The Response of Hormones, Reactive Oxygen Species and Nitric Oxide in the Polyethylene-Glycol-Promoted, Salt–Alkali-Stress-Induced Embryo Germination of Sorbus pohuashanensis
Abstract
:1. Introduction
2. Results
2.1. Effects of PEG and Na2CO3 on Embryo Germination of S. pohuashanensis
2.2. Changes in Endogenous Hormone Content during the Embryo Germination of S. pohuashanensis
2.3. Changes in Reactive Oxygen Species Content during the Embryonic Germination of S. pohuashanensis
2.4. Changes in Antioxidant Enzyme Activity during the Embryonic Germination of S. pohuashanensis
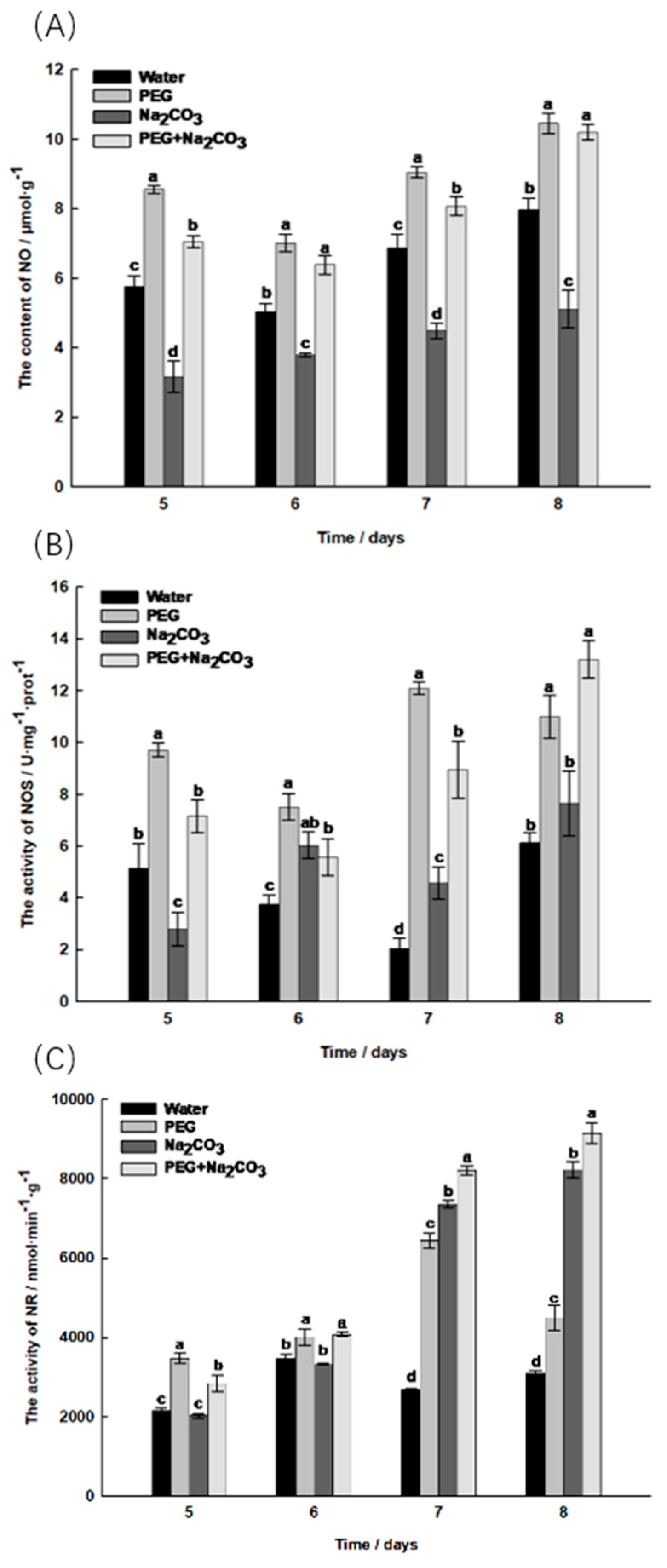
2.5. Changes in Active Nitrogen Content during the Germination of S. pohuashanensis
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Methods
4.2.1. PEG Pretreatment
4.2.2. PEG Treatment Germination Test
4.2.3. Salt–Alkali Stress Treatment
4.2.4. Germination Experiment under Salt–Alkali Stress Treatment
4.2.5. Endogenous Hormone Determination
4.2.6. Determination of Reactive Oxygen Species Content
4.2.7. Determination of Antioxidant Enzyme Activity
4.2.8. Determination of the Active Nitrogen Content
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.W.; Wu, L.Y.; Shen, H.B.; Tian, S.B.; Li, M. Progress in research and application of seed initiation technology. Shanghai Agric. J. 2020, 36, 153–160. [Google Scholar]
- Chen, K.; Arora, R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in Spinach (Spinacia oleracea). Plant Sci. 2011, 180, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.-D.; Xie, Q.; He, Z.-H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.X.; Li, X.; Li, C.; Zhao, L. The role of nitric oxide in plant responses to salt stress. Int. J. Mol. Sci. 2022, 23, 6167. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Wang, X.; Zhao, C.; Shen, H.; Yang, L. Nitric oxide regulates seed germination by integrating multiple signalling pathways. Int. J. Mol. Sci. 2023, 24, 9052. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, S.R.; Wang, J.N.; Shen, H.L.; Yang, L. Interaction between reactive oxygen species and hormones during the breaking of embryo dormancy in Sorbus pohuashanensis by exogenous nitric oxide. J. For. Res. 2022, 33, 435–444. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.; Liu, H.; Wei, C.; Wang, J.; Shen, H. Effects of a nitric oxide donor and nitric oxide scavengers on Sorbus pohuashanensis embryo germination. J. For. Res. 2018, 29, 631–638. [Google Scholar] [CrossRef]
- Dong, S.; Chen, L.N.; Zhang, M.; Liu, Q.Y.; Wu, Y.; Li, X.Y. The effects of inducing drying and storage conditions on the germination of sorghum seeds for liquor use. J. North. Agric. 2022, 50, 127–134. [Google Scholar]
- Wang, Q.Z.; Liu, Q.; Gao, Y.N.; Liu, X. Research progress on the response mechanism of plants to salt alkali stress. J. Ecol. 2017, 37, 5565–5577. [Google Scholar]
- Meng, X.H.; Liu, Y.G.; Zhang, Y.M.; Zhang, H.S.; Mu, P.; Lin, Q. Responses of antioxidant properties and root activity of different wheat varieties to salt stress at seedling stage. Triticeae Crops 2015, 35, 1168–1175. [Google Scholar]
- Xing, Y.M.; Zhou, G.Y.; Peng, X.J.; Mi, L.F.; Xie, Y. The effect of PEG on the germination of radish seeds under salt stress. Anhui Agric. Sci. 2022, 50, 52–55. [Google Scholar]
