Efficacy of Integrated Risk Score Using Omics-Based Biomarkers for the Prediction of Acute Rejection in Kidney Transplantation: A Randomized Prospective Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Clinical Information at 1 Year after KT
2.3. Changes in the Integrated Risk Score and Each Biomarker at 1 Year after KT
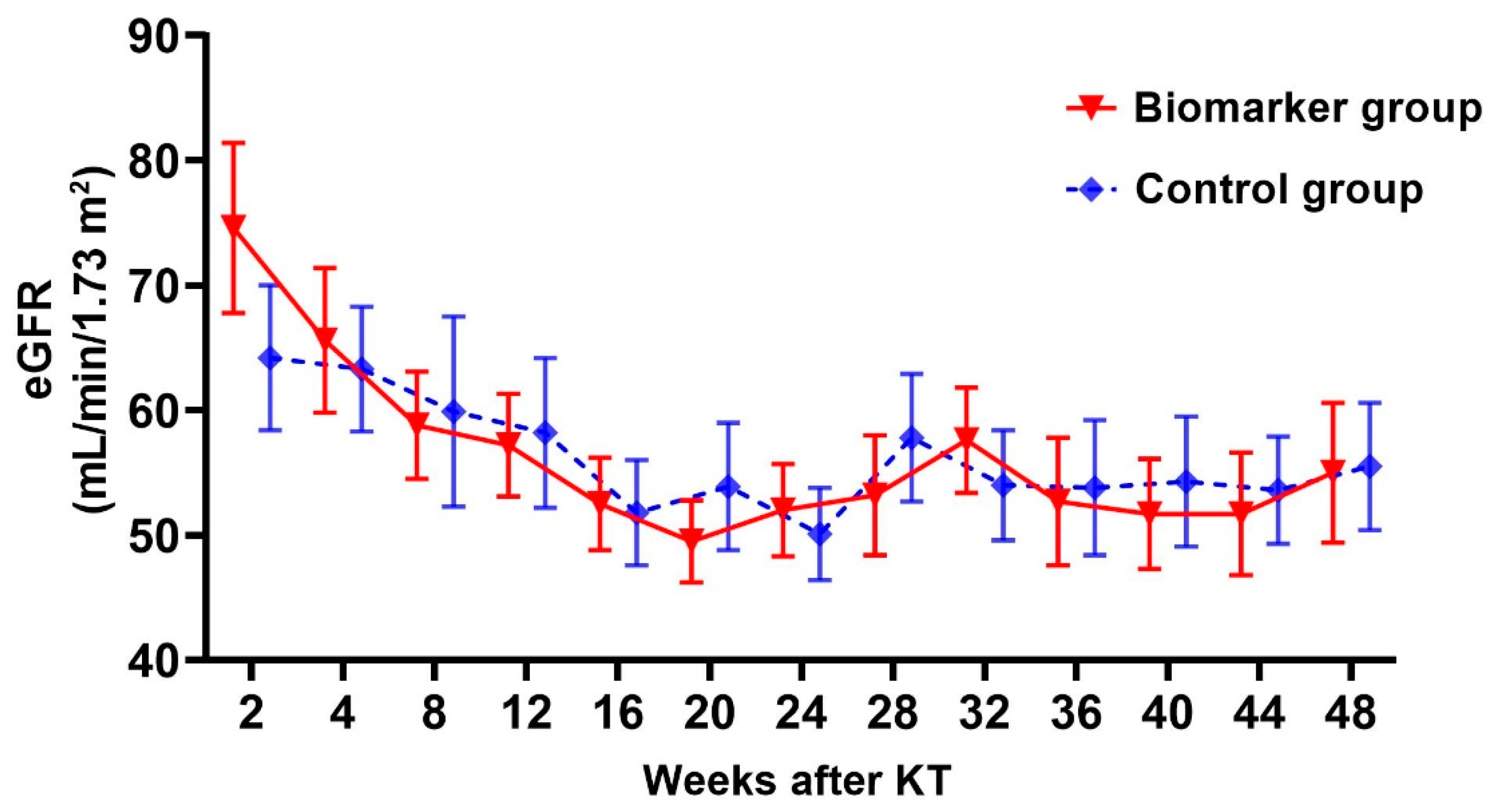
2.4. Changes in Graft Function and Tacrolimus trough Level 1 Year after KT
2.5. Changes in the Integrated Risk Score and Histopathologic Findings of KTRs with AR
3. Discussion
4. Materials and Methods
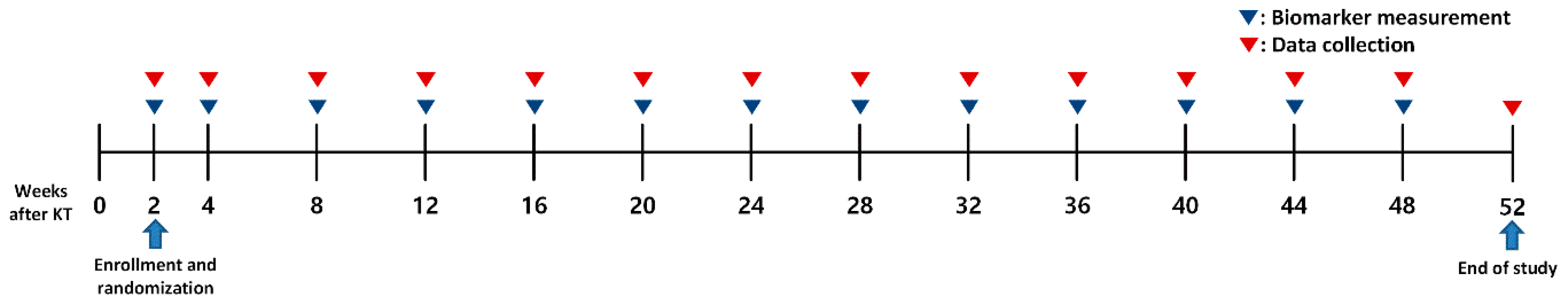
4.1. Study Design and Participants
4.2. Data Collection
4.3. Omics Biomarkers and Integrated Risk Score
4.4. Measurement of Omics Biomarkers
4.5. Outcomes
4.6. Immunohistochemistry for C4d
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Wolfe, R.A.; Mauger, E.A.; Berling, D.P.; Jiang, K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993, 270, 1339–1343. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, G.Y.; Jeon, Y.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, Y.L.; Kim, H.K.; Huh, S.; et al. Elderly kidney transplant recipients have favorable outcomes but increased infection-related mortality. Kidney Res. Clin. Pract. 2022, 41, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Jofre, R.; López-Gómez, J.M.; Valderrábano, F. Quality of life for patient groups. Kidney Int. 2000, 57, S121–S130. [Google Scholar] [CrossRef]
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long-term kidney transplant graft survival-Making progress when most needed. Am. J. Transplant. 2021, 21, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Evaluation and Treatment of Acute Rejection in Kidney Allografts. Clin. J. Am. Soc. Nephrol. 2020, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Lee, C.H.; Kim, K.Y.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, Y.L.; Baek, M.C.; Park, J.B.; et al. Novel urinary exosomal biomarkers of acute T cell-mediated rejection in kidney transplant recipients: A cross-sectional study. PLoS ONE 2018, 13, e0204204. [Google Scholar] [CrossRef] [PubMed]
