Porous Hydrogels for Immunomodulatory Applications
Abstract
:1. Introduction
2. Strategies to Develop Porous Hydrogels
2.1. Ice Templating
2.2. Pickering Emulsion Templating
2.3. Microgel Templating
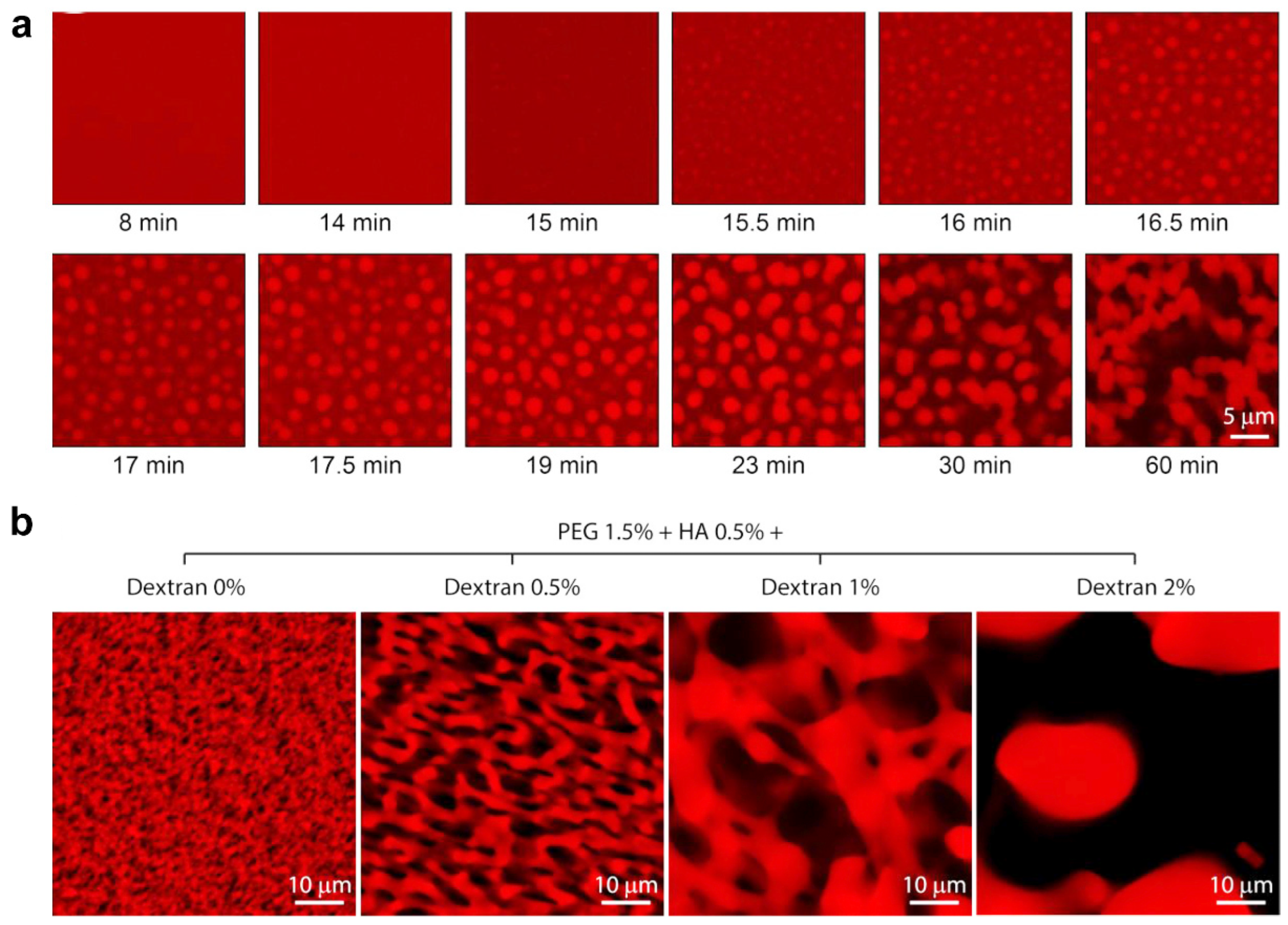
2.4. Phase Separation
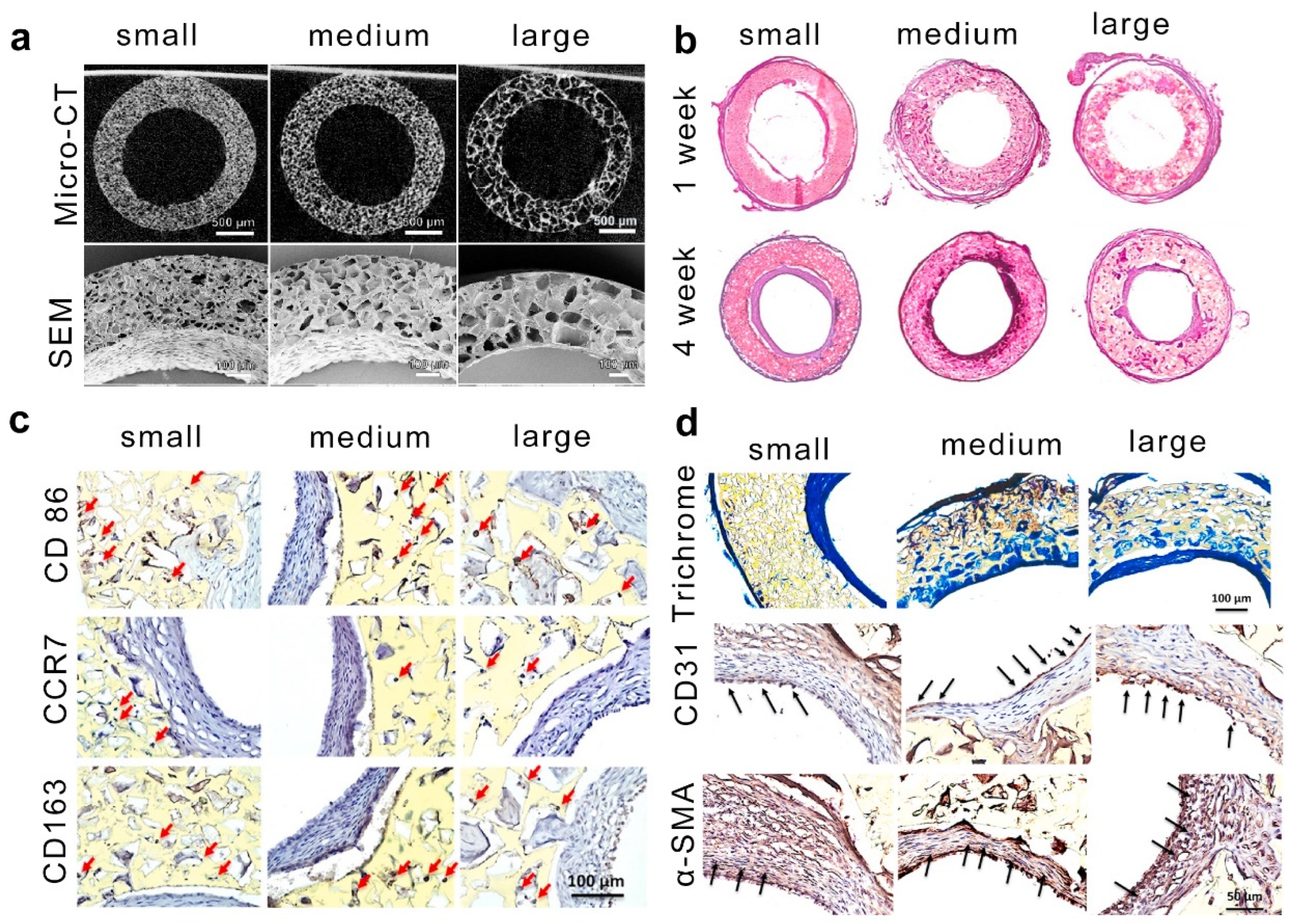
2.5. Salt Templating
2.6. Gas Foaming
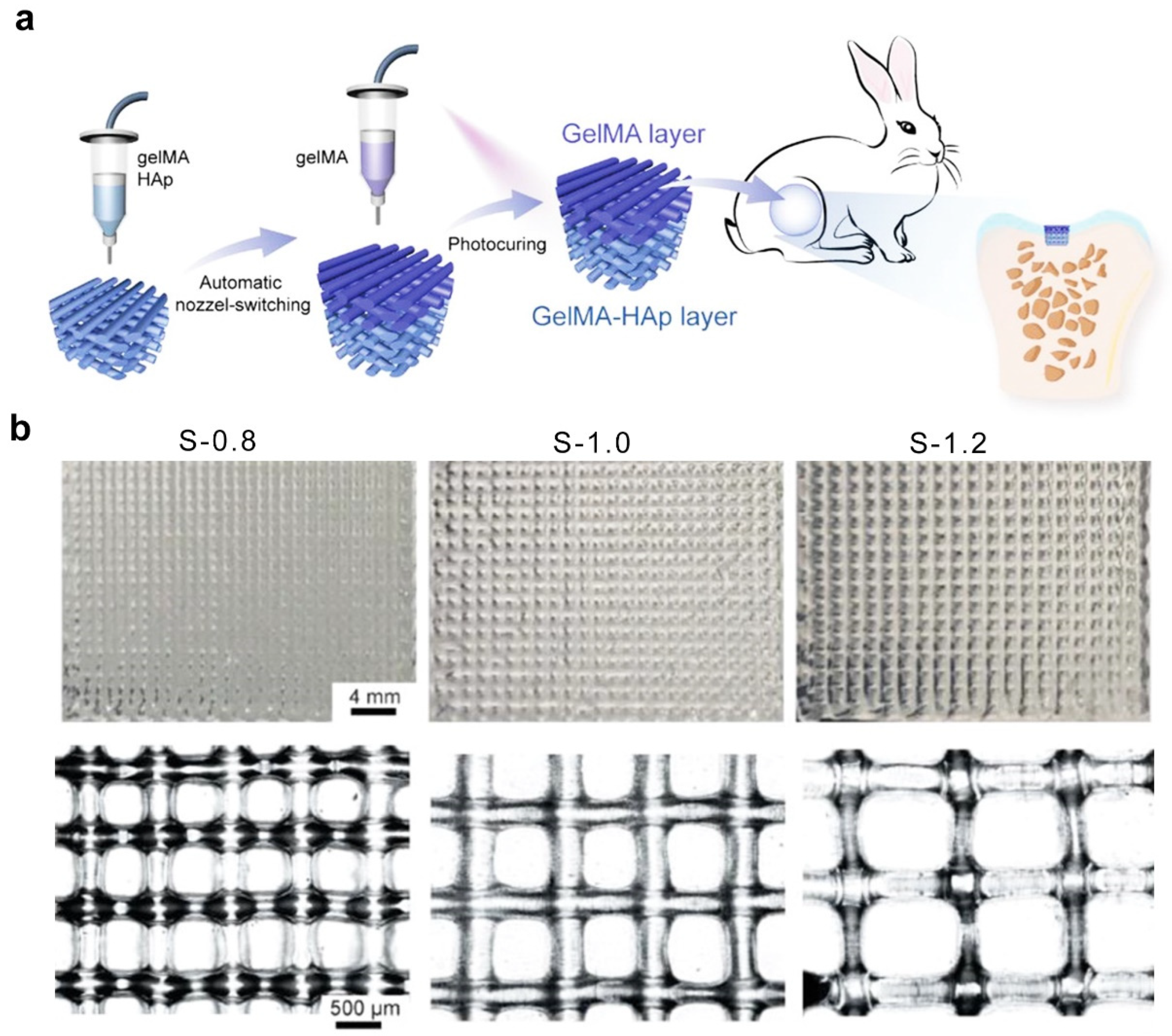
2.7. Three-Dimensional Printing Technique
3. Porous Hydrogel-Assisted Immunomodulation for Cancer Therapy
4. Porous Hydrogel-Assisted Immunomodulation for Tissue Regeneration
5. Concluding Remarks of Porous Hydrogel for Immunomodulatory Applications
6. Future Developments of Porous Hydrogel for Immunomodulatory Applications
Author Contributions
Funding
Conflicts of Interest
References
- Shields, C.W., IV; Wang, L.L.W.; Evans, M.A.; Mitragotri, S. Materials for immunotherapy. Adv. Mater. 2020, 32, 1901633. [Google Scholar] [CrossRef]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef]
- Xue, Y.; Che, J.; Ji, X.; Li, Y.; Xie, J.; Chen, X. Recent advances in biomaterial-boosted adoptive cell therapy. Chem. Soc. Rev. 2022, 51, 1766–1794. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Ji, P.; Sun, W.; Zhang, S.; Xing, Y.; Wang, C.; Wei, M.; Li, Q.; Ji, G.; Yang, G. Modular hydrogel vaccine for programmable and coordinate elicitation of cancer immunotherapy. Adv. Sci. 2023, 10, 2301789. [Google Scholar] [CrossRef] [PubMed]
- Zarubova, J.; Hasani-Sadrabadi, M.M.; Ardehali, R.; Li, S. Immunoengineering strategies to enhance vascularization and tissue regeneration. Adv. Drug Deliv. Rev. 2022, 184, 114233. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv. Mater. 2021, 33, 2100176. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.-H.; Misra, P.K.; Yang, J.M. Synthesis and characterization of modified poly (vinyl alcohol) membrane and study of its enhanced water-induced shape-memory behavior. J. Polym. Environ. 2022, 30, 3409–3419. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Designing hydrogels for immunomodulation in cancer therapy and regenerative medicine. Adv. Mater. 2023, 36, 2308894. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Wu, Y.; Ghaemmaghami, A.M.; Sun, H.; Mata, A. Rational design of hydrogels for immunomodulation. Regen. Biomater. 2022, 9, rbac009. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C. Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef]
- Xiong, Y.; Feng, Q.; Lu, L.; Zha, K.; Yu, T.; Lin, Z.; Hu, Y.; Panayi, A.C.; Nosrati Ziahmagi, V.; Chu, X. Immunomodulatory hydrogels: Advanced regenerative tools for diabetic foot ulcer. Adv. Funct. Mater. 2023, 33, 2213066. [Google Scholar] [CrossRef]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable cryogels for biomedical applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef]
- Djemaa, I.B.; Auguste, S.; Drenckhan-Andreatta, W.; Andrieux, S. Hydrogel foams from liquid foam templates: Properties and optimisation. Adv. Colloid. Interface Sci. 2021, 294, 102478. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Su, T.; Lu, F.; Chang, Q.; Gao, J. Recent advances in macroporous hydrogels for cell behavior and tissue engineering. Gels 2022, 8, 606. [Google Scholar] [CrossRef]
