Lemon Flavonoid Extract Eriomin Improves Pro/Antioxidant Status and Interferes with Cholesterol Metabolism without Affecting Serum Cholesterol Levels in Aged Rats
Abstract
:1. Introduction
2. Results
2.1. Effects of Lemon Extract on Oxidative Stress Parameters
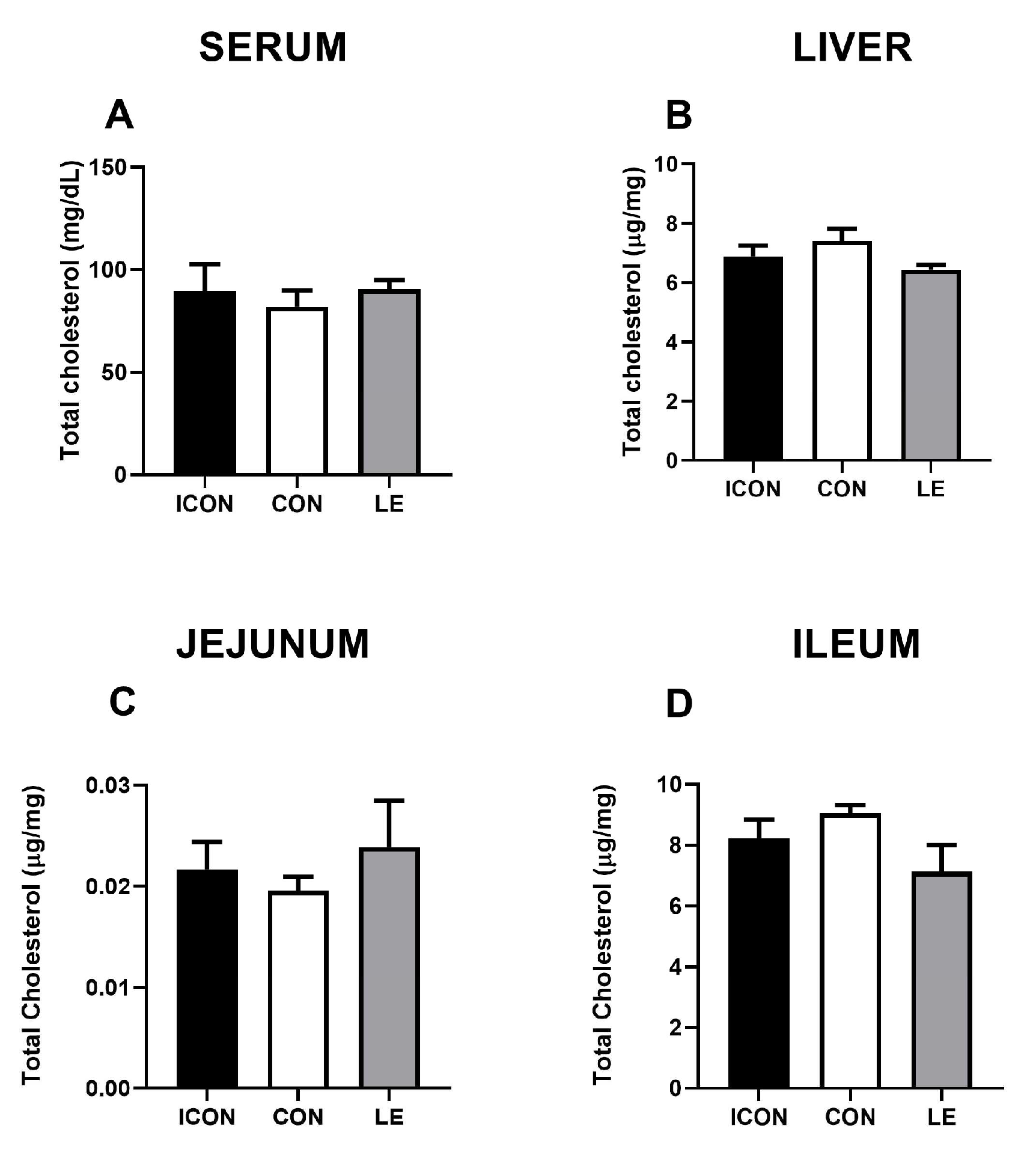
2.2. Effects of Lemon Extract on the Parameters of Cholesterol Metabolism
2.3. Liver Histology, Gene, and Immunohistochemical Expression of CYP7A1
2.4. In Silico Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Experiments and Study Design
4.2. Sample Collection
4.3. Quantification of Oxidative Stress Parameters in the Liver, Jejunum, and Ileum
4.4. Quantification of Sterols and Oxysterols in the Serum, Liver, Jejunum, and Ileum
4.5. Gene Expression Analyses of Cyp7a1 in the Liver
4.6. Liver Histology and Immunohistochemical Analysis of CYP71
4.7. In Silico Analysis
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and Regulation of Cholesterol Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Félix-Redondo, F.J.; Grau, M.; Fernández-Bergés, D. Cholesterol and Cardiovascular Disease in the Elderly. Facts and Gaps. Aging Dis. 2013, 4, 154. [Google Scholar] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of Chronic Liver Diseases in the USA in the Past Three Decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus Flavanones: What Is Their Role in Cardiovascular Protection? J. Agric. Food Chem. 2012, 60, 8809–8822. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Macarro, M.; Martínez Rodríguez, J.P.; Bernal Morell, E.; Pérez-Piñero, S.; Victoria-Montesinos, D.; García-Muñoz, A.M.; Cánovas García, F.; Castillo Sánchez, J.; López-Román, F.J. Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients 2020, 12, 1475. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Milenkovic, D.; Cesar, T.; Filipović, M.R.; Milošević, V. Lemon Extract Reduces the Hepatic Oxidative Stress and Persulfidation Levels by Upregulating the Nrf2 and Trx1 Expression in Old Rats. Biofactors 2024. [Google Scholar] [CrossRef]
- Hunt, N.J.; Kang, S.W.S.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Šošić-Jurjević, B.; Lütjohann, D.; Trifunović, S.; Pavlović, S.; Borković Mitić, S.; Jovanović, L.; Ristić, N.; Marina, L.; Ajdžanović, V.; Filipović, B. Differences in Cholesterol Metabolism, Hepato-Intestinal Aging, and Hepatic Endocrine Milieu in Rats as Affected by the Sex and Age. Int. J. Mol. Sci. 2023, 24, 12624. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress-Induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1–22. [Google Scholar] [CrossRef]