- Cho, U.; Suh, K.S.; Kie, J.H.; Choi, Y.J.; Renal Pathology Study Group of Korean Society of Pathologists. Investigation and Standardization on Current Practice of Renal Transplant Pathology in Korea. Korean J. Transplant. 2017, 31, 170–176. [Google Scholar] [CrossRef]
- Lim, J.H.; Chung, B.H.; Lee, S.H.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, Y.L.; Kim, C.D. Omics-based biomarkers for diagnosis and prediction of kidney allograft rejection. Korean J. Intern. Med. 2022, 37, 520–533. [Google Scholar] [CrossRef]
- Lee, A.; Jeong, J.C.; Choi, Y.W.; Seok, H.Y.; Kim, Y.G.; Jeong, K.H.; Moon, J.Y.; Lee, T.W.; Ihm, C.G.; Jeon, H.J.; et al. Validation study of peripheral blood diagnostic test for acute rejection in kidney transplantation. Transplantation 2014, 98, 760–765. [Google Scholar] [CrossRef]
- Seo, J.W.; Lee, Y.H.; Tae, D.H.; Park, S.H.; Moon, J.Y.; Jeong, K.H.; Kim, C.D.; Chung, B.H.; Park, J.B.; Kim, Y.H.; et al. Non-Invasive Diagnosis for Acute Rejection Using Urinary mRNA Signature Reflecting Allograft Status in Kidney Transplantation. Front. Immunol. 2021, 12, 656632. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Lee, Y.H.; Tae, D.H.; Kim, Y.G.; Moon, J.Y.; Jung, S.W.; Kim, J.S.; Hwang, H.S.; Jeong, K.H.; Jeong, H.Y.; et al. Development and validation of urinary exosomal microRNA biomarkers for the diagnosis of acute rejection in kidney transplant recipients. Front. Immunol. 2023, 14, 1190576. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Moon, H.; Kim, S.Y.; Moon, J.Y.; Jeong, K.H.; Lee, Y.H.; Kim, Y.G.; Lee, T.W.; Ihm, C.G.; Kim, C.D.; et al. Both absolute and relative quantification of urinary mRNA are useful for non-invasive diagnosis of acute kidney allograft rejection. PLoS ONE 2017, 12, e0180045. [Google Scholar] [CrossRef] [PubMed]
- Kidd, J.M.; Cooper, G.M.; Donahue, W.F.; Hayden, H.S.; Sampas, N.; Graves, T.; Hansen, N.; Teague, B.; Alkan, C.; Antonacci, F.; et al. Mapping and sequencing of structural variation from eight human genomes. Nature 2008, 453, 56–64. [Google Scholar] [CrossRef]
- Naesens, M.; Sarwal, M.M. Molecular diagnostics in transplantation. Nat. Rev. Nephrol. 2010, 6, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Horgan, R.P.; Kenny, L.C. ‘Omic’ technologies: Genomics, transcriptomics, proteomics and metabolomics. Obstet. Gynaecol. 2011, 13, 189–195. [Google Scholar] [CrossRef]
