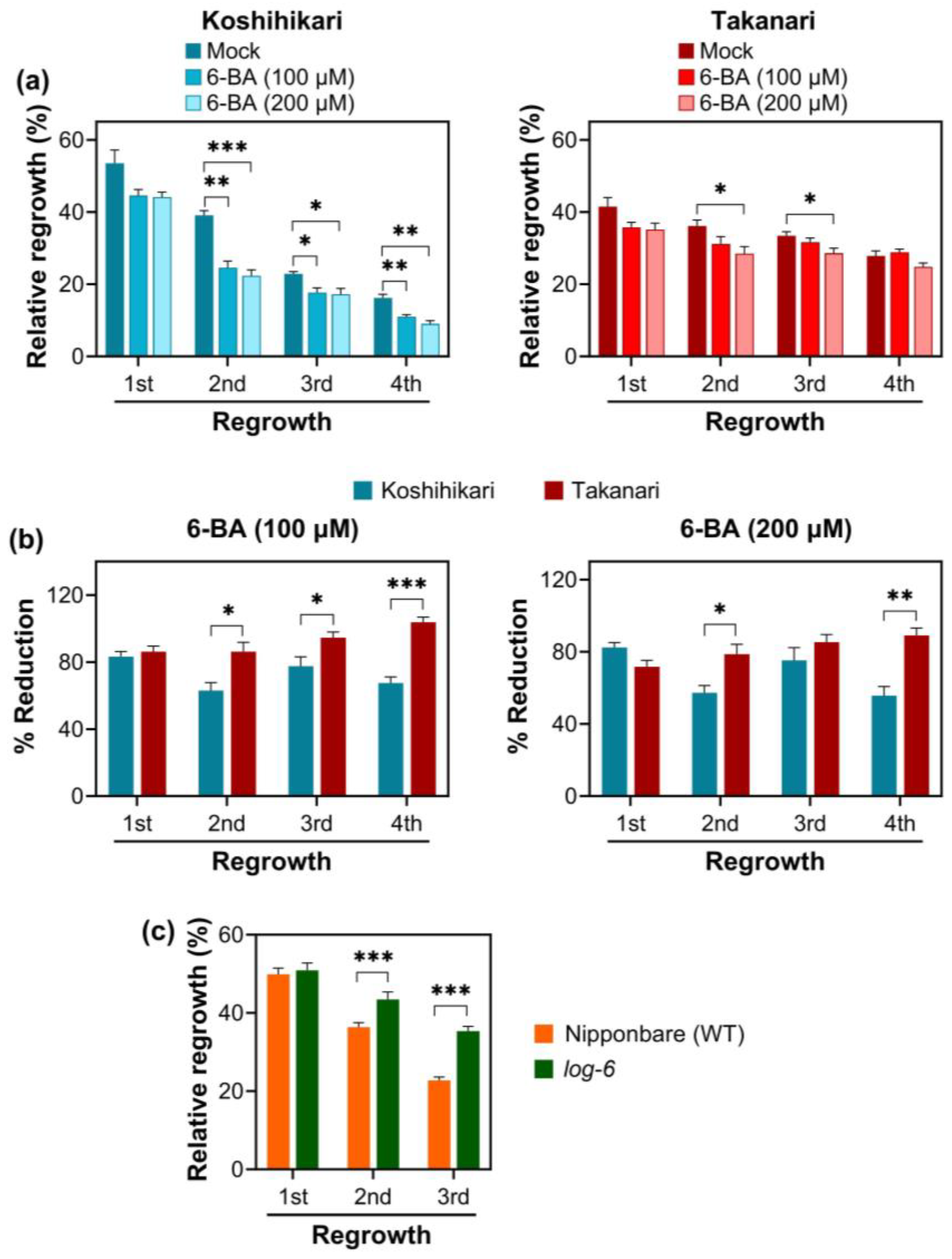
2.5. Effect of Cytokinin on Vegetative Regrowth after Defoliation in Rice
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Carbohydrate Assays
4.3. Quantitative RT-PCR Analysis
4.4. Enzyme Assays
4.5. Chlorophyll Content and Fluorescence Measurements
4.6. Cytokinin Response Assays
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morvan-Bertrand, A.; Pavis, N.; Boucaud, J.; Prud’Homme, M.-P. Partitioning of Reserve and Newly Assimilated Carbon in Roots and Leaf Tissues of Lolium perenne during Regrowth after Defoliation: Assessment by 13C Steady-state Labelling and Carbohydrate Analysis. Plant Cell Environ. 1999, 22, 1097–1108. [Google Scholar] [CrossRef]
- Donaghy, D.J.; Turner, L.R.; Adamczewski, K.A. Effect of Defoliation Management on Water-Soluble Carbohydrate Energy Reserves, Dry Matter Yields, and Herbage Quality of Tall Fescue. Agron. J. 2008, 100, 122–127. [Google Scholar] [CrossRef]
- Alderman, P.D.; Boote, K.J.; Sollenberger, L.E.; Coleman, S.W. Carbohydrate and Nitrogen Reserves Relative to Regrowth Dynamics of ‘Tifton 85’ Bermudagrass as Affected by Nitrogen Fertilization. Crop. Sci. 2011, 51, 1727–1738. [Google Scholar] [CrossRef]
- Mislevy, P.; Washko, J.B.; Harrington, J.D. Influence of plant stage at initial harvest and height of regrowth at cutting on forage yield and quality of timothy and orchardgrass. Agron. J. 1977, 69, 353–356. [Google Scholar] [CrossRef]
- Garay, A.H.; Matthew, C.; Hodgson, J. Tiller size/density compensation in perennial ryegrass miniature swards subject to differing defoliation heights and a proposed productivity index. Grass Forage Sci. 1999, 54, 347–356. [Google Scholar] [CrossRef]
- De Visser, R.; Vianden, H.; Schnyder, H. Kinetics and Relative Significance of Remobilized and Current C and N Incorporation in Leaf and Root Growth Zones of Lolium perenne after Defoliation: Assessment by 13C and 15N Steady-state Labelling. Plant Cell Environ. 1997, 20, 37–46. [Google Scholar] [CrossRef]
- Meuriot, F.; Morvan-Bertrand, A.; Noiraud-Romy, N.; Decau, M.-L.; Escobar-Gutiérrez, A.J.; Gastal, F.; Prud’homme, M.-P. Short-Term Effects of Defoliation Intensity on Sugar Remobilization and N Fluxes in Ryegrass. J. Exp. Bot. 2018, 69, 3975–3986. [Google Scholar] [CrossRef] [PubMed]
- Baumann, U.; Juttner, J.; Bian, X.; Langridge, P. Self-incompatibility in the grasses. Ann. Bot. 2000, 85, 203–209. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Humphreys, M.O. Progress in breeding perennial forage grasses for temperate agriculture. J. Agric. Sci. 2003, 140, 129–150. [Google Scholar] [CrossRef]
