Chemiresistors Based on Hybrid Nanostructures Obtained from Graphene and Conducting Polymers with Potential Use in Breath Methane Detection Associated with Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Technology for Obtaining Graphene on Copper Substrate
2.1. Materials and Preparation Methods
2.2. Characterization Equipment
2.3. Results and Discussion
2.3.1. SEM Analysis
2.3.2. AFM Analysis
3. Technology for Obtaining Hybrid Nanostructures from Graphene and Conducting Polymers
3.1. Materials and Preparation Methods
3.2. Results and Discussion
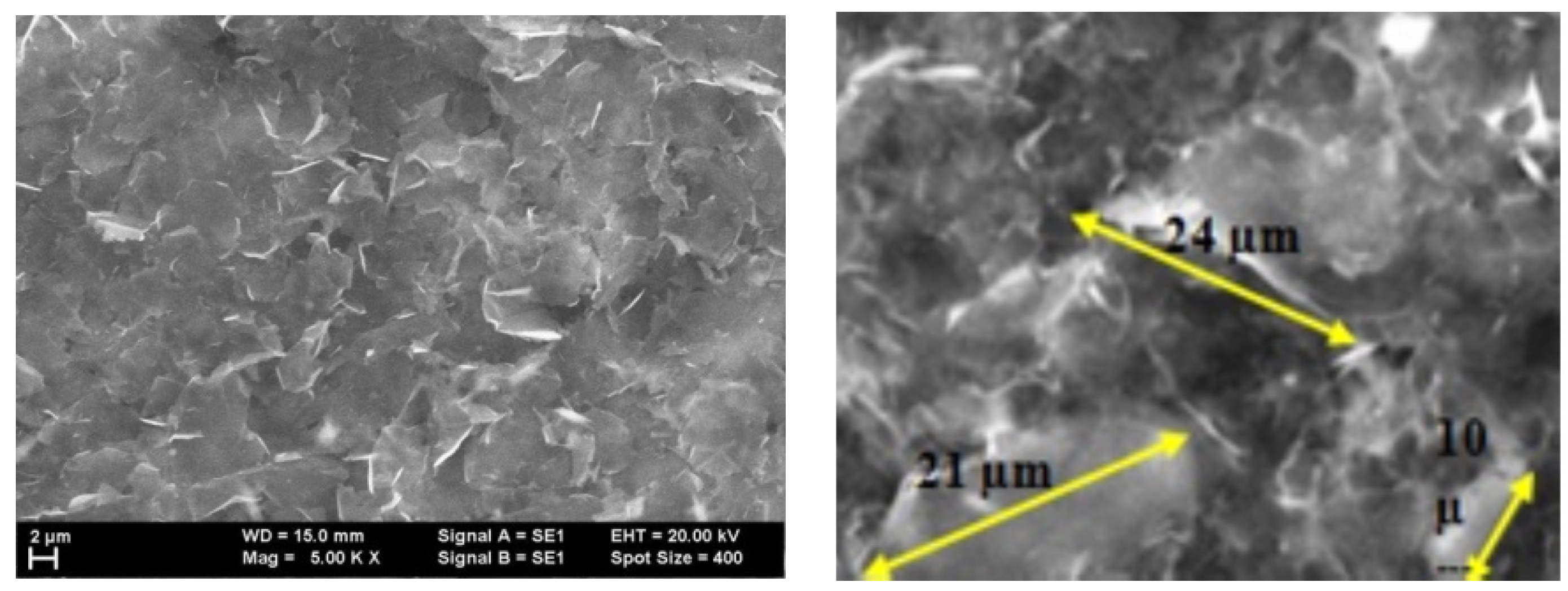
3.2.1. Hybrid Nanostructures from Graphene and P3HT
3.2.2. Hybrid Nanostructures from Graphene and F8T2
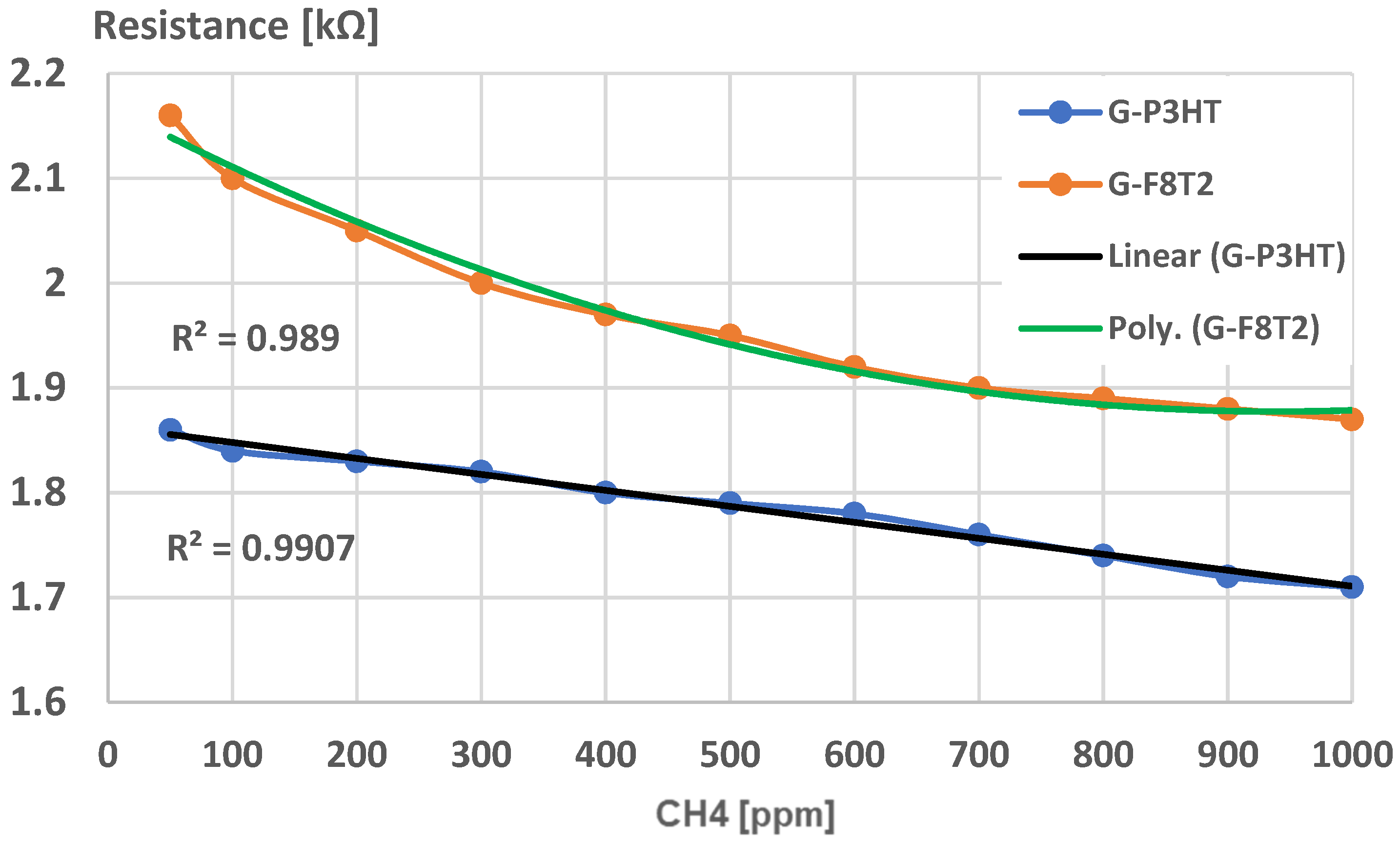
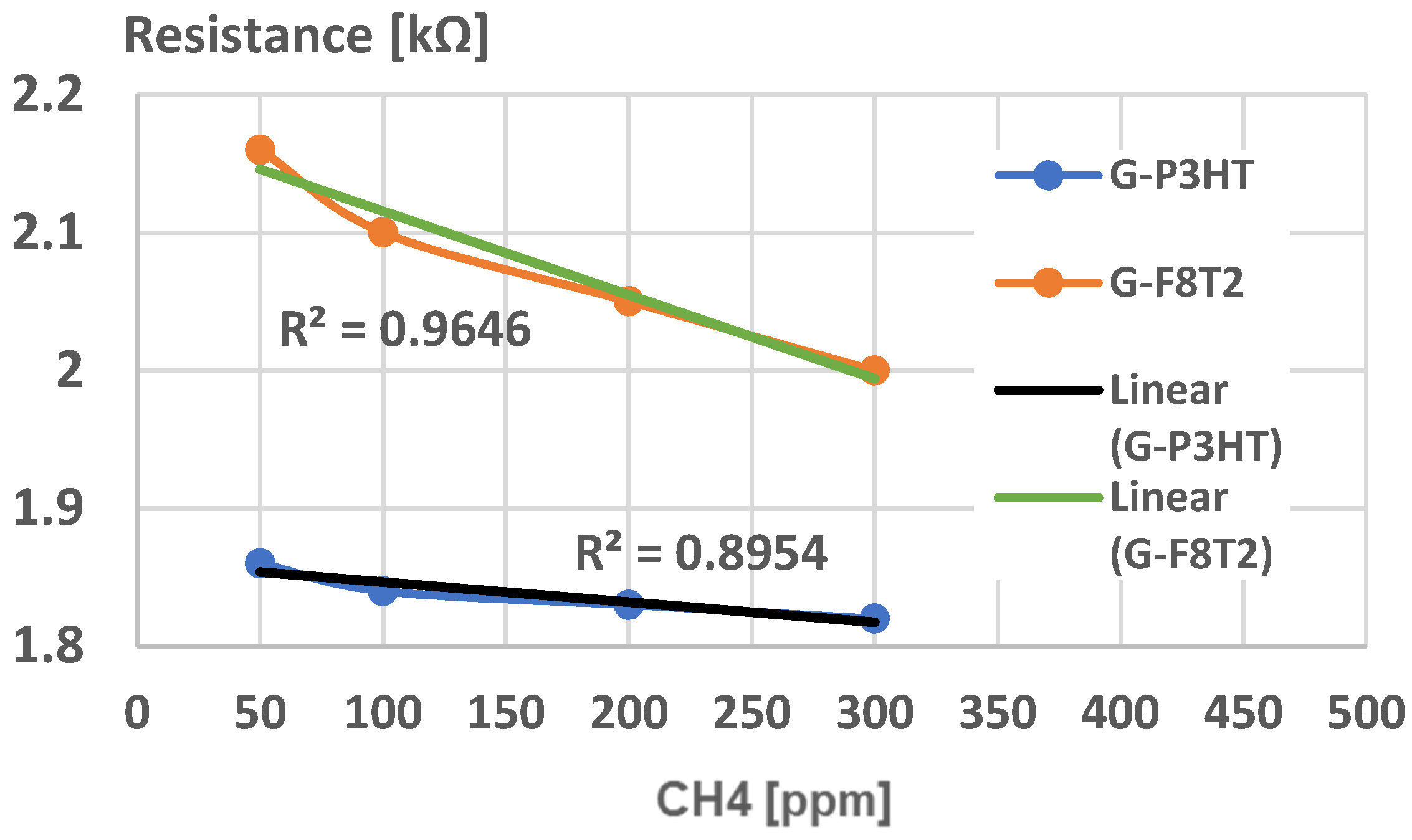
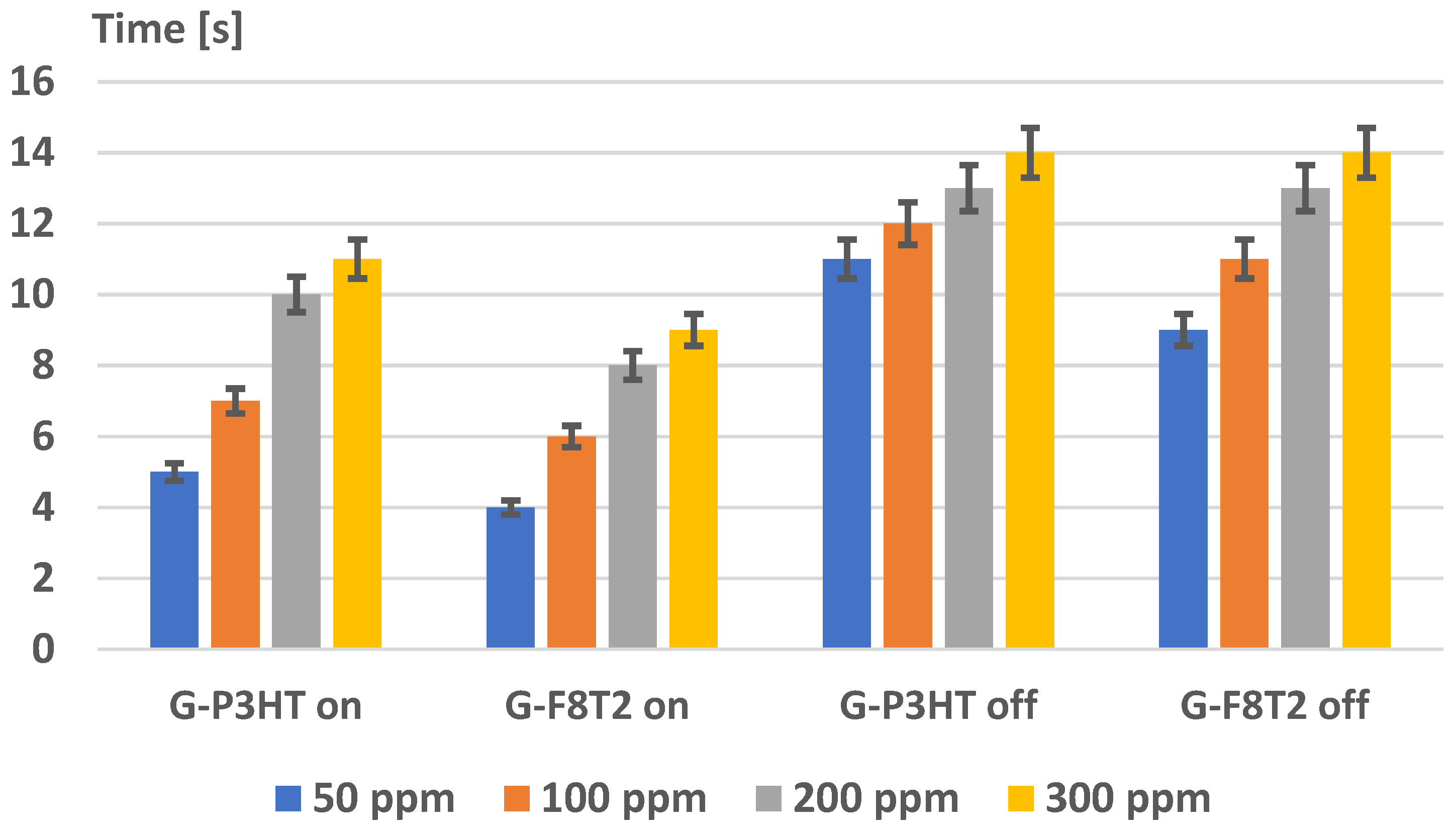
4. Analysis of Functionality as Gas Sensors for Methane