- Quaglia, M.; Merlotti, G.; Guglielmetti, G.; Castellano, G.; Cantaluppi, V. Recent Advances on Biomarkers of Early and Late Kidney Graft Dysfunction. Int. J. Mol. Sci. 2020, 21, 5404. [Google Scholar] [CrossRef]
- Wilkinson, A. Protocol Transplant Biopsies: Are They Really Needed? Clin. J. Am. Soc. Nephrol. 2006, 1, 130–137. [Google Scholar] [CrossRef]
- Lim, M.; Park, B.K.; Lee, K.W.; Park, J.B.; Kim, K.D.; Yang, J.; Kwon, J.; Jeong, E.S.; Lee, S. Two-Week Protocol Biopsy in Renal Allograft: Feasibility, Safety, and Outcomes. J. Clin. Med. 2022, 11, 785. [Google Scholar] [CrossRef]
- Silver, S.A.; Adhikari, N.K.; Bell, C.M.; Chan, C.T.; Harel, Z.; Kitchlu, A.; Meraz-Muñoz, A.; Norman, P.A.; Perez, A.; Zahirieh, A.; et al. Nephrologist Follow-Up versus Usual Care after an Acute Kidney Injury Hospitalization (FUSION): A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 1005–1014. [Google Scholar] [CrossRef]
- Agrawal, A.; Ison, M.G.; Danziger-Isakov, L. Long-Term Infectious Complications of Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2022, 17, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Jeon, Y.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; Hwang, D.; Yun, W.-S.; Kim, H.-K.; et al. Pretransplant C-reactive protein-to-albumin ratio predicts mortality in kidney transplant recipients: A retrospective cohort study. Korean J. Transplant. 2023, 37, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Elnokeety, M.M.; Hussein, W.M.; Ahmed Abdelrazek, S.; Momtaz, M. Cell cycle arrest biomarkers for the early detection of acute allograft dysfunction and acute rejection in living donor kidney transplantation: A cross-sectional study from Egypt. Korean J. Transplant. 2023, 37, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Anglicheau, D. Precision Transplant Medicine: Biomarkers to the Rescue. J. Am. Soc. Nephrol. 2018, 29, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Lim, J.H.; Cho, J.H.; Jung, H.Y.; Choi, J.Y.; Park, S.H.; Kim, Y.L.; Kim, H.K.; Huh, S.; Yoo, E.S.; Won, D.I.; et al. Excellent outcome after desensitization in high immunologic risk kidney transplantation. PLoS ONE 2019, 14, e0222537. [Google Scholar] [CrossRef]