- Zhou, Q.Y. The effects of different initiators on the germination of tomato and eggplant seeds. Shanxi Agric. Sci. 2021, 49, 947–951. [Google Scholar]
- Hou, M.C.; Ma, M. Effect of PEG-simulated Drought Stress on Seed Germination of Three Medicinal Liquorice (Glycyrrhiza) Species. Legume Res. 2022, 45, 1388–1393. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Bian, L.; Li, Y.; Shen, H. Cyclic secondary somatic embryogenesis and efficient plant regeneration in mountain ash (Sorbus pohuashanensis). Plant Cell Tissue Organ Cult. 2012, 111, 173–182. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Llanes, A.S.; Andrade, A.M.; Alemano, S.G.; Luna, M.V. Alterations of endogenous hormonal levels in plants under drought and salinity. Am. J. Plant Sci. 2016, 7, 1357–1371. [Google Scholar] [CrossRef]
- Chen, H.; Ruan, J.; Chu, P.; Fu, W.; Liang, Z.; Li, Y.; Tong, J.; Xiao, L.; Liu, J.; Li, C.; et al. AtPER1 enhances primary seed dormancy and reduces seed germination by suppressing the ABA catabolism and GA biosynthesis in Arabidopsis seeds. Plant J. 2020, 101, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Lin, J.; Zhang, M.; Li, L.; Zhao, C.; Chen, M. Phytohormone involved in salt tolerance regulation of Elaeagnus angustifolia L. seedlings. J. For. Res. 2019, 24, 235–242. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, E.; Yao, M.; Xue, D.; Zhao, N.; Zhou, Y.; Li, B.; Wang, K.; Miao, Y.; Gu, C.; et al. PEG-6000 Priming Improves Aged Soybean Seed Vigor via Carbon Metabolism, ROS Scavenging, Hormone Signaling, and Lignin Synthesis Regulation. Agronomy 2023, 13, 3021. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Wang, X.; Shen, H.; Yang, L. Exogenous Ethylene Alleviates the Inhibition of Sorbus pohuashanensis Embryo Germination in a Saline-Alkali Environment (NaHCO3). Int. J. Mol. Sci. 2023, 24, 4244. [Google Scholar] [CrossRef]
- Mangena, P. Effect of hormonal seed priming on germination, growth, yield and biomass allocation in soybean grown under induced drought stress. Indian J. Agric. Res. 2020, 54, 592–598. [Google Scholar] [CrossRef]
- Othman, F.; Naufaliansyah, M.A.; Hussain, F. Effect of water salinity on permeability alteration during CO2 sequestration. Adv. Water Resour. 2019, 127, 237–251. [Google Scholar] [CrossRef]
- Zhu, L.; Tian, S.; Huang, J.X.; Sun, S.Q.; Qiao, H.Y. Research progress on the response and regulation of plants to salt stress. Mol. Plant Breed. 2024, 1–12. [Google Scholar]
- Chen, H.; Jin, Z.; Huang, R.; He, L.; Tian, W.; Zhao, L.; Zhang, Z. Promotion of Growth of Alfalfa by Erwinia persicina Cp2 Exopolysaccharides under NaCl Stress. Agronomy 2023, 13, 2129. [Google Scholar] [CrossRef]
- Teh, C.Y.; Mahmood, M.; Shaharuddin, N.A.; Ho, C.L. In vitro rice shoot apices as simple model to study the effect of NaCl and the potential of exogenous proline and glutathione in mitigating salinity stress. Plant Growth Regul. 2014, 75, 771–781. [Google Scholar] [CrossRef]
- Xu, P.Y.; Wu, Y.X.; He, T.M. Research progress on the adaptation mechanism of plants to salt alkali stress. Chin. Wildl. Resour. 2020, 39, 41–49. [Google Scholar]
- Fang, S.M.; Hou, X.; Liang, X.L. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Zavariyan, A.M.; Rad, M.Y.; Asghari, M. Effect of seed priming by potassium nitrate on germination and biochemical indices in Silybum marianum L. under salinity stress. Int. J. Life Sci. 2015, 9, 23–28. [Google Scholar] [CrossRef]