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Hannon, J.B.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-Area Graphene Single Crystals Grown by Low-Pressure Chemical Vapor Deposition of Methane on Copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Layer Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 3087. [Google Scholar] [CrossRef]
- Lee, J.S.; Jang, C.W.; Kim, J.M.; Shin, D.H.; Kim, S.; Choi, S.-H.; Belay, K.; Elliman, R. Graphene synthesis by C implantation into Cu foils. Carbon 2014, 66, 267–271. [Google Scholar] [CrossRef]
- Cheng, Q.; Duan, J.; Zhang, Q.; Jiang, L. Learning from Nature: Constructing Integrated Graphene-Based Artificial Nacre. ACS Nano 2015, 9, 2231–2234. [Google Scholar] [CrossRef]
- Lim, W.S.; Kim, Y.Y.; Kim, H.; Jang, S.; Kwon, N.; Park, B.J.; Ahn, J.-H.; Chung, I.; Hong, B.H.; Yeom, G.Y. Atomic layer etching of graphene for full graphene device fabrication. Carbon 2012, 50, 429–435. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Xu, C.; Khatami, Y.; Banerjee, K. Synthesis of high-quality monolayer and bilayer graphene on copper using chemical vapor deposition. Carbon 2011, 49, 4122–4130. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Chen, S. Large area CVD growth of graphene. Synth. Met. 2015, 210 Pt A, 95–108. [Google Scholar] [CrossRef]
- Hazbun, R.; Hart, J.; Hickey, R.; Ghosh, A.; Fernando, N.; Zollner, S.; Adam, T.N.; Kolodzey, J. Silicon epitaxy using tetrasilane at low temperatures in ultra-high vacuum chemical vapor deposition. J. Cryst. Growth 2016, 444, 21–27. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Xu, X.; Dubuisson, E.; Bao, Q.; Lu, J.; Loh, K.P. Electrochemical Delamination of CVD-Grown Graphene Film: Toward the Recyclable Use of Copper Catalyst. ACS Nano 2011, 5, 9927–9933. [Google Scholar] [CrossRef]