- Szabo, L.; Molnar, R.; Tomesz, A.; Deutsch, A.; Darago, R.; Varjas, T.; Ritter, Z.; Szentpeteri, J.L.; Andreidesz, K.; Mathe, D.; et al. Olive Oil Improves While Trans Fatty Acids Further Aggravate the Hypomethylation of LINE-1 Retrotransposon DNA in an Environmental Carcinogen Model. Nutrients 2022, 14, 908. [Google Scholar] [CrossRef]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, W.; Bashir, M.; Farrukh Nisar, M.; Wan, C. Eriocitrin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin Ameliorates Diet-Induced Hepatic Steatosis with Activation of Mitochondrial Biogenesis. Sci. Rep. 2014, 4, 3708. [Google Scholar] [CrossRef]
- Duan, L.P.; Wang, H.H.; Wang, D.Q.H. Cholesterol Absorption Is Mainly Regulated by the Jejunal and Ileal ATP-Binding Cassette Sterol Efflux Transporters Abcg5 and Abcg8 in Mice. J. Lipid Res. 2004, 45, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Mulas, M.F.; Demuro, G.; Mulas, C.; Putzolu, M.; Cavallini, G.; Donati, A.; Bergamini, E.; Dessi, S. Dietary Restriction Counteracts Age-Related Changes in Cholesterol Metabolism in the Rat. Mech. Ageing Dev. 2005, 126, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Gälman, C.; Matasconi, M.; Persson, L.; Parini, P.; Angelin, B.; Rudling, M. Age-Induced Hypercholesterolemia in the Rat Relates to Reduced Elimination but Not Increased Intestinal Absorption of Cholesterol. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.D.; Csanaky, I.L.; Klaassen, C.D. Gender-Divergent Profile of Bile Acid Homeostasis during Aging of Mice. PLoS ONE 2012, 7, e32551. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.P.; Wang, H.H.; Ohashi, A.; Wang, D.Q.H. Role of Intestinal Sterol Transporters Abcg5, Abcg8, and Npc111 in Cholesterol Absorption in Mice: Gender and Age Effects. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, 269–276. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Xu, S.; Ishfaq, M.; Zhang, X. NF-E2-Related Factor 2 Deletion Facilitates Hepatic Fatty Acids Metabolism Disorder Induced by High-Fat Diet via Regulating Related Genes in Mice. Food Chem. Toxicol. 2016, 94, 186–196. [Google Scholar] [CrossRef]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Menassa, M.; Stronks, K.; Khatmi, F.; Roa Díaz, Z.M.; Espinola, O.P.; Gamba, M.; Itodo, O.A.; Buttia, C.; Wehrli, F.; Minder, B.; et al. Concepts and Definitions of Healthy Ageing: A Systematic Review and Synthesis of Theoretical Models. eClinicalMedicine 2023, 56, 101821. [Google Scholar] [CrossRef] [PubMed]
