Comparison of Synthetic Pathways for Obtaining Fluorescent Nanomaterials Based on Halloysite and Carbon Dots for Potential Biological Sensing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of N-Doped CDs Covalently Linked to HNTs External Surface
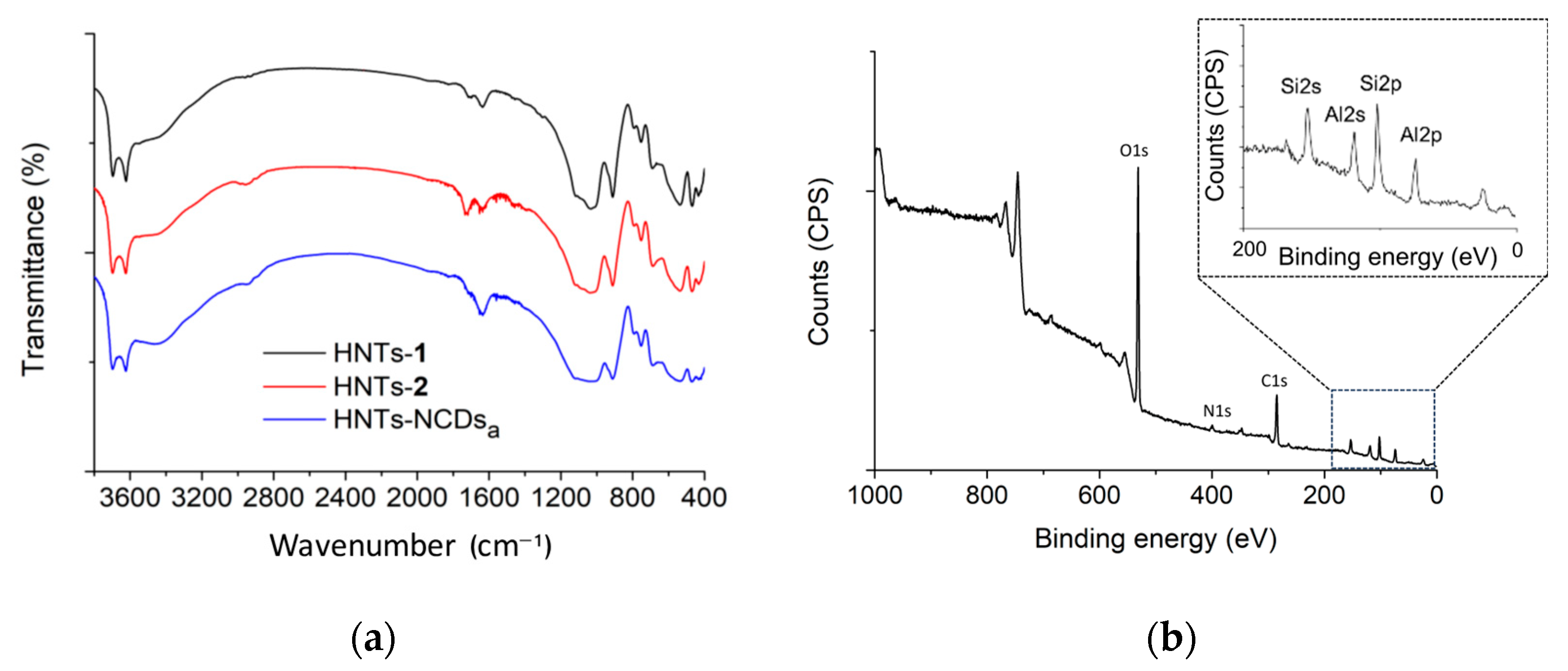
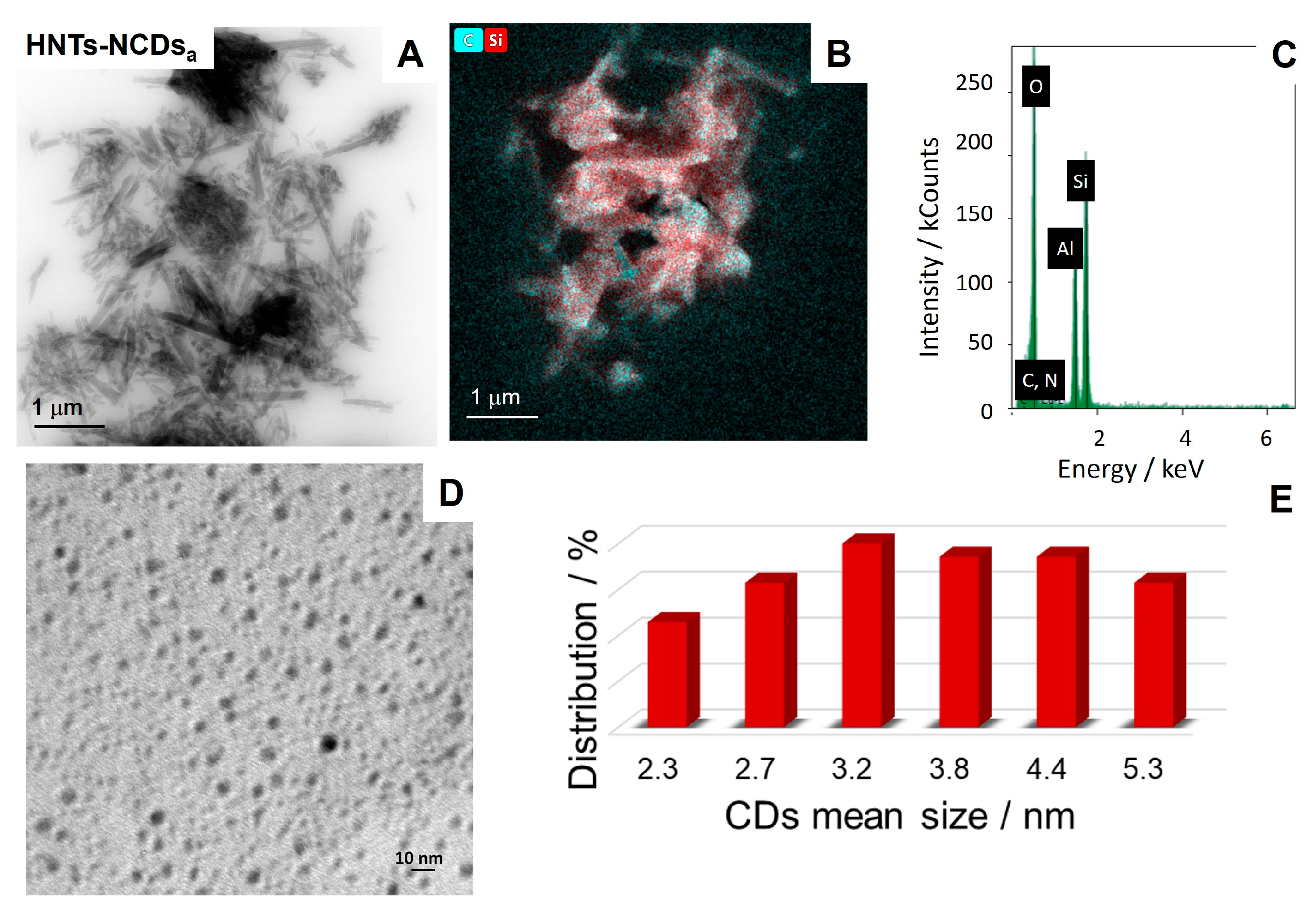
Characterization of HNTs-NCDsa–c Nanomaterials
2.2. Post-Modification of N-Doped CDs by Modified HNTs
2.3. Photoluminescence Properties of HNTs-NCDs
2.4. pH and Solvent Effects on Photoluminescence Properties of HNTs-NCDs Nanomaterials
2.5. Fluorescence Sensing of Fe3+ Ions
2.6. Biological Evaluation of HNTs-NCDs Nanomaterials
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of HNTs-COOH
3.1.2. Synthesis of HNTs-2
3.1.3. Synthesis of HNTs-NCDsa-c
3.1.4. Synthesis of HNTs-NCDs1
3.1.5. Synthesis of HNTs-NCDs2
3.1.6. Fluorescence Titration for Fe(III) Ions Detection
3.2. Cell Cultures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Adair, L.D.; Yang, K.; New, E.J. An Introduction to Small Molecule Fluorescent Sensors. In Molecular Fluorescent Sensors for Cellular Studies; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 1–35. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. 15—Fluorescent metal-organic frameworks for analytical applications. In Hybrid Nanomaterials for Sustainable Applications; Koduru, J.R., Karri, R.R., Mubarak, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 339–374. [Google Scholar] [CrossRef]
- Lin, X.; Xiong, M.; Zhang, J.; He, C.; Ma, X.; Zhang, H.; Kuang, Y.; Yang, M.; Huang, Q. Carbon dots based on natural resources: Synthesis and applications in sensors. Microchem. J. 2021, 160, 105604. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Peng, B.; Zhang, L.; Xie, N.; Yue, D.; Li, W.; Qin, B.; Du, W.; Wang, Z.; et al. Fluorescent carbon dots doped with nitrogen for rapid detection of Fe (III) and preparation of fluorescent films for optoelectronic devices. J. Mol. Struct. 2024, 1304, 137739. [Google Scholar] [CrossRef]