- Matsumura, H. Catalytic Chemical Vapor Deposition (CTC–CVD) Method Producing High Quality Hydrogenated Amorphous Silicon. Jpn. J. Appl. Phys. 1986, 25, L949. [Google Scholar] [CrossRef]
- Kathalingam, A.; Ajmal HM, S.; Ramesh, S.; Kim, H.S.; Kim, S.D.; Choi, S.H.; Yang, W.; Kim, K.K.; Kim, H.-S. Poly(methyl methacrylate)-derived graphene films on different substrates using rapid thermal process: A way to control the film properties through the substrate and polymer layer thickness. J. Mater. Res. Technol. 2019, 8, 3752–3763. [Google Scholar]
- Iwasaki, T.; Endo, K.; Watanabe, E.; Tsuya, D.; Morita, Y.; Nakaharai, S.; Noguchi, Y.; Wakayama, Y.; Watanabe, K.; Taniguchi, T.; et al. Bubble-Free Transfer Technique for High-Quality Graphene/Hexagonal Boron Nitride van der Waals Heterostructures. ACS Appl. Mater. Interfaces 2020, 12, 8533–8538. [Google Scholar] [CrossRef] [PubMed]
- Graphene. Available online: https://www.graphene.manchester.ac.uk/learn/applications/ (accessed on 12 March 2024).