- Najibi, A.J.; Shih, T.-Y.; Mooney, D.J. Cryogel vaccines effectively induce immune responses independent of proximity to the draining lymph nodes. Biomaterials 2022, 281, 121329. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Shokrollahi Barough, M.; Rahmanian, M.; Mojtabavi, N.; Sarrami Forooshani, R.; Seyfoori, A.; Akbari, M. Cancer immunotherapy using bioengineered micro/nano structured hydrogels. Adv. Healthc. Mater. 2023, 12, 2301174. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Wang, C.; Huang, Y.; Han, Y.; Guo, B. Injectable conductive micro-cryogel as a muscle stem cell carrier improves myogenic proliferation, differentiation and in situ skeletal muscle regeneration. Acta Biomater. 2022, 151, 197–209. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous hydrogels: Present challenges and future opportunities. Langmuir. 2023, 39, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Nicol, E. Photopolymerized porous hydrogels. Biomacromolecules 2021, 22, 1325–1345. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, B.; Yang, C.; Wu, J.; Zhao, Q. Macroporous hydrogels prepared by ice templating: Developments and challenges. Adv. Funct. Mater. 2023, 41, 3082–3096. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion templating: Porous polymers and beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef]
- Pentzer, E.; Cruz Barrios, E.; Starvaggi, N. Pickering emulsions as templates for architecting composite structures. Acc. Mater. Res. 2023, 4, 641–647. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Rao, V.V.; Golden, A.C.; Anseth, K.S. Porous bio-click microgel scaffolds control hMSC interactions and promote their secretory properties. Biomaterials 2020, 232, 119725. [Google Scholar] [CrossRef]
- Lu, M.; Liu, F.; Tan, R.; Xiao, Z.; Dong, X.-h.; Wang, H.; Tang, L.; Chen, T.; Wu, Z.L.; Hong, W. Phase-separation-induced porous hydrogels from amphiphilic triblock copolymer with high permeability and mechanical strength. Chem. Mater. 2022, 34, 10995–11006. [Google Scholar] [CrossRef]
- Coogan, K.R.; Stone, P.T.; Sempertegui, N.D.; Rao, S.S. Fabrication of micro-porous hyaluronic acid hydrogels through salt leaching. Eur. Polym, J. 2020, 135, 109870. [Google Scholar] [CrossRef]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef]
- Puza, F.; Lienkamp, K. 3D printing of polymer hydrogels—From basic techniques to programmable actuation. Adv. Funct. Mater. 2022, 32, 2205345. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice templating soft matter: Fundamental principles and fabrication approaches to tailor pore structure and morphology and their biomedical applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Xia, K.; Qian, T.; Hu, Z.; Hong, L.; Liao, Y.; Peng, G.; Yuan, Z.; Chen, Y.; Zeng, Z. Shape-recoverable macroporous nanocomposite hydrogels created via ice templating polymerization for noncompressible wound hemorrhage. ACS Biomater. Sci. Eng. 2022, 8, 2076–2087. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice inhibition for cryopreservation: Materials, strategies, and challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.; Chen, L.; Dai, B. Control of ice crystal growth and its effect on porous structure of chitosan cryogels. Chem. Eng. Sci. 2019, 201, 50–57. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.S.; Bae, S.; Lee, H.; Hwang, N.S. Heparin functionalized injectable cryogel with rapid shape-recovery property for neovascularization. Biomacromolecules 2018, 19, 2257–2269. [Google Scholar] [CrossRef]
- Murray, K.A.; Gibson, M.I. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lyu, C.; Zhao, P.; Li, W.; Kong, W.; Huang, C.; Genin, G.M.; Du, Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019, 10, 3491. [Google Scholar] [CrossRef]
- Tam, R.Y.; Fisher, S.A.; Baker, A.E.; Shoichet, M.S. Transparent porous polysaccharide cryogels provide biochemically defined, biomimetic matrices for tunable 3D cell culture. Chem. Mater. 2016, 28, 3762–3770. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Shao, Z.; Mao, A.; Gao, W.; Bai, H. Smart sponge for fast liquid absorption and thermal responsive self-squeezing. Adv. Mater. 2020, 32, 1908249. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Agresti, F.; Fedele, L.; Barison, S.; Hermida-Merino, C.; Losada-Barreiro, S.; Bobbo, S.; Piñeiro, M. Review on phase change material emulsions for advanced thermal management: Design, characterization and thermal performance. Renew. Sustain. Energy Rev. 2022, 159, 112238. [Google Scholar] [CrossRef]
- Dai, H.; Luo, Y.; Huang, Y.; Ma, L.; Chen, H.; Fu, Y.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Recent advances in protein-based emulsions: The key role of cellulose. Food Hydrocolloids 2023, 136, 108260. [Google Scholar] [CrossRef]
- Rodriguez, A.M.B.; Binks, B.P. High internal phase pickering emulsions. Curr. Opin. Colloid. Interface Sci. 2022, 57, 101556. [Google Scholar] [CrossRef]
- Jie, Y.; Chen, F.; Zhu, T.; Lv, D. High internal phase emulsions stabilized solely by carboxymethyl chitosan. Food Hydrocolloids 2022, 127, 107554. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, S.; Zheng, W.; Chen, M.; Zhou, S.; Liao, M.; Huang, W.; Hu, Y.; Zhou, W. Formation of poly (ε-caprolactone)-embedded bioactive nanoparticles/collagen hierarchical scaffolds with the designed and customized porous structures. J. Appl. Polym. Sci. 2022, 139, e52749. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid. Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High internal phase emulsions stabilised by supramolecular cellulose nanocrystals and their application as cell-adhesive macroporous hydrogel monoliths. J. Mater. Chem. 2017, 5, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.; Chen, Y.; Teng, L.; Qi, D.; Ren, L. Air-in-water emulsion solely stabilized by gelatin methacryloyl and templating for macroporous nanocomposite hydrogels. Macromol. Chem. Phys. 2019, 220, 1800500. [Google Scholar] [CrossRef]