- Sadeghi-chahnasir, F.; Amiripour, F.; Ghasemi, S. Orange peel-derived carbon dots/Cu-MOF nanohybrid for fluorescence determination of l-ascorbic acid and Fe3+. Anal. Chim. Acta 2024, 1287, 342066. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Qi, J.; Miao, Q.; Deng, D.; He, H.; Yan, X.; Luo, L. A paper-based ratiometric fluorescence sensor based on carbon dots modified with Eu3+ for the selective detection of tetracycline in seafood aquaculture water. Analyst 2024, 149, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Ghaemy, M. One-step hydrothermal-assisted synthesis of highly fluorescent N-doped carbon dots from gum tragacanth: Luminescent stability and sensitive probe for Au3+ ions. Opt. Mater. 2019, 97, 109356. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Park, S.-J.; Zhang, Y.; Kim, T.; Chae, S.; Park, M.; Kim, H.-Y. One-step synthesis of robust nitrogen-doped carbon dots: Acid-evoked fluorescence enhancement and their application in Fe3+ detection. J. Mater. Chem. A 2015, 3, 17747–17754. [Google Scholar] [CrossRef]
- Rahmani, Z.; Ghaemy, M.; Olad, A. Removal of heavy metals from polluted water using magnetic adsorbent based on κ-carrageenan and N-doped carbon dots. Hydrometallurgy 2022, 213, 105915. [Google Scholar] [CrossRef]
- Arcudi, F.; Dordevic, L.; Prato, M. Synthesis, separation, and characterization of small and highly fluorescent nitrogen-doped carbon nanodots. Angew. Chem. Int. Ed. 2016, 55, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, B.; Prato, M. The Importance of the Purification Step and the Characterization of the Products in the Synthesis of Carbon Nanodots. Small 2023, 19, 2206714. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, B.; Bogo, A.; Amato, F.; Ragazzon, G.; Prato, M. Nuclear Magnetic Resonance Reveals Molecular Species in Carbon Nanodot Samples Disclosing Flaws. Angew. Chem. Int. Ed. 2022, 61, e202200038. [Google Scholar] [CrossRef]
- Essner, J.B.; Kist, J.A.; Polo-Parada, L.; Baker, G.A. Artifacts and Errors Associated with the Ubiquitous Presence of Fluorescent Impurities in Carbon Nanodots. Chem. Mater. 2018, 30, 1878–1887. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Guernelli, S.; Lazzara, G.; Liu, M.; Nicotra, G.; Noto, R.; Parisi, F.; Pibiri, I.; Spinella, C.; et al. Photoluminescent hybrid nanomaterials from modified halloysite nanotubes. J. Mater. Chem. C 2018, 6, 7377–7384. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Reis, S.; Veiga, F.; Saleh, M.; Lvov, Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Expert Opin. Drug Deliv. 2019, 16, 1169–1182. [Google Scholar] [CrossRef]