- López-Almada, G.; Domínguez-Avila, J.A.; Mejía-León, M.E.; Robles-Sánchez, M.; González-Aguilar, G.A.; Salazar-López, N.J. Could Naringenin Participate as a Regulator of Obesity and Satiety? Molecules 2023, 28, 1450. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Z.; Qi, X.; Cui, J.; Zhou, Y.; Tan, Y.; Huang, X.; Ye, H. Naringin Ameliorates Obesity via Stimulating Adipose Thermogenesis and Browning, and Modulating Gut Microbiota in Diet-Induced Obese Mice. Curr. Res. Food Sci. 2024, 8, 100683. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.S.; Manthey, J.A.; Nery, M.S.; Spolidorio, L.C.; Cesar, T.B. Low Doses of Eriocitrin Attenuate Metabolic Impairment of Glucose and Lipids in Ongoing Obesogenic Diet in Mice. J. Nutr. Sci. 2020, 9, e59. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, Y.; Hiramitsu, M.; Okada, M.; Hayashi, S.; Nabeno, Y.; Osawa, T.; Naito, M. Lemon Polyphenols Suppress Diet-Induced Obesity by Up-Regulation of MRNA Levels of the Enzymes Involved in β-Oxidation in Mouse White Adipose Tissue. J. Clin. Biochem. Nutr. 2008, 43, 201–209. [Google Scholar] [CrossRef]
- Go, R.E.; Hwang, K.A.; Kim, Y.S.; Kim, S.H.; Nam, K.H.; Choi, K.C. Effects of Palm and Sunflower Oils on Serum Cholesterol and Fatty Liver in Rats. J. Med. Food 2015, 18, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O. Oilseed Crop Sunflower (Helianthus Annuus) as a Source of Food: Nutritional and Health Benefits. Food Sci. Nutr. 2020, 8, 4666. [Google Scholar] [CrossRef]
- Walczewska, A.; Dziedzic, B.; Stepien, T.; Swiatek, E.; Nowak, D. Effect of Dietary Fats on Oxidative-Antioxidative Status of Blood in Rats. J. Clin. Biochem. Nutr. 2010, 47, 18–26. [Google Scholar] [CrossRef]
- Lawrence, G.D. Perspective: The Saturated Fat-Unsaturated Oil Dilemma: Relations of Dietary Fatty Acids and Serum Cholesterol, Atherosclerosis, Inflammation, Cancer, and All-Cause Mortality. Adv. Nutr. 2021, 12, 647–656. [Google Scholar] [CrossRef]
- Valastyan, S.; Thakur, V.; Johnson, A.; Kumar, K.; Manor, D. Novel Transcriptional Activities of Vitamin E: Inhibition of Cholesterol Biosynthesis. Biochemistry 2024, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Terlecky, S.R.; Koepke, J.I.; Walton, P.A. Peroxisomes and Aging. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Le Xu, X. Naringenin Alleviates Nonalcoholic Steatohepatitis in Middle-Aged Apoe/Mice: Role of SIRT1. Phytomedicine 2020, 81, 153412. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Fecka, I.; Cisowski, W. Antiradical and Anti-H2O2 Properties of Polyphenolic Compounds from an Aqueous Peppermint Extract. Z. Naturforsch. C. 2005, 60, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, Y.; Shen, S.; Zhi, Z.; Cheng, H.; Chen, S.; Ye, X. Antioxidant and Pancreatic Lipase Inhibitory Effects of Flavonoids from Different Citrus Peel Extracts: An in Vitro Study. Food Chem. 2020, 326, 126785. [Google Scholar] [CrossRef] [PubMed]
