Enhanced Efficacy against Drug-Resistant Tumors Enabled by Redox-Responsive Mesoporous-Silica-Nanoparticle-Supported Lipid Bilayers as Targeted Delivery Vehicles
Abstract
:1. Introduction
2. Results and Discussion
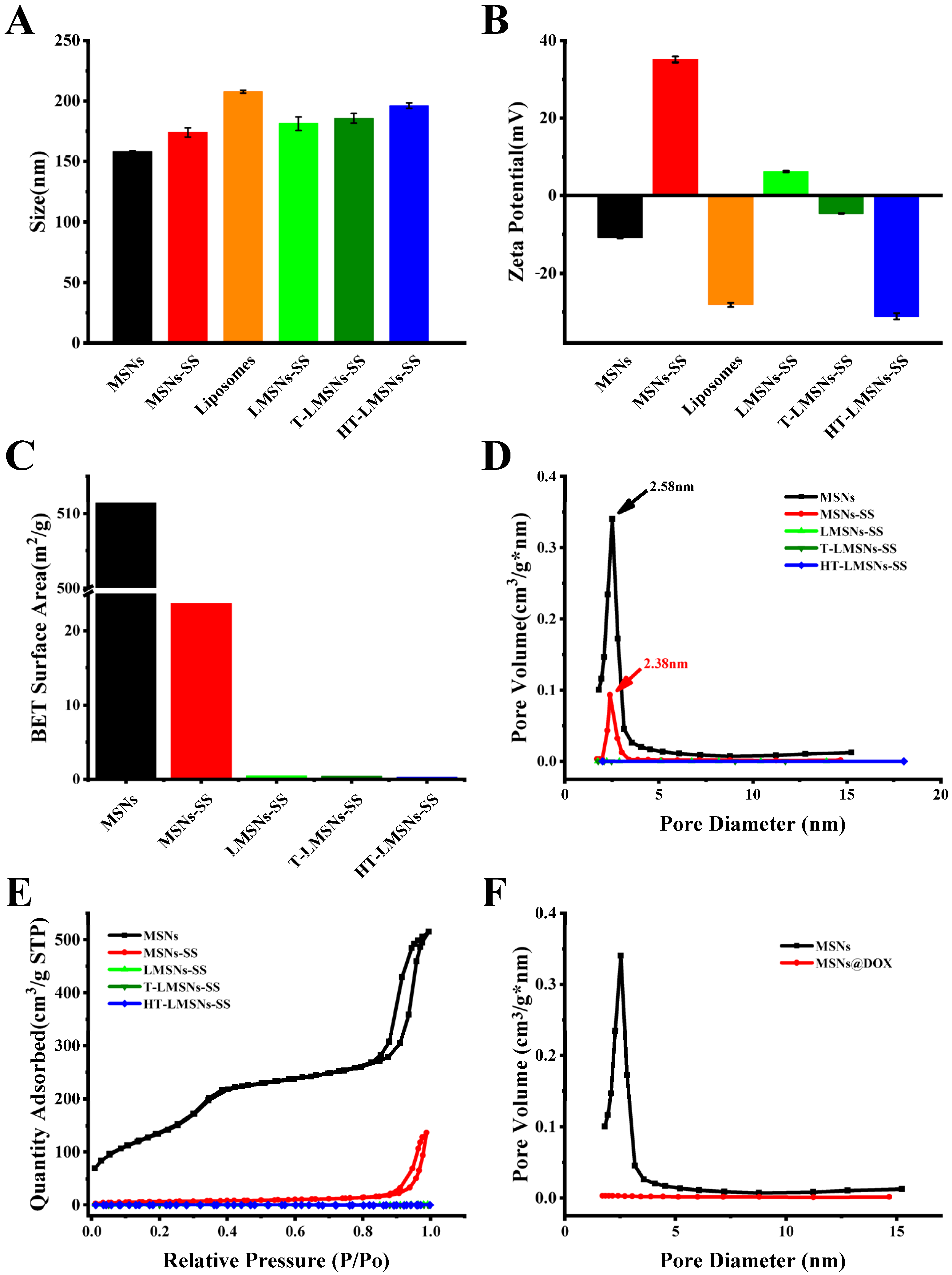
2.1. Preparation and Characterization of Various MSN-Based Nanocarriers
2.2. Drug-Loading Efficiency and In Vitro Drug Release
2.3. In Vitro Cytotoxicity
2.4. Intracellular Uptake and Internalization Mechanism
2.5. Cell Apoptosis and Cell Cycle Analysis
2.5.1. Apoptosis Analysis through FACS
2.5.2. Cell Cycle Analysis Using FACS
2.6. P-gp Expression Detection Using Cellular Immunofluorescence Assay
2.7. Analysis for Reversal of MDR
2.8. Intracellular Tracking in MCF-7/ADR Cells
2.9. Intracellular ROS Detection of MCF-7/ADR Cells
2.10. Assessment of the Antitumor Efficacy In Vivo
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Experimental Animals
3.4. Synthesis of MSNs and Preparation of Hybrid and Functionalized LMSNs
3.5. Labeling Using FITC and Loading of DOX for Nanocarriers
3.6. Characterization of Various Nanocarriers
3.7. In Vitro Drug Release
3.8. Cell Viability
3.9. Cellular Uptake and Internalization Mechanism Analysis
3.10. Cell Apoptosis and Cell Cycle Assay
3.11. Cellular Immunofluorescence
3.12. Western Blot Analysis and Wound Healing Assay
3.13. Intracellular ROS Detection
3.14. In Vivo Antitumor Effect Evaluation
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, A.M.; Zhang, M.; Wei, D.; Stueber, D.; Taratula, O.; Minko, T.; He, H. Co-delivery of Doxorubicin and Bcl-2 siRNA by Mesoporous Silica Nanoparticles Enhances the Efficacy of Chemotherapy in Multidrug-Resistant Cancer Cells. Small 2009, 5, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; LeValley, P.J.; Luo, T.; Kloxin, A.M.; Kiick, K.L. Manipulation of Glutathione-Mediated Degradation of Thiol Maleimide Conjugates. Bioconjugate Chem. 2018, 29, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, Z.; Zheng, D.; Li, Z.; Zhao, Z. A targeted and redox/pH-responsive chitosan oligosaccharide derivatives based nanohybrids for overcoming multidrug resistance of breast cancer cells. Carbohydr. Polym. 2021, 251, 117008. [Google Scholar] [CrossRef]
- He, Q.; Gao, Y.; Zhang, L.; Zhang, Z.; Gao, F.; Ji, X.; Li, Y.; Shi, J. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials 2011, 32, 7711–7720. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Buheazaha, T.M.; AlHomoud, H.S.; Al-Nasif, H.A.; Sarafroz, M. A comparative ex vivo permeation evaluation of a novel 5-Fluorocuracil nanoemulsion-gel by topically applied in the different excised rat, goat, and cow skin. Saudi J. Biol. Sci. 2020, 27, 1024–1040. [Google Scholar] [CrossRef]