- Peixoto, D.; Pereira, I.; Pereira-Silva, M.; Veiga, F.; Hamblin, M.R.; Lvov, Y.; Liu, M.; Paiva-Santos, A.C. Emerging role of nanoclays in cancer research, diagnosis, and therapy. Coord. Chem. Rev. 2021, 440, 213956. [Google Scholar] [CrossRef]
- Falanga, A.P.; Massaro, M.; Borbone, N.; Notarbartolo, M.; Piccialli, G.; Liotta, L.F.; Sanchez-Espejo, R.; Viseras Iborra, C.; Raymo, F.M.; Oliviero, G.; et al. Carrier capability of halloysite nanotubes for the intracellular delivery of antisense PNA targeting mRNA of neuroglobin gene. J. Colloid Interface Sci. 2024, 663, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Laura Alfieri, M.; Rizzo, G.; Babudri, F.; Barbosa de Melo, R.; Faddetta, T.; Gallo, G.; Napolitano, A.; Sanchèz-Espejo, R.; Viseras Iborra, C.; et al. Modification of halloysite lumen with dopamine derivatives as filler for antibiofilm coating. J. Colloid Interface Sci. 2023, 646, 910–921. [Google Scholar] [CrossRef]
- Wong, L.W.; Tan, J.B.L. Halloysite nanotube-polymer nanocomposites: A review on fabrication and biomedical applications. J. Manuf. Process. 2024, 118, 76–88. [Google Scholar] [CrossRef]
- Boraei, S.B.A.; Eshghabadi, F.; Hosseinpour, R.; Zare, Y.; Munir, M.T.; Rhee, K.Y. Halloysite nanotubes in biomedical applications: Recent approaches and future trends. Appl. Clay Sci. 2024, 253, 107346. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, S.; Liu, J.; Sun, X. Recent Advances of Halloysite Nanotubes in Biomedical Applications. Small 2024, 20, 2306169. [Google Scholar] [CrossRef] [PubMed]
- Rozhina, E.; Panchal, A.; Akhatova, F.; Lvov, Y.; Fakhrullin, R. Cytocompatibility and cellular uptake of alkylsilane-modified hydrophobic halloysite nanotubes. Appl. Clay Sci. 2020, 185, 105371. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Fakhrullina, G.; Nigamatzyanova, L.; Sitmukhanova, E.; Khusnetdenova, E.; Fakhrullin, R.; Vinokurov, V. Biodistribution of Quantum Dots-Labelled Halloysite Nanotubes: A Caenorhabditis elegans In Vivo Study. Materials 2021, 14, 5469. [Google Scholar] [CrossRef] [PubMed]
- Stavitskaya, A.; Rubtsova, M.; Glotov, A.; Vinokurov, V.; Vutolkina, A.; Fakhrullin, R.; Lvov, Y. Architectural design of core–shell nanotube systems based on aluminosilicate clay. Nanoscale Adv. 2022, 4, 2823–2835. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Gong, W.; Li, C.; Deng, Z.; Jin, Y.; Meng, X. Sustained antioxidant properties of epigallocatechin gallate loaded halloysite for PLA as potentially durable materials. J. Appl. Polym. Sci. 2023, 140, e53411. [Google Scholar] [CrossRef]