- Ačimovič, J.; Goyal, S.; Košir, R.; Goličnik, M.; Perše, M.; Belič, A.; Urlep, Ž.; Guengerich, F.P.; Rozman, D. Cytochrome P450 Metabolism of the Post-Lanosterol Intermediates Explains Enigmas of Cholesterol Synthesis. Sci. Rep. 2016, 6, 28462. [Google Scholar] [CrossRef] [PubMed]
- Heverin, M.; Ali, Z.; Olin, M.; Tillander, V.; Joibari, M.M.; Makoveichuk, E.; Leitersdorf, E.; Warner, M.; Olivercrona, G.; Gustafsson, J.Å.; et al. On the Regulatory Importance of 27-Hydroxycholesterol in Mouse Liver. J. Steroid Biochem. Mol. Biol. 2017, 169, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid Peroxidation Biomarkers for Evaluating Oxidative Stress and Assessing Antioxidant Capacity in Vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef]
- Iuliano, L. Pathways of Cholesterol Oxidation via Non-Enzymatic Mechanisms. Chem. Phys. Lipids 2011, 164, 457–468. [Google Scholar] [CrossRef]
- Zmysłowski, A.; Szterk, A. Oxysterols as a Biomarker in Diseases. Clin. Chim. Acta 2019, 491, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Meaney, S.; Lütjohann, D.; Diczfalusy, U.; Björkhem, I. Formation of Oxysterols from Different Pools of Cholesterol as Studied by Stable Isotope Technique: Cerebral Origin of Most Circulating 24S-Hydroxycholesterol in Rats, but Not in Mice. Biochim. Biophys. Acta 2000, 1486, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D.S. Preclinical Evidence for the Pharmacological Actions of Naringin: A Review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- van Iersel, L.E.; Stevens, Y.R.; Conchillo, J.M.; Troost, F.J. The Effect of Citrus Flavonoid Extract Supplementation on Anaerobic Capacity in Moderately Trained Athletes: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D.M.; Hicks, B.J.; Reed, A.W. Phenolic content of olive oil is reduced in extraction and refining. Asia Pac. J. Clin. Nutr. 1996, 5, 105–107. [Google Scholar] [PubMed]
- Cueva, C.; Silva, M.; Pinillos, I.; Bartolomé, B.; Moreno-Arribas, M.V. Interplay between Dietary Polyphenols and Oral and Gut Microbiota in the Development of Colorectal Cancer. Nutrients 2020, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Abiaka, C.; Al-Awadi, F.; Olusi, S. Effect of Prolonged Storage on the Activities of Superoxide Dismutase, Glutathione Reductase, and Glutathione Peroxidase. Clin. Chem. 2000, 46, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Tamura, M.; Oshino, N.; Chance, B. Some Characteristics of Hydrogen- and Alkylhydroperoxides Metabolizing Systems in Cardiac Tissue. J. Biochem. 