- Xu, M.; Li, Q.; Fang, Z.; Jin, M.; Zeng, Q.; Huang, G.; Jia, Y.-G.; Wang, L.; Chen, Y. Conductive and antimicrobial macroporous nanocomposite hydrogels generated from air-in-water pickering emulsions for neural stem cell differentiation and skin wound healing. Biomater. Sci. 2020, 8, 6957–6968. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zheng, Z.; Pu, S.; Jia, Y.G.; Wang, L.; Chen, Y. Macroporous adhesive nano-enabled hydrogels generated from air-in-water emulsions. Macromol. Biosci. 2022, 22, 2100491. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, X.; Luo, J.; Yang, Q.; Chen, Y.; Wang, Y. Double-network DNA macroporous hydrogel enables aptamer-directed cell recruitment to accelerate bone healing. Adv. Sci. 2023, 11, 2303637. [Google Scholar] [CrossRef]
- Feng, Q.; Li, D.; Li, Q.; Cao, X.; Dong, H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioact. Mater. 2022, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lin, F.-Y.; Jiang, K.; Nguyen, H.; Chang, C.-Y.; Lin, C.-C. Dissolvable microgel-templated macroporous hydrogels for controlled cell assembly. Biomater. Adv. 2022, 134, 112712. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Wojciechowski, J.P.; Tang, J.; Guo, Y.; Stevens, M.M. Tunable microgel-templated porogel (MTP) bioink for 3D bioprinting applications. Adv. Healthc. Mater. 2022, 11, 2200027. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Seymour, A.J.; Shin, S.; Heilshorn, S.C. 3D printing of microgel scaffolds with tunable void fraction to promote cell infiltration. Adv. Healthc. Mater. 2021, 10, 2100644. [Google Scholar] [CrossRef] [PubMed]
- Ataie, Z.; Horchler, S.; Jaberi, A.; Koduru, S.V.; El-Mallah, J.C.; Sun, M.; Kheirabadi, S.; Kedzierski, A.; Risbud, A.; Silva, A.R.A.E. Accelerating patterned vascularization using granular hydrogel scaffolds and surgical micropuncture. Small 2023, 20, 2307928. [Google Scholar] [CrossRef] [PubMed]
- Kittel, Y.; Kuehne, A.J.; De Laporte, L. Translating therapeutic microgels into clinical applications. Adv. Healthc. Mater. 2022, 11, 2101989. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.; Pappu, R.V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 2022, 82, 2201–2214. [Google Scholar] [CrossRef]
- Xiao, Q.; McAtee, C.K.; Su, X. Phase separation in immune signalling. Nat. Rev. Immunol. 2022, 22, 188–199. [Google Scholar] [CrossRef]
- Ryu, J.K.; Hwang, D.E.; Choi, J.M. Current understanding of molecular phase separation in chromosomes. Int. J. Mol. Sci. 2021, 22, 10736. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Zhang, J. Liquid–liquid phase separation drives cellular function and dysfunction in cancer. Nat. Rev. Cancer 2022, 22, 239–252. [Google Scholar] [CrossRef]
- Broguiere, N.; Husch, A.; Palazzolo, G.; Bradke, F.; Madduri, S.; Zenobi-Wong, M. Macroporous hydrogels derived from aqueous dynamic phase separation. Biomaterials 2019, 200, 56–65. [Google Scholar] [CrossRef]