- Riela, S.; Borrego-Sánchez, A.; Cauteruccio, S.; de Melo Barbosa, R.; Massaro, M.; Sainz-Díaz, C.I.; Sánchez-Espejo, R.; Viseras-Iborra, C.; Licandro, E. Exploiting the interaction between halloysite and charged PNAs for their controlled release. J. Mater. Chem. B 2023, 11, 6685–6696. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, J.; Wu, Y.-P.; Yang, X.; Kuang, X.-P.; Li, W.-X.; Li, Y.-F.; He, R.-R.; Liu, M. Multifunctional HNT@Fe3O4@PPy@DOX Nanoplatform for Effective Chemo-Photothermal Combination Therapy of Breast Cancer with MR Imaging. ACS Biomater. Sci. Eng. 2020, 6, 3361–3374. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Machowska, A.; Kasprzyk, M.; Ledwójcik, G. Application of Halloysite Nanotubes in Cancer Therapy—A Review. Materials 2021, 14, 2943. [Google Scholar] [CrossRef]
- Long, Z.; Wu, Y.-P.; Gao, H.-Y.; Li, Y.-F.; He, R.-R.; Liu, M. Functionalization of Halloysite Nanotubes via Grafting of Dendrimer for Efficient Intracellular Delivery of siRNA. Bioconj. Chem. 2018, 29, 2606–2618. [Google Scholar] [CrossRef]
- Massaro, M.; Notarbartolo, M.; Raymo, F.M.; Cavallaro, G.; Lazzara, G.; Mazza, M.M.A.; Viseras-Iborra, C.; Riela, S. Supramolecular Association of Halochromic Switches and Halloysite Nanotubes in Fluorescent Nanoprobes for Tumor Detection. ACS Appl. Nano Mater. 2022, 5, 13729–13736. [Google Scholar] [CrossRef]
- Ramanan, V.; Subray, S.H.; Ramamurthy, P. A green synthesis of highly luminescent carbon dots from itaconic acid and their application as an efficient sensor for Fe3+ ions in aqueous medium. New J. Chem. 2018, 42, 8933–8942. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The Role of Iron in Cancer Progression. Front. Oncol. 2021, 11, 778492. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Choong, C.-J.; Baba, K. Parkinson’s disease and iron. J. Neural Transm. 2020, 127, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; DiGiacomo, P.; Born, D.E.; Georgiadis, M.; Zeineh, M. Iron and Alzheimer’s Disease: From Pathology to Imaging. Front. Hum. Neurosci. 2022, 16, 838692. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, C.; Zhang, X.; Zhou, H.; Li, H.; Zhu, X.; Wang, Y. A novel molecularly imprinted material based on magnetic halloysite nanotubes for rapid enrichment of 2,4-dichlorophenoxyacetic acid in water. J. Hazard. Mater. 