- What Is the Graphene Flagship? Available online: https://graphene-flagship.eu/ (accessed on 12 March 2024).
- Ghuge, A.D.; Shirode, A.R.; Kadam, V.J. Graphene: A Comprehensive Review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, G.; Haseeb, A.S.M.A. Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials 2020, 13, 3311. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Sun, K.; Qiu, Y.; Jiao, X. Recent Advances in Graphene and Conductive Polymer Composites for Supercapacitor Electrodes: A Review. Crystals 2021, 11, 947. [Google Scholar] [CrossRef]
- Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene oxide/conducting polymer composite hydrogels. J. Mater. Chem. 2011, 21, 18653–18658. [Google Scholar] [CrossRef]
- Conducting Polymer/Graphene-Based Material Composites, and Methods for Preparing the Composites. Available online: https://patents.google.com/patent/US20140087192A1/en (accessed on 12 March 2024).
- Chauhan, A.K.; Gupta, S.K.; Taguchi, D.; Manaka, T.; Jha, P.; Veerender, P.; Sridevi, C.; Koiry, S.P.; Gadkari, S.C.; Iwamoto, M. Enhancement of the carrier mobility of conducting polymers by formation of their graphene composites. RSC Adv. 2017, 7, 11913–11920. [Google Scholar] [CrossRef]
- Kausar, A. Conjugated Polymer/Graphene Oxide Nanocomposites—State-of-the-Art. J. Compos. Sci. 2021, 5, 292. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Bissessur, R. Nanocomposites based on graphene analogous materials and conducting polymers: A review. J. Mater. Sci. 2020, 55, 6721–6753. [Google Scholar] [CrossRef]
- Adedoja, O.S.; Sadiku, E.R.; Hamam, Y. Prospects of Hybrid Conjugated Polymers Loaded Graphene in Electrochemical Energy Storage Applications. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3915–3934. [Google Scholar] [CrossRef]
- Shalini, A.; Kothai, S.; Jaisankar, V. Graphene Deposited Conducting Polymers: Synthesis and Sensing Applications. Int. J. Curr. Res. Aca. Rev. 2021, 9, 94–103. [Google Scholar]
- Zhu, Z.; Lin, W.-D.; Lin, Z.-Y.; Chuang, M.-H.; Wu, R.-J.; Chavali, M. Conductive Polymer (Graphene/PPy)–BiPO4 Composite Applications in Humidity Sensors. Polymers 2021, 13, 2013. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Chakraborty, K.; Pan, A.; Ghosh, S.; Pal, T. Conductivity relaxation and photocurrent generation in reduced graphene ox-ide-poly(9,9′-dioctyl-fluorene-co-bithiophene) composite with application in temperature sensing. J. Appl. Phys. 2019, 125, 085104. [Google Scholar] [CrossRef]
- Pinelli, F.; Nespoli, T.; Fiorati, A.; Farè, S.; Magagnin, L.; Rossi, F. Graphene nanoplatelets can improve the performances of graphene oxide—Polyaniline composite gas sensing aerogels. Carbon Trends 2021, 5, 100123. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, S.J.; Jeon, I.-Y. Graphene/Polymer Nanocomposites: Preparation, Mechanical Properties, and Application. Polymers 2022, 14, 4733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, Y.; Zhang, R.; Wang, H.; Sun, C.; Wang, T. Recent Progress in Gas Sensors Based on P3HT Polymer Field-Effect Transistors. Sensors 2023, 23, 8309. [Google Scholar] [CrossRef]
- Cheon, H.J.; Shin, S.Y.; Van Tran, V.; Park, B.; Yoon, H.; Chang, M. Preparation of conjugated polymer/reduced graphene oxide nano-composites for high-performance volatile organic compound sensors. Chem. Eng. J. 2021, 425, 131424. [Google Scholar] [CrossRef]
- Khanh, T.S.T.; Trung, T.Q.; Giang, L.T.T.; Nguyen, T.Q.; Lam, N.D.; Dinh, N.N. Ammonia Gas Sensing Characteristic of P3HT-rGO-MWCNT Composite Films. Appl. Sci. 2021, 11, 6675. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.; Verma, A.S.; Kumar, T. A review of composite conducting polymer-based sensors for detection of industrial waste gases. Sens. Actuators Rep. 2023, 5, 100143. [Google Scholar] [CrossRef]