- Anwar, M.; Shaikh Abdul, M.A.; Khan, U.M.; Hassan, M.; Khoja, A.H.; Muchtar, A. A Review of X-ray Photoelectron Spectroscopy Technique to Analyze the Stability and Degradation Mechanism of Solid Oxide Fuel Cell Cathode Materials. Materials 2022, 15, 2540. [Google Scholar] [CrossRef]
- Apostolou, M.; Assi, S.; Fatokun, A.A.; Khan, I. The Effects of Solid and Liquid Lipids on the Physicochemical Properties of Nanostructured Lipid Carriers. J. Pharm. Sci. 2021, 110, 2859–2872. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef]
- Ashique, S.; Sandhu, N.K.; Chawla, V.; Chawla, P.A. Targeted Drug Delivery: Trends and Perspectives. Curr. Drug Deliv. 2021, 18, 1400–1420. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, P.; Huan, F.; Wang, J.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Toxicity-attenuated mesoporous silica Schiff-base bonded anticancer drug complexes for chemotherapy of drug resistant cancer. Colloids Surf. B Biointerfaces 2021, 205, 111839. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.D.; Yu, Y.W.; Li, N.; Chen, W.Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, W.; Wang, X.; Wang, Y.; Wang, P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 2018, 111, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Li, N.; Xing, J.H.; Zheng, M.B.; Zheng, M.B.; Luo, Y.M.; Ma, A.Q.; Cui, L.; Cai, L.T. Review on Cell/Bacteria-Driven Drug Delivery Systems. Prog. Biochem. Biophys. 2019, 46, 1162–1170. [Google Scholar]
- Chen, M.J.; Gong, N.Q.; Sun, W.; Chen, M.; Gong, N.; Sun, W.; Han, J.; Liu, Y.; Zhang, S.; Zheng, A.; et al. Red-Light-Responsive Metallopolymer Nanocarriers with Conjugated and Encapsulated Drugs for Phototherapy Against Multidrug-Resistant Tumors. Small 2022, 18, 2201672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Jiang, J.H.; Chen, Z.L.; Yang, L. Editorial: Unveiling the Impact of Local or Systemic Therapeutic Strategies on the Tumor Microenvironment. Front. Oncol. 2022, 11, 832036. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liang, C.; Wang, X.; Tsai, H.I.; Liu, G.; Peng, Y.; Nie, J.; Huang, L.; Mei, L.; Zeng, X. A drug-self-gated and tumor microenvironment-responsive mesoporous silica vehicle: “four-in-one” versatile nanomedicine for targeted multidrug-resistant cancer therapy. Nanoscale 2017, 9, 17063–17073. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liang, C.; Xu, L.; Liu, G.; Gao, N.; Tao, W.; Luo, L.; Zuo, Y.; Wang, X.; Zhang, X.; et al. TPGS-Functionalized Polydopamine-Modified Mesoporous Silica as Drug Nanocarriers for Enhanced Lung Cancer Chemotherapy against Multidrug Resistance. Small 2017, 13, 1700623. [Google Scholar] [CrossRef]
- Chouaib, S.; Lorens, J. Editorial: Targeting the Tumor Microenvironment for a More Effective and Efficient Cancer Immunotherapy. Front. Immunol. 2020, 11, 933. [Google Scholar] [CrossRef]