| Biomarker (n = 16) | Control (n = 19) | p | |
|---|---|---|---|
| Age, years | 49.4 ± 11.0 | 52.3 ± 13.4 | 0.497 |
| Sex, male n, % | 12 (75.0) | 13 (68.4) | 0.668 |
| Body mass index, kg/m2 | 22.0 ± 2.5 | 21.6 ± 3.5 | 0.665 |
| Primary renal disease, n (%) | 0.260 | ||
| Diabetes mellitus | 6 (37.5) | 3 (15.8) | |
| Hypertension | 1 (6.3) | 4 (21.1) | |
| Glomerulonephritis | 3 (18.8) | 1 (5.3) | |
| Chronic glomerulonephritis | 5 (31.3) | 8 (42.1) | |
| Others | 1 (6.3) | 3 (15.8) | |
| Number of HLA mismatch | 4.1 ± 1.3 | 3.7 ± 1.9 | 0.507 |
| Direct cross-match positive, n (%) | 9 (56.3) | 7 (36.8) | 0.251 |
| CDC-XM positive | 5 (31.3) | 4 (21.1) | 0.700 |
| FCXM positive | 7 (43.8) | 7 (36.8) | 0.678 |
| ABO incompatible, n (%) | 5 (31.3) | 11 (57.9) | 0.115 |
| Pretransplant DSA, n (%) | 7 (43.8) | 4 (21.1) | 0.150 |
| Pretransplant desensitization, n (%) | 13 (81.3) | 14 (73.7) | 0.595 |
| Calculated PRA ≥ 30%, n (%) | 2 (12.5) | 3 (15.8) | 1.000 |
| Comorbid conditions, n (%) | |||
| Diabetes mellitus | 7 (43.8) | 3 (15.8) | 0.132 |
| Hypertension | 8 (50.0) | 14 (73.7) | 0.149 |
| Cardiovascular disease | 2 (12.5) | 1 (5.3) | 0.582 |
| Induction therapy, n (%) | 0.142 | ||
| Basiliximab | 7 (43.8) | 13 (68.4) | |
| Anti-thymocyte globulin | 9 (56.3) | 6 (31.6) | |
| Laboratory findings at discharge | |||
| White blood cell, ×109/L | 9.3 ± 3.5 | 7.9 ± 3.2 | 0.218 |
| Hemoglobin, g/dL | 10.8 ± 1.1 | 11.6 ± 1.3 | 0.065 |
| Platelet, ×109/L | 196.7 ± 62.2 | 188.1 ± 78.1 | 0.724 |
| Sodium, mEq/L | 139.4 ± 2.6 | 138.3 ± 3.1 | 0.263 |
| Potassium, mEq/L | 4.5 ± 0.5 | 4.7 ± 0.5 | 0.225 |
| Creatinine, mg/dL | 0.94 ± 0.37 | 1.14 ± 0.47 | 0.538 |
| eGFR, mL/min/1.73 m2 | 79.9 ± 29.0 | 70.5 ± 24.8 | 0.418 |
| Donor age, years | 49.9 ± 9.8 | 52.6 ± 7.1 | 0.354 |
| Donor type, n (%) | 0.238 | ||
| Living | 13 (81.3) | 12 (63.2) | |
| Deceased | 3 (18.8) | 7 (36.8) | |
| KDPI score > 60% | 2 (12.5) | 7 (36.8) | 0.135 |
| KT from expanded criteria donor, n (%) | 2 (12.5) | 5 (26.3) | 0.415 |