1982, 92, 1019–1031. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Glatzle, D.; Vuilleumier, J.P.; Weber, F.; Decker, K. Glutathione Reductase Test with Whole Blood, a Convenient Procedure for the Assessment of the Riboflavin Status in Humans. Experientia 1974, 30, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rehncrona, S.; Smith, D.S.; Åkesson, B.; Westerberg, E.; Siesjö, B.K. Peroxidative Changes in Brain Cortical Fatty Acids and Phospholipids, as Characterized during Fe2+- and Ascorbic Acid-Stimulated Lipid Peroxidation in Vitro. J. Neurochem. 1980, 34, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-Dinitrophenylhydrazine Spectrophotometric Assay for Quantification of Carbonyls in Oxidized Proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid. Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Zar, J.H. Statistical Procedures for Biological Research; Prentice Hall: Upper On River City, NJ, USA, 1974; Volume 5, p. 620. [Google Scholar]



| ICON | CON | LE | p-Value (CON vs. ICON) | p-Value (CON vs. LE) | |
|---|---|---|---|---|---|
| Initial body weight (g) | 450 ± 16 | 467 ± 8 | 456 ± 9 | 0.902 | 0.950 |
| Weight gain (g) | −12.0 ± 3 | −16.9 ± 2 | −15.0 ± 2 | 0.841 | 0.987 |
| Food intake (g) | 17.8 ± 1.0 | 18.1 ± 1.1 | 17.5 ± 1.2 | 0.834 | 0.739 |
| Absolute liver weight (g) | 13 ± 1 | 11 ± 1 | 12 ± 1 | 0.241 | 0.676 |
| Relative liver weight (% b.m.) | 2.7 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 | 0.147 | 0.601 |
| LIVER | |||
|---|---|---|---|
| ICON | CON | LE | |
| SOD | 3349.8 ± 68.6 | 3300.5 ± 230.5 | 3543.7 ± 196.6 |
| CAT | 52,352.5 ± 6942.4 | 48,246.4 ± 8190.7 | 56,875.0 ± 4214.8 |
| GSH-Px | 142,292.6 ± 21,438.2 | 121,866.5 ± 18,844.4 | 144,966.5 ± 15,314.2 |
| GR | 6857.3 ± 401.4 | 3766.9 ± 1486.9 | 5390.3 ± 1330.5 |
| GST | 173,576.6 ± 10,808.7 | 166,185.9 ± 26,910.3 | 169,963.8 ± 11,768.9 |
| GSH | 3854.4 ± 235.0 * | 5959.1 ± 203.4 | 5664.7 ± 406.0 |
| SH | 3681.8 ± 315.6 * | 4600.6 ± 147.4 | 4674.4 ± 211.6 |
| LPO | 11.9 ± 1.4 | 11.9 ± 1.2 | 13.6 ± 0.8 |
| PCO | 3340.7 ± 161.2 | 4271.4 ± 420.0 | 3653.8 ± 266.6 |
| TOS | 34.4 ± 0.6 | 37.8 ± 2.5 | 14.1 ± 0.6 **** |
| TAS | 873.3 ± 95.0 | 1002.1 ± 105.1 | 706.2 ± 56.9 *** |
| OSI | 3.96 ± 0.37 | 3.90 ± 0.55 | 2.03 ± 0.11 *** |
| JEJUNUM | |||
| SOD | 942.5 ± 99.1 | 1585.4 ± 222.6 | 1384.1 ± 349.6 |
| CAT | 302.6 ± 81.2 | 338.4 ± 46.4 | 347.6 ± 89.1 |