2014, 276, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes as nanoreactors for heterogeneous micellar catalysis. J. Colloid Interface Sci. 2022, 608, 424–434. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Wang, J.; Zheng, X.; Chang, D.; Wang, S.; Jiang, T. A novel nanogel delivery of poly-α, β-polyasparthydrazide by reverse microemulsion and its redox-responsive release of 5-Fluorouridine. Asian J. Pharm. Sci. 2016, 11, 735–743. [Google Scholar] [CrossRef]
- Tang, X.-D.; Yu, H.-M.; Nguyen, W.; Amador, E.; Cui, S.-P.; Ma, K.; Chen, M.-L.; Wang, S.-Y.; Hu, Z.-Z.; Chen, W. New Observations on Concentration-Regulated Carbon Dots. Adv. Photonics Res. 2023, 4, 2200314. [Google Scholar] [CrossRef]
- Sawalha, S.; Assali, M.; Raddad, M.; Ghneem, T.; Sawalhi, T.; Almasri, M.; Zarour, A.; Misia, G.; Prato, M.; Silvestri, A. Broad-Spectrum Antibacterial Activity of Synthesized Carbon Nanodots from d-Glucose. ACS Appl. Bio Mater. 2022, 5, 4860–4872. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.-H.; Chen, X.-B.; Wei, J.-S.; Li, X.-B.; Xiong, H.-M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 127, 231101. [Google Scholar] [CrossRef]
- Campisciano, V.; Burger, R.; Calabrese, C.; Liotta, L.F.; Lo Meo, P.; Gruttadauria, M.; Giacalone, F. Straightforward preparation of highly loaded MWCNT–polyamine hybrids and their application in catalysis. Nanoscale Adv. 2020, 2, 4199–4211. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Saladino, M.L.; Chillura Martino, D.; Lo Meo, P.; Noto, R. Polyaminocyclodextrin nanosponges: Synthesis, characterization and pH-responsive sequestration abilities. RSC Adv. 2016, 6, 49941–49953. [Google Scholar] [CrossRef]
- Filippini, G.; Amato, F.; Rosso, C.; Ragazzon, G.; Vega-Peñaloza, A.; Companyó, X.; Dell’Amico, L.; Bonchio, M.; Prato, M. Mapping the Surface Groups of Amine-Rich Carbon Dots Enables Covalent Catalysis in Aqueous Media. Chem 2020, 6, 3022–3037. [Google Scholar] [CrossRef]
- Ullal, N.; Muthamma, K.; Sunil, D. Carbon dots from eco-friendly precursors for optical sensing application: An up-to-date review. Chem. Pap. 2022, 76, 6097–6127. [Google Scholar] [CrossRef]