- Simeão, R.M.; Resende, M.D.V.; Alves, R.S.; Pessoa-Filho, M.; Azevedo, A.L.S.; Jones, C.S.; Pereira, J.F.; Machado, J.C. Genomic selection in tropical forage grasses: Current status and future applications. Front. Plant Sci. 2021, 12, 665195. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Q. Rice genome research: Current status and future perspectives. Plant Genome 2008, 1, 71–76. [Google Scholar] [CrossRef]
- Lyu, J. An indoor rice model. Mol. Plant 2018, 4, 740. [Google Scholar] [CrossRef] [PubMed]
- Harrell, D.L.; Bond, J.A.; Blanche, S. Evaluation of main-crop stubble height on ratoon rice growth and development. Field Crop. Res. 2009, 114, 396–403. [Google Scholar] [CrossRef]
- Faruq, G.; Taha, R.M.; Prodhan, Z.H. Rice ratoon crop: A sustainable rice production system for tropical hill agriculture. Sustainability 2014, 6, 5785–5800. [Google Scholar] [CrossRef]
- Paterson, A.H.; Freeling, M.; Sasaki, T. Grains of knowledge: Genomics of model cereals. Genome Res. 2005, 15, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, B. Twenty years of rice genomics research: From sequencing and functional genomics to quantitative genomics. Mol. Plant 2022, 15, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Tanisaka, W.; Yamauchi, T.; Tsuda, K. Genetic basis controlling rice plant architecture and its modification for breeding. Breed. Sci. 2023, 73, 3–45. [Google Scholar] [CrossRef]
- Kobayashi, A.; Hori, K.; Yamamoto, T.; Yano, M. Koshihikari: A premium short-grain rice cultivar—Its expansion and breeding in Japan. Rice 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Ikka, T.; Kondo, K.; Nonoue, Y.; Ono, N.; Arai-Sanoh, Y.; Yoshinaga, S.; Nakano, H.; Yano, M.; Kondo, M.; et al. Genetic Mechanisms Underlying Yield Potential in the Rice High-Yielding Cultivar Takanari, Based on Reciprocal Chromosome Segment Substitution Lines. BMC Plant Biol. 2014, 14, 295. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A Variable Cluster of Ethylene Response Factor–Like Genes Regulates Metabolic and Developmental Acclimation Responses to Submergence in Rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthase in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, Y.; Liu, C.; Sun, Y.; Zhang, T.; Hou, M.; Huang, S.; Yuan, H. The Evolutionary History of the Sucrose Synthase Gene Family in Higher Plants. BMC Plant Biol. 2019, 19, 566. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M.; Hirose, T.; Hashida, Y.; Ohsugi, R.; Aoki, N. Suppression of starch accumulation in ‘sugar leaves’ of rice affects plant productivity under field conditions. Plant Prod. Sci. 2017, 20, 102–110. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, J.; Li, T.; Yang, H.; Wang, P.; Su, L.; Zheng, Y.; Bao, C.; Zhou, C. SDG711 Is Involved in Rice Seed Development through Regulation of Starch Metabolism Gene Expression in Coordination with Other Histone Modifications. Rice 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin Signaling Networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinin Signaling in Plant Development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Barratt, D.H.P.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef]
- Mundy, J.; Hejgaard, J.; Svendsen, I. Characterization of a Bifunctional Wheat Inhibitor of Endogenous α-amylase and Subtilisin. FEBS Lett. 1984, 167, 210–214. [Google Scholar] [CrossRef]
- Yamagata, H.; Kunimatsu, K.; Kamasaka, H.; Kuramoto, T.; Iwasaki, T. Rice Bifunctional α-Amylase/Subtilisin Inhibitor: Characterization, Localization, and Changes in Developing and Germinating Seeds. Biosci. Biotechnol. Biochem. 1998, 62, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Turnbull, M.H.; Song, J.; Roche, J.; Novak, O.; Späth, J.; Jameson, P.E.; Love, J. Depletion of Carbohydrate Reserves Limits Nitrate Uptake during Early Regrowth in Lolium perenne L. J. Exp. Bot. 2017, 68, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Liu, D.; Li, Z.-Q. Effects of the Coordination Mechanism between Roots and Leaves Induced by Root-Breaking and Exogenous Cytokinin Spraying on the Grazing Tolerance of Ryegrass. J. Plant Res. 2012, 125, 407–416. [Google Scholar] [CrossRef]
- Wang, X.-L.; Wang, J.; Li, Z.-Q. Correlation of Continuous Ryegrass Regrowth with Cytokinin Induced by Root Nitrate Absorption. J. Plant Res. 2013, 126, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Hake, S. Control of Phyllotaxy in Maize by the Abphyl1 Gene. Development 1999, 126, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Johnston, R.; Yang, Y.; Gallavotti, A.; Kojima, M.; Travençolo, B.A.N.; Costa, L.D.F.; Sakakibara, H.; Jackson, D. Studies of Aberrant Phyllotaxy1 Mutants of Maize Indicate Complex Interactions between Auxin and Cytokinin Signaling in the Shoot Apical Meristem. Plant Physiol. 2009, 150, 205–216. [Google Scholar] [CrossRef]
- Itoh, J.; Hibara, K.; Kojima, M.; Sakakibara, H.; Nagato, Y. Rice DECUSSATEControls Phyllotaxy by Affecting the Cytokinin Signaling Pathway. Plant J. 2012, 72, 869–881. [Google Scholar] [CrossRef]