- Lei, W.; Si, W.; Xu, Y.; Gu, Z.; Hao, Q. Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim. Acta 2014, 181, 707–722. [Google Scholar] [CrossRef]
- Kim, Y.; An, T.K.; Kim, J.; Hwang, J.; Park, S.; Nam, S.; Cha, H.; Park, W.J.; Baik, J.M.; Park, C.E. A composite of a graphene oxide derivative as a novel sensing layer in an organic field-effect transistor. J. Mater. Chem. C 2014, 2, 4539–4544. [Google Scholar] [CrossRef]
- Rahman, A.; Pal, R.K.; Islam, N.; Freeman, R.; Berthiaume, F.; Mazzeo, A.; Ashraf, A. A Facile Graphene Conductive Polymer Paper Based Biosensor for Dopamine, TNF-α, and IL-6 Detection. Sensors 2023, 23, 8115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, G.H.; Singh, G.; More, N.; Keerthana, M.; Sharma, A.; Jawade, D.; Balu, A.; Kapusetti, G. Ultrahigh sensitive graphene oxide/conducting polymer composite based biosensor for cholesterol and bilirubin detection. Biosens. Bioelectron. X 2023, 13, 100290. [Google Scholar] [CrossRef]
- Al-Graiti, W.; Foroughi, J.; Liu, Y.; Chen, J. Hybrid Graphene/Conducting Polymer Strip Sensors for Sensitive and Selective Electrochemical Detection of Serotonin. ACS Omega 2019, 4, 22169–22177. [Google Scholar] [CrossRef]
- Văduva, M.; Baibarac, M.; Cramariuc, O. Functionalization of Graphene Derivatives with Conducting Polymers and Their Applications in Uric Acid Detection. Molecules 2023, 28, 135. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.; Kang, K.; Lee, S.; Shin, M.; Son, D. Stretchable and Self-Healable Graphene–Polymer Conductive Composite for Wearable EMG Sensor. Polymers 2022, 14, 3766. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, M.L.; Colombo, E.; Papaleo, E.D.; Maya-Vetencourt, J.F.; Manfredi, G.; Lanzani, G.; Benfenati, F. A hybrid P3HT-Graphene interface for efficient photostimulation of neurons. Carbon 2020, 162, 308–317. [Google Scholar] [CrossRef]
- Yazid, S.N.A.M.; Adnan, A.A.C.; Isa, I.M.; Saidin, M.I.; Ahmad, M.S.; Fun, C.S. Conducting polymer functionalized graphene-based electrochemical sensors for sensing pollutants in water. J. Electrochem. Sci. Eng. 2023, 13, 251–274. [Google Scholar] [CrossRef]
- Diédhiou, I.; Fall, B.; Gaye, C.; Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Fall, M.; Raouafi, N. Preparations and applications of organic conducting polymers/graphene composites in heavy metal ion sensing: A review. Int. J. Mater. Res. 2023, 114, 79–99. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lin, J.-H.; Chen, C.-F.; Ito, Y.; Luo, S.-C. Conducting polymer-based sensors for food and drug analysis. J. Food Drug Anal. 2021, 29, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Mulyono, T.; Siswoyo, A.; Lestari, P.B.; Zulfikar; Mufliha, Y.M. Development of A Resistive Sensor Array Based on Graphene and Conducting Polymer Composites for Coffee Aroma Classification. E3S Web Conf. 2024, 481, 06012. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Minta, D.; Gryglewicz, G. Conductive Polymer/Graphene-based Composites for Next Generation Energy Storage and Sensing Applications. ChemElectroChem 2023, 10, e202201145. [Google Scholar] [CrossRef]
- Shen, F.; Pankratov, D.; Chi, Q. Graphene-conducting polymer nanocomposites for enhancing electrochemical capacitive energy storage. Curr. Opin. Electrochem. 2017, 4, 133–144. [Google Scholar] [CrossRef]
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar] [CrossRef]
- Achary, S.; Barik, B.; Dash, P. Graphene Oxide-Polymer Nanocomposites Towards Sensing and Photocatalytic Applications, Handbook of Polymer and Ceramic Nanotechnology; Springer: Cham, Switzerland, 2021; pp. 965–986. [Google Scholar] [CrossRef]
- Hydrogen/Methane Breath Test. Available online: https://www.nationaljewish.org/conditions/tests-procedures/gastroenterology/hydrogen-methane-breath-test (accessed on 12 March 2024).