- Massaro, M.; Poma, P.; Cavallaro, G.; García-Villén, F.; Lazzara, G.; Notarbartolo, M.; Muratore, N.; Sánchez-Espejo, R.; Iborra, C.V.; Riela, S. Prodrug based on halloysite delivery systems to improve the antitumor ability of methotrexate in leukemia cell lines. Colloids Surf. B Biointerfaces 2022, 213, 112385. [Google Scholar] [CrossRef]











| Nanomaterial | Apparent Hydrodynamic Diameter (nm) | –Potential (mV) |
|---|---|---|
| HNTs | 295 ± 16 | −18.0 ± 1.0 |
| HNTs-2 | 686 ± 57 | −25.2 ± 1.0 |
| HNTs-NCDsa | 523 ± 35 | −25.7 ± 1.9 |
| HNTs-NCDsb | 433 ± 64 | −18.2 ± 1.7 |
| HNTs-NCDsc | 450 ± 55 | −23.3 ± 1.0 |
| Entry | Nanomaterial | FLQY (%) |
|---|---|---|
| 1 | N-doped CDs | 26.0 |
| 2 | HNTs-NCDsa | 24.0 |
| 3 | HNTs-NCDsb | 11.0 |
| 4 | HNTs-NCDsc | 8.0 |
| 5 | HNTs-NCDs1 | 3.0 |
| 6 | HNTs-NCDs2 | 3.0 |
| Entry | Sample | pH a | pKa b | na/g (mmol g−1) | c | nb1/g (mmol g−1) | c | nb2/g (mmol g−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | HNTs-NCDsa | 8.2 | 4.2 | 2.6 | 7.3 | 2.4 | 10.3 | 1.1 |
| 2 | N-doped CDs | 8.6 | 4.2 | 4.8 | 8.2 | 4.3 | / | / |
| 3 | HNTs-NCDs1 | 4.8 | 3.8 | 3.6 | 9.9 | 9.5 | / | / |
| 4 | HNTs-COOH | 4.2 | 3.5 | 3.4 | 9.0 | 9.6 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, M.; Cinà, G.; Cavallaro, G.; Lazzara, G.; Silvestri, A.; Barbosa, R.d.M.; Sànchez-Espejo, R.; Viseras-Iborra, C.; Notarbartolo, M.; Riela, S. Comparison of Synthetic Pathways for Obtaining Fluorescent Nanomaterials Based on Halloysite and Carbon Dots for Potential Biological Sensing. Int. J. Mol. Sci. 2024, 25, 5370. https://doi.org/10.3390/ijms25105370
Massaro M, Cinà G, Cavallaro G, Lazzara G, Silvestri A, Barbosa RdM, Sànchez-Espejo R, Viseras-Iborra C, Notarbartolo M, Riela S. Comparison of Synthetic Pathways for Obtaining Fluorescent Nanomaterials Based on Halloysite and Carbon Dots for Potential Biological Sensing. International Journal of Molecular Sciences. 2024; 25(10):5370. https://doi.org/10.3390/ijms25105370
Chicago/Turabian StyleMassaro, Marina, Giuseppe Cinà, Giuseppe Cavallaro, Giuseppe Lazzara, Alessandro Silvestri, Raquel de Melo Barbosa, Rita Sànchez-Espejo, César Viseras-Iborra, Monica Notarbartolo, and Serena Riela. 2024. "Comparison of Synthetic Pathways for Obtaining Fluorescent Nanomaterials Based on Halloysite and Carbon Dots for Potential Biological Sensing" International Journal of Molecular Sciences 25, no. 10: 5370. https://doi.org/10.3390/ijms25105370