- Musacchio, A. On the role of phase separation in the biogenesis of membraneless compartments. EMBO J. 2022, 41, e109952. [Google Scholar] [CrossRef]
- Cao, H.; Li, H.; Liu, L.; Xue, K.; Niu, X.; Hou, J.; Chen, L. Salt-templated nanoarchitectonics of CoSe(2)-NC nanosheets as an efficient bifunctional oxygen electrocatalyst for water splitting. Int. J. Mol. Sci. 2022, 23, 5239. [Google Scholar] [CrossRef]
- Dušková-Smrčková, M.; Zavřel, J.; Bartoš, M.; Kaberova, Z.; Filová, E.; Zárubová, J.; Šlouf, M.; Michálek, J.; Vampola, T.; Kubies, D. Communicating macropores in PHEMA-based hydrogels for cell seeding: Probabilistic open pore simulation and direct micro-CT proof. Mater. Des. 2021, 198, 109312. [Google Scholar] [CrossRef]
- De France, K.J.; Xu, F.; Hoare, T. Structured macroporous hydrogels: Progress, challenges, and opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, S.; Yin, S.; Jiang, F.; Zhou, M.; Yang, G.; Sun, N.; Zhang, W.; Jiang, X. In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials 2020, 232, 119727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, M.; Liao, S.; Yuan, B.; Shi, R.; Hu, X.; Wang, Y. An injectable macroporous hydrogel templated by gasification reaction for enhanced tissue regeneration. Supramol. Mater. 2023, 2, 100037. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D bioprinting of human tissues: Biofabrication, bioinks, and bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yue, Z.; Lucarelli, E.; Wallace, G.G. Hybrid printing using cellulose nanocrystals reinforced GelMA/HAMA hydrogels for improved structural integration. Adv. Healthc. Mater. 2020, 9, 2001410. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-art of 3D printing technology of alginate-based hydrogels—An emerging technique for industrial applications. Adv. Colloid. Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ding, X.; Yu, X.; Chen, X.; Zhang, X.; Cui, S.; Shi, J.; Chen, J.; Yu, L.; Chen, S. Cell-free bilayered porous scaffolds for osteochondral regeneration fabricated by continuous 3D-printing using nascent physical hydrogel as ink. Adv. Healthc. Mater. 2021, 10, 2001404. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef]
- Wales, D.J.; Keshavarz, M.; Howe, C.; Yeatman, E. 3D Printability assessment of poly (octamethylene maleate (anhydride) citrate) and poly (ethylene glycol) diacrylate copolymers for biomedical applications. ACS Appl. Polym. Mater. 2022, 4, 5457–5470. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.; Papneja, N.; Miller, W. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer immunotherapy: Challenges and limitations. Pathol. Res. Pract. 2022, 229, 153723. [Google Scholar] [CrossRef]
- Erfani, A.; Diaz, A.E.; Doyle, P.S. Hydrogel-enabled, local administration and combinatorial delivery of immunotherapies for cancer treatment. Mater. Today 2023, 65, 227–243. [Google Scholar] [CrossRef]