- Skalák, J.; Vercruyssen, L.; Claeys, H.; Hradilová, J.; Černý, M.; Novák, O.; Plačková, L.; Saiz-Fernández, I.; Skaláková, P.; Coppens, F.; et al. Multifaceted Activity of Cytokinin in Leaf Development Shapes Its Size and Structure in Arabidopsis. Plant J. 2019, 97, 805–824. [Google Scholar] [CrossRef]
- Alpuerto, J.B.; Hussain, R.M.F.; Fukao, T. The Key Regulator of Submergence Tolerance, SUB1A, Promotes Photosynthetic and Metabolic Recovery from Submergence Damage in Rice Leaves. Plant Cell Environ. 2016, 39, 672–684. [Google Scholar] [CrossRef]
- Fukao, T.; Yeung, E.; Bailey-Serres, J. The Submergence Tolerance Gene SUB1A Delays Leaf Senescence under Prolonged Darkness through Hormonal Regulation in Rice. Plant Physiol. 2012, 160, 1795–1807. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, G.; Wang, Y.; Zhang, R.; Wang, Y.; Feng, S.; Luo, K.; Peng, L. Sucrose synthase enhances hull size and grain weight by regulating cell division and starch accumulation in transgenic rice. Int. J. Mol. Sci. 2019, 20, 4971. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Michiyama, H.; Hirano, T. Involvement of α-amylase genes in starch degradation in rice leaf sheaths at the post-heading stage. Plant Prod. Sci. 2015, 18, 277–283. [Google Scholar] [CrossRef]
- He, Y.; Zhu, M.; Li, Z.; Jiang, S.; He, Z.; Xu, S.; Chen, X.; Hu, Z.; Zhang, Z. IPA1 negatively regulates early rice seedling development by interfering with starch metabolism via the GA and WRKY pathways. Int. J. Mol. Sci. 2021, 22, 6605. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakashita, Y.; Kurashima, H.; Fukuda, M.; Hirano, H.; Lamsal, S.; Katayama, N.; Fukao, T. Possible Roles of Carbohydrate Management and Cytokinin in the Process of Defoliation–Regrowth Cycles in Rice. Int. J. Mol. Sci. 2024, 25, 5070. https://doi.org/10.3390/ijms25105070
Sakashita Y, Kurashima H, Fukuda M, Hirano H, Lamsal S, Katayama N, Fukao T. Possible Roles of Carbohydrate Management and Cytokinin in the Process of Defoliation–Regrowth Cycles in Rice. International Journal of Molecular Sciences. 2024; 25(10):5070. https://doi.org/10.3390/ijms25105070
Chicago/Turabian StyleSakashita, Yuki, Hikaru Kurashima, Mika Fukuda, Haru Hirano, Sagar Lamsal, Naoki Katayama, and Takeshi Fukao. 2024. "Possible Roles of Carbohydrate Management and Cytokinin in the Process of Defoliation–Regrowth Cycles in Rice" International Journal of Molecular Sciences 25, no. 10: 5070. https://doi.org/10.3390/ijms25105070