- Cosco, D.; Mare, R.; Paolino, D.; Salvatici, M.C.; Cilurzo, F.; Fresta, M. Sclareol-loaded hyaluronan-coated PLGA nanoparticles: Physico-chemical properties and in vitro anticancer features. Int. J. Biol. Macromol. 2019, 132, 550–557. [Google Scholar] [CrossRef]
- De Vera, A.A.; Gupta, P.; Lei, Z.; Liao, D.; Narayanan, S.; Teng, Q.; Reznik, S.E.; Chen, Z.S. Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo. Cancer Lett. 2019, 442, 91–103. [Google Scholar] [CrossRef]
- Ding, C.; Wu, H.; Yin, Z.Z.; Gao, J.; Wu, D.; Qin, Y.; Kong, Y. Disulfide-cleavage- and pH-triggered drug delivery based on a vesicle structured amphiphilic self-assembly. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 107, 110366. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Niu, Y.; Li, S.; Lv, M.; Liu, J.; Zhang, L.; Cui, L.; Qu, L. TPGS and Doca dual-modified mesoporous silica nanoparticle-supported lipid bilayers enhance the efficient delivery and in vivo absorption of Coenzyme Q10. J. Drug Deliv. Sci. Technol. 2023, 81, 104243. [Google Scholar] [CrossRef]
- Guo, J.; Huang, L. Membrane-core nanoparticles for cancer nanomedicine. Adv Drug Deliv Rev. 2020, 156, 23–39. [Google Scholar] [CrossRef]
- Du, J.Z.; Lane, L.A.; Nie, S.M. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, W.; Yang, S.; Zhao, D.; Li, Z.; Luan, Y. Co-delivery of docetaxel and verapamil by reduction-sensitive PEG-PLGA-SS-DTX conjugate micelles to reverse the multi-drug resistance of breast cancer. Colloids Surf. B-Biointerfaces 2017, 151, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Vallet-Regi, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526. [Google Scholar] [CrossRef]
- Obeid, S.; Guyomarc’h, F. Atomic force microscopy of food assembly: Structural and mechanical insights at the nanoscale and potential opportunities from other fields. Food Biosci. 2020, 36, 100654. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, S.; Li, Y.; Parshad, B.; Li, W.; Haag, R. Novel dendritic polyglycerol-conjugated, mesoporous silica-based targeting nanocarriers for co-delivery of doxorubicin and tariquidar to overcome multidrug resistance in breast cancer stem cells. J. Control. Release 2021, 330, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, Z.; Massoumi, B.; Jaymand, M. A Novel Stimuli-Responsive Magnetite Nanocomposite as De Novo Drug Delivery System. Polym. Plast. Technol. Mater. 2019, 58, 405–418. [Google Scholar] [CrossRef]