- Iudin, D.; Vasilieva, M.; Knyazeva, E.; Korzhikov-Vlakh, V.; Demyanova, E.; Lavrentieva, A.; Skorik, Y.; Korzhikova-Vlakh, E. Hybrid nanoparticles and composite hydrogel systems for delivery of peptide antibiotics. Int. J. Mol. Sci. 2022, 23, 2771. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Warren Sands, R.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.-Y.; Verbeke, C.S.; Bhatta, D.; Dranoff, G. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef] [PubMed]
- Bauleth-Ramos, T.; Shih, T.Y.; Shahbazi, M.A.; Najibi, A.J.; Mao, A.S.; Liu, D.; Granja, P.; Santos, H.A.; Sarmento, B.; Mooney, D.J. Acetalated dextran nanoparticles loaded into an injectable alginate cryogel for combined chemotherapy and cancer vaccination. Adv. Funct. Mater. 2019, 29, 1903686. [Google Scholar] [CrossRef]
- Shah, N.J.; Najibi, A.J.; Shih, T.-Y.; Mao, A.S.; Sharda, A.; Scadden, D.T.; Mooney, D.J. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat. Biomed. Eng. 2020, 4, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Najibi, A.J.; Sobral, M.C.; Seo, B.R.; Lee, J.Y.; Wu, D.; Li, A.W.; Verbeke, C.S.; Mooney, D.J. Biomaterial-based scaffold for in situ chemo-immunotherapy to treat poorly immunogenic tumors. Nat. Commun. 2020, 11, 5696. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Y.; Chen, J.; Yu, P.; Tang, F.; Hu, Z.; Zhou, J.; Liu, L.; Qiu, W.; Ye, Y. Regulation of biomaterial implantation-induced fibrin deposition to immunological functions of dendritic cells. Materials Today Bio 2022, 14, 100224. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Gao, W.; Gong, Y.; Guo, P.; Li, W.; Shu, X.; Lü, S.; Zeng, Z.; Zhang, Y.; Long, M. Trail formation alleviates excessive adhesion and maintains efficient neutrophil migration. ACS Appl. Mater. Interfaces 2023, 15, 17577–17591. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, Y.; Si, X.; Yao, H.; Ma, S.; Xu, Y.; Zhao, J.; Ma, C.; He, C.; Tang, Z. Biopolymer immune implants’ sequential activation of innate and adaptive immunity for colorectal cancer postoperative immunotherapy. Adv. Mater. 2021, 33, 2004559. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Han, J.; Liu, Y.; Bo, Y.; Wang, H. T cell-responsive macroporous hydrogels for in situ T cell expansion and enhanced antitumor efficacy. Biomaterials 2023, 293, 121972. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-T.; Cho, S.M.; Youn, D.H.; Hong, E.P.; Park, C.H.; Lee, Y.; Jung, H.; Jeon, J.P. Therapeutic effect of a hydrogel-based neural stem cell delivery sheet for mild traumatic brain injury. Acta Biomater. 2023, 167, 335–347. [Google Scholar] [CrossRef]
- Pazhouhnia, Z.; Beheshtizadeh, N.; Namini, M.S.; Lotfibakhshaiesh, N. Portable hand-held bioprinters promote in situ tissue regeneration.Bioeng. Transl. Med. 2022, 7, e10307. [Google Scholar]
- Bakhshandeh, B.; Ranjbar, N.; Abbasi, A.; Amiri, E.; Abedi, A.; Mehrabi, M.R.; Dehghani, Z.; Pennisi, C.P. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng. Transl. Med. 2023, 8, e10383. [Google Scholar] [CrossRef]