- Costello, B.P.J.d.L.; Ledochowski, M.; Ratcliffe, N.M. The importance of methane breath testing: A review. J. Breath Res. 2013, 7, 024001. [Google Scholar] [CrossRef] [PubMed]
- John, R.A.B.; Kumar, A.R. A review on resistive-based gas sensors for the detection of volatile organic compounds using metal-oxide nanostructures. Inorg. Chem. Commun. 2021, 133, 108893. [Google Scholar] [CrossRef]
- Resistive Gas Sensor. Available online: https://www.electricity-magnetism.org/resistive-gas-sensor/ (accessed on 12 March 2024).
- Ansari, H.R.; Mirzaei, A.; Shokrollahi, H.; Kumar, R.; Kim, J.-Y.; Kim, H.W.; Kumar, M.; Kim, S.S. Flexible/wearable resistive gas sensors based on 2D materials. J. Mater. Chem. C 2023, 11, 6528–6549. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C. MOX-Based Resistive Gas Sensors with Different Types of Sensitive Materials (Powders, Pellets, Films), Used in Environmental Chemistry. Chemosensors 2023, 11, 95. [Google Scholar] [CrossRef]
- Baier, D.; Priamushko, T.; Weinberger, C.; Kleitz, F.; Tiemann, M. Selective Discrimination between CO and H2 with Copper–Ceria-Resistive Gas Sensors. ACS Sens. 2023, 8, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Hu, W.; Zhao, Z.; Cheng, P.; Haick, H.; Yao, M.; Wu, W. Gas Sensors Based on Chemi-Resistive Hybrid Functional Nanomaterials. Nano-Micro Lett. 2020, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Tansel, A.; Levinthal, D.J. Understanding Our Tests: Hydrogen-Methane Breath Testing to Diagnose Small Intestinal Bacterial Overgrowth. Clin. Transl. Gastroenterol. 2023, 14, e00567. [Google Scholar] [CrossRef] [PubMed]
- ISO 14644-1:2015 Cleanrooms and Associated Controlled Environments. Available online: https://www.iso.org/standard/53394.html (accessed on 10 March 2024).
- Rafailović, L.D.; Jovanović, A.Z.; Gutić, S.J.; Wehr, J.; Rentenberger, C.; Trišović, T.L.; Pašti, I.A. New Insights into the Metallization of Graphene-Supported Composite Materials─from 3D Cu-Grown Structures to Free-Standing Electrodeposited Porous Ni Foils. ACS Omega 2022, 7, 4352–4362. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Manalili, D.; Khodabakhsh, A.; Cristescu, S.M. Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor. Sensors 2024, 24, 1077. [Google Scholar] [CrossRef] [PubMed]
- The Methane Breath CH4ECK™. Available online: https://thefunctionalgutclinic.com/blog/news/the-methane-breath-ch4eck/ (accessed on 12 March 2024).