| Biomarker (n = 16) | Control (n = 19) | p | |
|---|---|---|---|
| Scr at 1 year after KT, mg/dL | 1.36 ± 0.81 | 1.37 ± 0.50 | 0.984 |
| eGFR at 1 year after KT, mL/min/m2 | 55.0 ± 16.0 | 55.5 ± 18.5 | 0.946 |
| Scr change (Δ) at 1 year after KT, mg/dL | –0.17 ± 0.61 | 0.01 ± 0.47 | 0.452 |
| Scr > 1.5 mg/dL at 1 year after KT | 1 (6.3) | 6 (31.6) | 0.062 |
| Graft biopsy during 1 year after KT, n (%) | 2 (12.5) | 9 (47.4) | 0.027 |
| BPAR during 1 year after KT, n (%) | 1 (6.3) | 2 (10.5) | 1.000 |
| TCMR | 1 (6.3) | 0 | 0.457 |
| ABMR | 0 | 2 (10.5) | 0.489 |
| Development of dnDSA during 1 year after KT, n (%) | 1 (6.3) | 2 (10.5) | 1.000 |
| Patient No. | Diagnosis | t | i | g | ptc | MVI | v | C4d | ci | ct |
|---|---|---|---|---|---|---|---|---|---|---|
| B-1 | Acute TCMR, IB | 3 | 2 | 1 | 2 | 3 | 0 | 0 | 0 | 0 |
| C-1 | Active ABMR | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 0 |
| C-2 | Active ABMR | 1 | 2 | 2 | 2 | 4 | 0 | 0 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-H.; Chung, B.H.; Lee, S.-H.; Lee, J.S.; Kim, Y.H.; Han, M.-H.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; et al. Efficacy of Integrated Risk Score Using Omics-Based Biomarkers for the Prediction of Acute Rejection in Kidney Transplantation: A Randomized Prospective Pilot Study. Int. J. Mol. Sci. 2024, 25, 5139. https://doi.org/10.3390/ijms25105139
Lim J-H, Chung BH, Lee S-H, Lee JS, Kim YH, Han M-H, Jung H-Y, Choi J-Y, Cho J-H, Park S-H, et al. Efficacy of Integrated Risk Score Using Omics-Based Biomarkers for the Prediction of Acute Rejection in Kidney Transplantation: A Randomized Prospective Pilot Study. International Journal of Molecular Sciences. 2024; 25(10):5139. https://doi.org/10.3390/ijms25105139
Chicago/Turabian StyleLim, Jeong-Hoon, Byung Ha Chung, Sang-Ho Lee, Jong Soo Lee, Yeong Hoon Kim, Man-Hoon Han, Hee-Yeon Jung, Ji-Young Choi, Jang-Hee Cho, Sun-Hee Park, and et al. 2024. "Efficacy of Integrated Risk Score Using Omics-Based Biomarkers for the Prediction of Acute Rejection in Kidney Transplantation: A Randomized Prospective Pilot Study" International Journal of Molecular Sciences 25, no. 10: 5139. https://doi.org/10.3390/ijms25105139