- Yang, R.; Xue, W.; Ma, X.; Ren, Y.; Xu, L.; Kong, W.; Zhang, W.; Wang, P.; Tan, X.; Chi, B. Engineering the dynamics of biophysical cues in supramolecular hydrogels to facile control stem cell chondrogenesis for cartilage regeneration. Compos. Part. B 2023, 250, 110429. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Gao, Y.; Xu, Z.; Dai, C.; Li, G.; Sun, C.; Yang, Y.; Zhang, K. Conductive biocomposite hydrogels with multiple biophysical cues regulate schwann cell behaviors. J. Mater. Chem. B 2022, 10, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and assessment of biodegradable macroporous cryogels as advanced tissue engineering and drug carrying materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, F.; Tang, Y.; Wang, J.; Chen, X.; Li, X.; Zhang, X. Regulation of macrophage polarization and functional status by modulating hydroxyapatite ceramic micro/nano-topography. Mater. Des. 2022, 213, 110302. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, Y.; Zhang, J.; Wang, Y.; Zhang, R.; Yang, L. Versatile hydrogel dressings that dynamically regulate the healing of infected deep burn wounds. Adv. Healthc. Mater. 2023, 12, 2301224. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Q.; Shi, C.; Chen, M.; Ma, K.; Wan, J.; Liu, R. Dealing with the foreign-body response to implanted biomaterials: Strategies and applications of new materials. Adv. Funct. Mater. 2021, 31, 2007226. [Google Scholar] [CrossRef]
- Yu, J.; Lin, Y.; Wang, G.; Song, J.; Hayat, U.; Liu, C.; Raza, A.; Huang, X.; Lin, H.; Wang, J.-Y. Zein-induced immune response and modulation by size, pore structure and drug-loading: Application for sciatic nerve regeneration. Acta Biomater. 2022, 140, 289–301. [Google Scholar] [CrossRef]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-specific macrophages: How they develop and choreograph tissue biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef]
- Mao, J.; Chen, L.; Cai, Z.; Qian, S.; Liu, Z.; Zhao, B.; Zhang, Y.; Sun, X.; Cui, W. Advanced biomaterials for regulating polarization of macrophages in wound healing. Adv. Funct. Mater. 2022, 32, 2111003. [Google Scholar] [CrossRef]
- Yin, Y.; He, X.-T.; Wang, J.; Wu, R.-X.; Xu, X.-Y.; Hong, Y.-L.; Tian, B.-M.; Chen, F.-M. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today 2020, 18, 100466. [Google Scholar] [CrossRef]
- Li, W.; Dai, F.; Zhang, S.; Xu, F.; Xu, Z.; Liao, S.; Zeng, L.; Song, L.; Ai, F. Pore size of 3D-printed polycaprolactone/polyethylene glycol/hydroxyapatite scaffolds affects bone regeneration by modulating macrophage polarization and the foreign body response. ACS Appl. Mater. Interfaces 2022, 14, 20693–20707. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Iwaki, R.; Reinhardt, J.W.; Chang, Y.-C.; Miyamoto, S.; Kelly, J.; Zbinden, J.; Blum, K.; Mirhaidari, G.; Ulziibayar, A. The effect of pore diameter on neo-tissue formation in electrospun biodegradable tissue-engineered arterial grafts in a large animal model. Acta Biomater. 2020, 115, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Hao, D.; Barrera, J.; Henn, D.; Lin, S.; Moeinzadeh, S.; Kim, S.; Maloney, W.; Gurtner, G.; Wang, A. A bioactive compliant vascular graft modulates macrophage polarization and maintains patency with robust vascular remodeling. Bioact. Mater. 2023, 19, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Moeller, J.; Vogel, V. Mechanobiology of macrophages: How physical factors coregulate macrophage plasticity and phagocytosis. Annu. Rev. Biomed. Eng. 2019, 21, 267–297. [Google Scholar] [CrossRef]