- Ozkan, M.C.; Kaymaz, B.T.; Gunes, A.; Can, B.K.; Soyer, N.A.; Yilmaz, A.F.; Vural, F.; Sahin, F.; Saydam, G. Investigating the cytotoxic and apoptotic effects of sunitinib upon K-562 chronic myelogenous leukemia cell line and assessment of gene profiling. J. Cancer Res. Ther. 2020, 16, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Oroojalian, F.; Karimzadeh, S.; Javanbakht, S.; Hejazi, M.; Baradaran, B.; Webster, T.J.; Mokhtarzadeh, A.; Varma, R.S.; Kesharwani, P.; Sahebkar, A. Current trends in stimuli-responsive nanotheranostics based on metal-organic frameworks for cancer therapy. Mater. Today 2022, 57, 192–224. [Google Scholar] [CrossRef]
- Yang, S.; Song, S.; Han, K.; Wu, X.; Chen, L.; Hu, Y.; Wang, J.; Liu, B. Characterization, in vitro evaluation and comparative study on the cellular internalization of mesoporous silica nanoparticle-supported lipid bilayers. Microporous Mesoporous Mater. 2019, 284, 212–224. [Google Scholar] [CrossRef]
- Jacinto, T.A.; Rodrigues, C.F.; Moreira, A.F.; Miguel, S.P.; Costa, E.C.; Ferreira, P.; Correia, I.J. Hyaluronic acid and vitamin E polyethylene glycol succinate functionalized gold-core silica shell nanorods for cancer targeted photothermal therapy. Colloids Surf. B-Biointerfaces 2020, 188, 110778. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Gholami Derami, H.; Gupta, P.; Wang, Z.; Rathi, P.; Gupta, R.; Cao, T.; Morrissey, J.; Singamaneni, S. Polydopamine-Mesoporous Silica Core-Shell Nanoparticles for Combined Photothermal Immunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 42499–42510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Esser, L.; Vasani, R.B.; Thissen, H.; Voelcker, N.H. Porous silicon nanomaterials: Recent advances in surface engineering for controlled drug-delivery applications. Nanomedicine 2019, 14, 3213–3230. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ji, X.; Wang, H.; Sun, B.; Chu, B.; Shi, Y.; Su, Y.; He, Y. Silicon nanowire-based multifunctional platform for chemo-photothermal synergistic cancer therapy. J. Mater. Chem. B 2018, 6, 3876–3883. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Jiang, J.; Cui, N.; Xue, X.; Wang, T.; Wang, X.; He, Y.; Wang, D. Doxorubicin-Loaded Carbon Dots Lipid-Coated Calcium Phosphate Nanoparticles for Visual Targeted Delivery and Therapy of Tumor. Int. J. Nanomed. 2020, 15, 433–444. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Li, Y.; Gong, Y.; Shi, H.; Huo, Q.; Xu, Q. Stimuli-responsive delivery vehicles based on mesoporous silica nanoparticles: Recent advances and challenges. J. Mater. Chem. B 2017, 5, 1339–1352. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, R.Q.; Jia, G.F.; Wang, Y.H.; Luo, Y.L.; Xu, F.; Chen, Y.S. Controlled release of DOX mediated by glutathione and pH dual-responsive hollow mesoporous silicon coated with polydopamine graft poly(poly(ethylene glycol) methacrylate) nanoparticles for cancer therapy. J. Taiwan Inst. Chem. Eng. 2020, 115, 60–70. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, L.; Li, Z.; Shen, Y.; Guan, X.; Yue, J.; Cong, L.; Xu, W.; Shi, W.; Liang, C.; et al. Multi-functionalized Nano-conjugate for combating multidrug resistant breast Cancer via starvation-assisted chemotherapy. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 116, 111127. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Peng, S.; Lin, W.; Wang, J.; Zhang, L. Multistage pH-responsive mesoporous silica nanohybrids with charge reversal and intracellular release for efficient anticancer drug delivery. J. Colloid Interface Sci. 2019, 555, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, J.; Yu, X.; Liu, X.; Cheng, Y.; Zhou, C.; Li, M.; Shi, L.; Shi, L.; Liu, H.; et al. Tumor-targeting pH/redox dual-responsive nanosystem epigenetically reverses cancer drug resistance by co-delivering doxorubicin and GCN5 siRNA. Acta Biomater. 2021, 135, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Z.; Wang, Y.; Zhu, H.; Li, F.; Shen, Y.; Guo, S. A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy. Acta Biomater. 2017, 59, 170–180. [Google Scholar] [CrossRef]
- Yang, X.; Li, M.; Liang, J.; Hou, X.; He, X.; Wang, K. NIR-Controlled Treatment of Multidrug-Resistant Tumor Cells by Mesoporous Silica Capsules Containing Gold Nanorods and Doxorubicin. ACS Appl. Mater. Interfaces 2021, 13, 14894–14910. [Google Scholar] [CrossRef]
- Yang, L.; Kim, T.H.; Cho, H.Y.; Luo, J.; Lee, J.M.; Chueng, S.T.D.; Hou, Y.; Yin, P.; Han, J.; Kim, J.; et al. Hybrid Graphene-Gold Nanoparticle-Based Nucleic Acid Conjugates for Cancer-Specific Multimodal Imaging and Combined Therapeutics. Adv. Funct. Mater. 2021, 31, 2006918. [Google Scholar] [CrossRef]
- Yan, J.; Long, X.; Liang, Y.; Li, F.; Yu, H.; Li, Y.; Li, Z.; Tian, Y.; He, B.; Sun, Y. Nanodrug delivery systems and cancer stem cells: From delivery carriers to treatment. Colloids Surf. B-Biointerfaces 2022, 217, 112701. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.; Qin, X.; Huang, Y.; Zhang, J.; Chen, T.; Wang, Y.; Ding, Y.; Yao, Y. NIR-II light triggered nitric oxide release nanoplatform combined chemo-photothermal therapy for overcoming multidrug resistant cancer. J. Mater. Chem. B 2021, 9, 1698–1706. [Google Scholar] [CrossRef]
- Yan, H.; Du, X.; Wang, R.; Zhai, G. Progress in the study of D-alpha-tocopherol polyethylene glycol 1000 succinate (TPGS) reversing multidrug resistance. Colloids Surf. B-Biointerfaces 2021, 205, 111914. [Google Scholar] [CrossRef] [PubMed]
- Natesh, J.; Chandola, C.; Meeran, S.M.; Neerathilingam, M. Targeted delivery of doxorubicin through CD44 aptamer to cancer cells. Ther. Deliv. 2021, 12, 693–703. [Google Scholar] [CrossRef] [PubMed]












| Samples | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| MSNs | 85.33 ± 0.54 | 0.13 ± 0.02 | −10.87 ± 0.12 |
| MSNs-SS | 123.93 ± 3.61 | 0.31 ± 0.02 | 35.13 ± 0.73 |