| GSH-Px | 2416.4 ± 532.8 | 3668.0 ± 492.5 | 3689.7 ± 370.1 |
| GR | 3376.2 ± 515.0 | 3885.0 ± 285.9 | 3569.1 ± 726.8 |
| GST | 11,935.4 ± 759.6 | 16,866.7 ± 1540.4 | 13,458.3 ± 5636.5 |
| GSH | 615.8 ± 44.5 * | 425 ± 63.0 | 425.8 ± 55.3 |
| SH | 1048.1 ± 149.4 | 1108.5 ± 192.9 | 1325.1 ± 308.3 |
| LPO | 8.8 ± 1.5 ** | 2.3 ± 0.6 | 1.6 ± 0.6 ** |
| PCO | 1304.5 ± 205.8 | 2093.4 ± 647.0 | 1869.3 ± 335.9 |
| TOS | 29.9 ± 1.8 * | 35.7 ± 1.4 | 16.9 ± 1.9 *** |
| TAS | 1695.0 ± 127.4 * | 1333.3 ± 32.8 | 2103.3 ± 49.1 * |
| OSI | 1.16 ± 0.47 * | 2.86 ± 0.20 | 0.66 ± 0.06 *** |
| ILEUM | |||
| SOD | 566.5 ± 17.4 | 715.8 ± 77.6 | 721.2 ± 36.0 |
| CAT | 142.6 ± 40.0 | 127.6 ± 21.6 | 120.9 ± 17.3 |
| GSH-Px | 2866.6 ± 167.9 | 2483.9 ± 192.3 | 2681.7 ± 273.2 |
| GR | 4420.4 ± 636.3 | 5020.9 ± 295.7 | 4336.0 ± 620.6 |
| GST | 5512.5 ± 507.2 | 4422.9 ± 760.3 | 3995.8 ± 226.0 |
| GSH | 295.0 ± 6.2 * | 420.0 ± 71.7 | 404.6 ± 45.9 |
| SH | 1034.6 ± 115.9 | 1000.7 ± 39.1 | 883.7 ± 103.6 |
| LPO | 5.1 ± 0.4 | 5.2 ± 0.4 | 7.5 ± 1.4 |
| PCO | 1609.3 ± 349.5 | 1557.7 ± 284.4 | 1490.5 ± 341.4 |
| TOS | 32.2 ± 3.3 | 37.6 ± 0.7 | 29.9 ± 1.6 * |
| TAS | 1760.0 ± 70.7 *** | 1030.0 ± 13.3 | 1856.7 ± 123.5 ** |
| OSI | 1.872 ± 0.26 ** | 3.653 ± 0.02 | 1.04 ± 0.31 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šošić-Jurjević, B.; Borković-Mitić, S.; Pavlović, S.; Vlahović, D.; Miler, M.; Cesar, T.; Ajdžanović, V.; Milenkovic, D.; Stellaard, F.; Trifunović, S.; et al. Lemon Flavonoid Extract Eriomin Improves Pro/Antioxidant Status and Interferes with Cholesterol Metabolism without Affecting Serum Cholesterol Levels in Aged Rats. Int. J. Mol. Sci. 2024, 25, 5221. https://doi.org/10.3390/ijms25105221
Šošić-Jurjević B, Borković-Mitić S, Pavlović S, Vlahović D, Miler M, Cesar T, Ajdžanović V, Milenkovic D, Stellaard F, Trifunović S, et al. Lemon Flavonoid Extract Eriomin Improves Pro/Antioxidant Status and Interferes with Cholesterol Metabolism without Affecting Serum Cholesterol Levels in Aged Rats. International Journal of Molecular Sciences. 2024; 25(10):5221. https://doi.org/10.3390/ijms25105221
Chicago/Turabian StyleŠošić-Jurjević, Branka, Slavica Borković-Mitić, Slađan Pavlović, Dragana Vlahović, Marko Miler, Thais Cesar, Vladimir Ajdžanović, Dragan Milenkovic, Frans Stellaard, Svetlana Trifunović, and et al. 2024. "Lemon Flavonoid Extract Eriomin Improves Pro/Antioxidant Status and Interferes with Cholesterol Metabolism without Affecting Serum Cholesterol Levels in Aged Rats" International Journal of Molecular Sciences 25, no. 10: 5221. https://doi.org/10.3390/ijms25105221