- Liu, Y.; Suarez-Arnedo, A.; Riley, L.; Miley, T.; Xia, J.; Segura, T. Spatial confinement modulates macrophage response in microporous annealed particle (MAP) scaffolds. Adv. Healthc. Mater. 2023, 12, 2300823. [Google Scholar] [CrossRef]
- Jain, N.; Vogel, V. Spatial confinement downsizes the inflammatory response of macrophages. Nat. Mater. 2018, 17, 1134–1144. [Google Scholar] [CrossRef]














| Strategies | Hydrogel | Pore Size (μm) | Gelation Mechanisms | Cell Type | Cellular Response | Refs. |
|---|---|---|---|---|---|---|
| Ice templating | Gelatin/PA | 26–155 | covalent interactions | Macrophages | Macrophages within smaller and softer pores exhibit proinflammatory phenotype, whereas anti-inflammatory phenotype is induced by larger and stiffer pores. | [33,38,40] |
| Pickering emulsions templating | GelMA | 50–150 | covalent interactions | BMSCs | Macroporous hydrogel speeds up stem cell migration to bone defects, promoting osteogenic differentiation and bone regeneration. | [48,51] |
| Microgel templating | Gelatin/GHS | 10–100 | covalent interactions | Osteoblast-like Saos-2 cells | A higher ratio of microgel-matrix would result in higher metabolic activity and a faster proliferation rate. | [54,57] |
| Phase separation | PEG and high viscous polysaccharides | 0.5–50 | noncovalent interactions | DRGs | The macroporous gels supported axonal growth in a rat sciatic nerve injury model. | [63] |
| Salt templating | HEMA copolymerized with EOEMA | 185–485 | covalent interactions | Osteoblast-like MG63 cells | The growth and survival of MG63 cells are mainly influenced by the higher elasticity of HEMA/EOEMA hydrogels and lack of positive charge, with pore size having a minimal impact. | [66] |
| Gas foaming | Gelatin | 5–30 | covalent interactions | L929 | Macro-porous hydrogel can promote cell vitality and proliferation. | [69] |
| 3D printing technique | GelMA | 800–1200 | covalent interactions | - | - | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, H.; Guo, Y.; Sun, X.; Hu, Z.; Teng, L.; Zeng, Z. Porous Hydrogels for Immunomodulatory Applications. Int. J. Mol. Sci. 2024, 25, 5152. https://doi.org/10.3390/ijms25105152
Wu C, Zhang H, Guo Y, Sun X, Hu Z, Teng L, Zeng Z. Porous Hydrogels for Immunomodulatory Applications. International Journal of Molecular Sciences. 2024; 25(10):5152. https://doi.org/10.3390/ijms25105152
Chicago/Turabian StyleWu, Cuifang, Honghong Zhang, Yangyang Guo, Xiaomin Sun, Zuquan Hu, Lijing Teng, and Zhu Zeng. 2024. "Porous Hydrogels for Immunomodulatory Applications" International Journal of Molecular Sciences 25, no. 10: 5152. https://doi.org/10.3390/ijms25105152