- Ndong, P.O.; Boutallaka, H.; Marine-Barjoan, E.; Ouizeman, D.; Mroue, R.; Anty, R.; Vanbiervliet, G.; Piche, T. Prevalence of small intestinal bacterial overgrowth in irritable bowel syndrome (IBS): Correlating H-2 or CH4 production with severity of IBS. JGH Open 2023, 7, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Shah, A.; Jones, M.P.; Koloski, N.; Talley, N.J.; Morrison, M.; Holtmann, G. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: A systematic review and meta-analysis. Gut Microbes 2021, 13, 1933313. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, Q.; Wan, F.; Gao, T. Gas Sensing Properties and Mechanism of Nano-SnO2-Based Sensor for Hydrogen and Carbon Monoxide. J. Nanomater. 2012, 2012, 612420. [Google Scholar] [CrossRef]
- Niyat, F.Y.; Abadi, M.H.S. COMSOL-Based Modeling and Simulation of SnO2/rGO Gas Sensor for Detection of NO2. Sci. Rep. 2018, 8, 2149. [Google Scholar] [CrossRef]

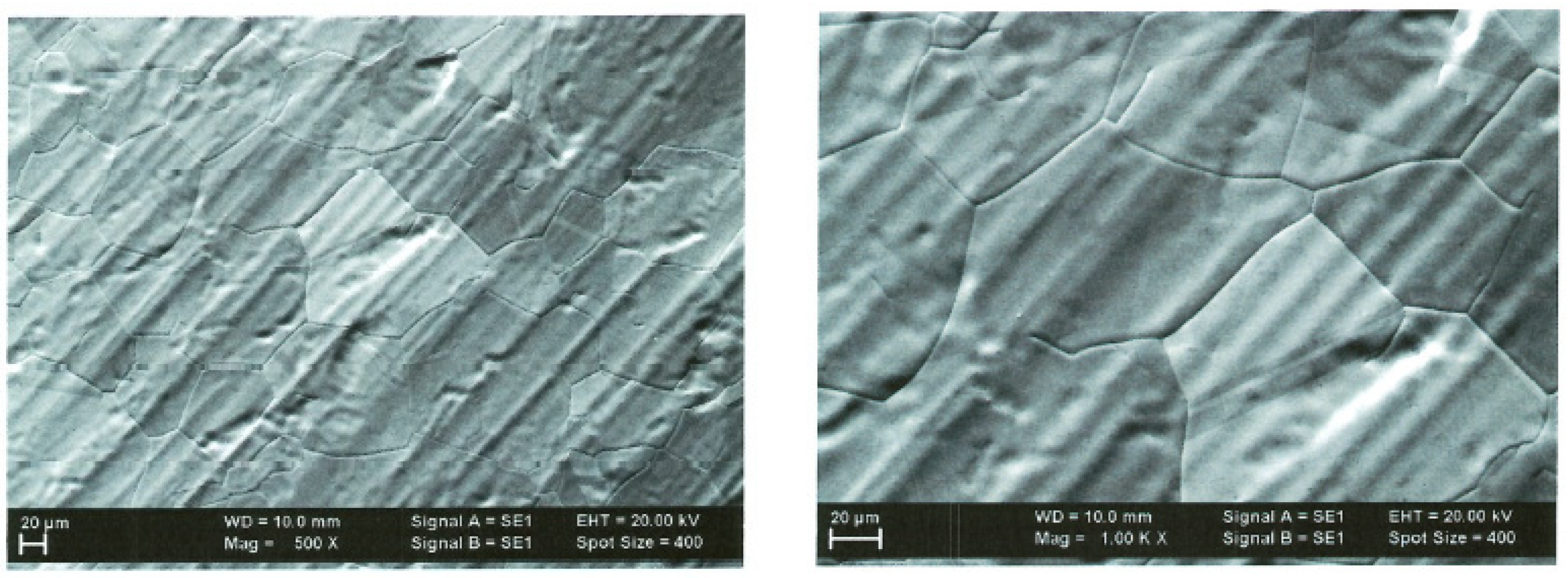









| Scanned Area | RMS (nm) | Ra (nm) | RSk | RKu |
|---|---|---|---|---|
| 40 × 40 μm | 489 | 376 | 0.447 | 3.63 |
| Scanned Area | RMS (nm) | Ra (nm) | RSk | RKu |
|---|---|---|---|---|
| 40 × 40 μm | 339 | 243 | 0.261 | 4.26 |
| Scanned Area | RMS (nm) | Ra (nm) | RSk | RKu |
|---|---|---|---|---|
| 40 × 40 μm | 64 | 53 | 0.143 | 3.31 |
| Scanned Area | RMS (nm) | Ra (nm) | RSk | RKu |
|---|---|---|---|---|
| 40 × 40 μm | 705 | 546 | 0.191 | 3.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trandabat, A.F.; Ciobanu, R.C.; Schreiner, O.D.; Schreiner, T.G.; Aradoaei, S. Chemiresistors Based on Hybrid Nanostructures Obtained from Graphene and Conducting Polymers with Potential Use in Breath Methane Detection Associated with Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 5552. https://doi.org/10.3390/ijms25105552
Trandabat AF, Ciobanu RC, Schreiner OD, Schreiner TG, Aradoaei S. Chemiresistors Based on Hybrid Nanostructures Obtained from Graphene and Conducting Polymers with Potential Use in Breath Methane Detection Associated with Irritable Bowel Syndrome. International Journal of Molecular Sciences. 2024; 25(10):5552. https://doi.org/10.3390/ijms25105552
Chicago/Turabian StyleTrandabat, Alexandru F., Romeo C. Ciobanu, Oliver Daniel Schreiner, Thomas Gabriel Schreiner, and Sebastian Aradoaei. 2024. "Chemiresistors Based on Hybrid Nanostructures Obtained from Graphene and Conducting Polymers with Potential Use in Breath Methane Detection Associated with Irritable Bowel Syndrome" International Journal of Molecular Sciences 25, no. 10: 5552. https://doi.org/10.3390/ijms25105552