- Loiacono, F.V.; De Tullio, M.C. Why we should stop inferring simple correlations between antioxidants and plant stress resistance: Towards the antioxidomic. A J. Integr. Biol. 2012, 16, 160–167. [Google Scholar] [CrossRef]
- Xu, M.P.; He, P.; Duan, C.X.; Yang, M. Study on relieving effects of exogenous SNP, Spd on Belamcanda chinensis under salt-alkalline stress. China J. Chin. Mater. Medica 2014, 39, 4553–4558. [Google Scholar]
- Wang, Y.; Wang, J.; Guo, D.; Zhang, H.; Che, Y.; Li, Y.; Tian, B.; Wang, Z.; Sun, G.; Zhang, H. Physiological and comparative transcriptome analysis of leaf response and physiological adaption to saline alkali stress across pH values in alfalfa (Medicago sativa). Plant Physiol. Biochem. 2021, 167, 140–152. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.N.; Bian, L.; Shen, H.L. The effect of exogenous NO on embryo germination and early accumulation of reactive oxygen species during seedling development of Sorbus pohuashanensis tree. For. Sci. 2013, 49, 60–67. [Google Scholar]
- Wen, H.; Jiang, D.; Nan, W.B.; Liang, Y.S.; Zhang, H.M.; Qing, X.J. Identification and related physiological characteristics analysis of a rice long spike mutant. Southwest Agric. J. 2021, 34, 461–468. [Google Scholar]
- Peng, Y.; Liu, M.X.; Zhao, X.H. Research progress on hydrogen peroxide content determination methods. Chem. Propellants Polym. Mater. 2022, 20, 24–29. [Google Scholar]
- Cheng, Y.; Chen, L.; Mi, Y.H.; Duan, H.P.; Cha, Y.S.; Shao, J.L.; Du, L.J. Comparative study on methods for measuring antioxidant enzyme activity in rice. Jiangxi Agric. J. 2018, 30, 108–111. [Google Scholar]





| Index | Water | PEG | Na2CO3 | PEG + Na2CO3 |
|---|---|---|---|---|
| Germination percentage/% | 64.44 ± 5.56 b | 80.00 ± 6.94 a | 7.78 ± 2.94 d | 24.44 ± 1.11 c |
| Mean germination speed/days | 5.64 ± 0.08 b | 4.55 ± 0.05 c | 6.58 ± 0.36 a | 6.06 ± 0.38 ab |
| Germination index | 3.64 ± 0.26 b | 5.44 ± 0.44 a | 0.35 ± 0.11 d | 1.25 ± 0.04 c |
| Germination potential/% | 34.44 ± 2.94 b | 65.56 ± 14.57 a | 6.67 ± 3.33 c | 20.00 ± 3.85 bc |
| Hypocotyl and radicle length/cm | 1.13 ± 0.07 a | 1.23 ± 0.25 a | 0.38 ± 0.02 b | 0.39 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shen, H.; Yang, L. The Response of Hormones, Reactive Oxygen Species and Nitric Oxide in the Polyethylene-Glycol-Promoted, Salt–Alkali-Stress-Induced Embryo Germination of Sorbus pohuashanensis. Int. J. Mol. Sci. 2024, 25, 5128. https://doi.org/10.3390/ijms25105128
Wang X, Shen H, Yang L. The Response of Hormones, Reactive Oxygen Species and Nitric Oxide in the Polyethylene-Glycol-Promoted, Salt–Alkali-Stress-Induced Embryo Germination of Sorbus pohuashanensis. International Journal of Molecular Sciences. 2024; 25(10):5128. https://doi.org/10.3390/ijms25105128
Chicago/Turabian StyleWang, Xiaodong, Hailong Shen, and Ling Yang. 2024. "The Response of Hormones, Reactive Oxygen Species and Nitric Oxide in the Polyethylene-Glycol-Promoted, Salt–Alkali-Stress-Induced Embryo Germination of Sorbus pohuashanensis" International Journal of Molecular Sciences 25, no. 10: 5128. https://doi.org/10.3390/ijms25105128