| Liposomes | 207.60 ± 1.26 | 0.43 ± 0.01 | −2.20 ± 0.51 |
| LMSNs-SS | 181.23 ± 5.74 | 0.35 ± 0.04 | 6.25 ± 0.16 |
| T-LMSNs-SS | 185.67 ± 3.92 | 0.24 ± 0.01 | −4.60 ± 0.03 |
| HT-LMSNs-SS | 196.20 ± 2.32 | 0.22 ± 0.01 | −31.07 ± 0.83 |
| Samples | EE (%) | LE (μg/mg) |
|---|---|---|
| MSNs@DOX | 82.79 | 356.74 |
| MSNs-SS@DOX | 81.67 | 342.83 |
| Liposomes@DOX | 85.03 | 358.64 |
| LMSNs-SS@DOX | 87.98 | 380.09 |
| T-LMSNs-SS@DOX | 89.01 | 388.48 |
| HT-LMSNs-SS@DOX | 90.87 | 396.79 |
| Samples | IC50 Values (µg/mL) | |||
|---|---|---|---|---|
| MCF-7 | MCF-7/ADR | |||
| 24 h | 48 h | 24 h | 48 h | |
| Control (DOX) | 28.86 | 18.15 | 159.27 | 92.6 |
| MSNs@DOX | 20.93 * | 14.94 * | 66.42 ** | 52.47 ** |
| MSNs-SS@DOX | 15.45 * | 10.97 * | 35.20 ** | 29.89 ** |
| LMSNs-SS@DOX | 12.27 ** | 8.36 ** | 23.26 ** | 16.99 ** |
| T-LMSNs-SS@DOX | 10.16 ** | 7.83 ** | 15.07 *** | 12.21 *** |
| HT-LMSNs-SS@DOX | 8.08 *** | 5.02 *** | 8.03 *** | 4.79 *** |
| Samples | RI | |
|---|---|---|
| 24 h | 48 h | |
| DOX | 5.51 | 5.1 |
| MSNs@DOX | 3.17 | 3.5 |
| MSNs-SS@DOX | 2.27 | 2.72 |
| LMSNs-SS@DOX | 1.89 | 2.03 |
| T-LMSNs-SS@DOX | 1.56 | 1.55 |
| HT-LMSNs-SS@DOX | 0.99 | 0.95 |
| Samples | MCF-7 | MCF-7/ADR | ||||
|---|---|---|---|---|---|---|
| Early Apoptosis | Late Apoptosis | Total Apoptosis | Early Apoptosis | Late Apoptosis | Total Apoptosis | |
| Control | 1.78% | 2.72% | 4.5% | 2.19% | 2.90% | 5.09% |
| DOX | 10.9% | 19.9% | 30.8% | 3.00% | 4.45% | 7.45% |
| MSNs-SS@DOX | 10.3% | 18.1% | 28.4% | 4.31% | 7.72% | 12.03% |
| LMSNs-SS@DOX | 12.3% | 19.7% | 32% | 4.65% | 9.37% | 14.02% |
| T-LMSNs-SS@DOX | 9.28% | 20.0% | 29.28% | 4.49% | 19.1% | 23.59% |
| HT-LMSNs-SS@DOX | 13.0% | 24.1% | 37.1% | 5.85% | 32.4% | 38.25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, B.; Zhao, X.; Zhang, M.; Zhang, M.; Cui, L.; Zhang, L. Enhanced Efficacy against Drug-Resistant Tumors Enabled by Redox-Responsive Mesoporous-Silica-Nanoparticle-Supported Lipid Bilayers as Targeted Delivery Vehicles. Int. J. Mol. Sci. 2024, 25, 5553. https://doi.org/10.3390/ijms25105553
Yang S, Zhang B, Zhao X, Zhang M, Zhang M, Cui L, Zhang L. Enhanced Efficacy against Drug-Resistant Tumors Enabled by Redox-Responsive Mesoporous-Silica-Nanoparticle-Supported Lipid Bilayers as Targeted Delivery Vehicles. International Journal of Molecular Sciences. 2024; 25(10):5553. https://doi.org/10.3390/ijms25105553
Chicago/Turabian StyleYang, Shuoye, Beibei Zhang, Xiangguo Zhao, Mengwei Zhang, Mengna Zhang, Lan Cui, and Lu Zhang. 2024. "Enhanced Efficacy against Drug-Resistant Tumors Enabled by Redox-Responsive Mesoporous-Silica-Nanoparticle-Supported Lipid Bilayers as Targeted Delivery Vehicles" International Journal of Molecular Sciences 25, no. 10: 5553. https://doi.org/10.3390/ijms25105553





