How We Interpret Thrombosis with Thrombocytopenia Syndrome?
Abstract
:1. Introduction
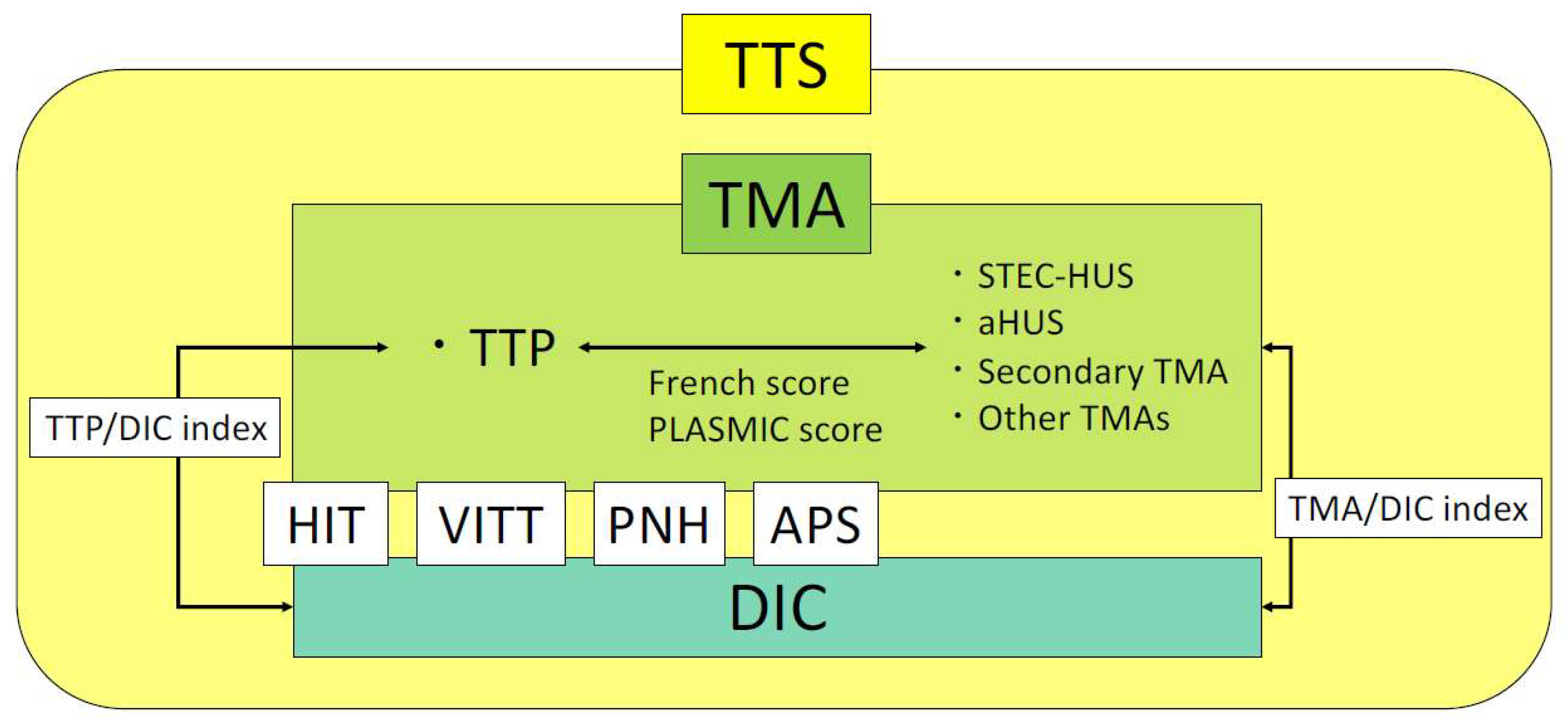
2. Characteristics of Thrombosis with Thrombocytopenia Syndrome
2.1. HIT
2.2. VITT
- Onset approximately 5–30 days after SARS-CoV-2 vaccination;
- Presence of thrombosis;
- Decrease in platelet count (<150,000/μL);
- D-dimer increase to ≥4 μg/mL;
- Anti-PF4 antibody-positive results from enzyme-linked immunosorbent assay (ELISA) assay.
2.3. PNH
2.4. APS
2.5. TMA
2.5.1. TTP
2.5.2. STEC-HUS
2.5.3. aHUS
2.5.4. Secondary TMA and Other TMAs
2.6. DIC
3. How to Distinguish Thrombosis with Thrombocytopenia Syndrome
3.1. French Score
- Platelet count <30,000/μL;
- Serum creatinine <200 μmol/L (<2.26 mg/dL);
- Positive results for antinuclear antibody.
3.2. PLASMIC Score
- Platelet count <30,000/μL;
- Serum creatinine <2.0 mg/dL;
- Hemolytic findings (indirect bilirubin >2 mg/dL or reticulocytes >2.5% or haptoglobin detection sensitivity <2.5%);
- No active cancer;
- No history of solid-organ or stem cell transplantation;
- Mean corpuscular volume (MCV) <90 fL;
- PT-INR < 1.5.
3.3. Scoring System for Differentiating TTP and Septic DIC
3.4. Differentiation of TTS in Daily Clinical Practice Utilizing Scoring Systems
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Portielje, J.E.; Westendorp, R.G.; Kluin-Nelemans, H.C.; Brand, A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001, 97, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.C.; Djulbegovic, B.; Shamai-Lubovitz, O.; Mozes, B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch. Intern. Med. 2000, 160, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.J.; MacLean, C.; Beer, P.A.; Buck, G.; Wheatley, K.; Kiladjian, J.J.; Forsyth, C.; Harrison, C.N.; Green, A.R. Correlation of blood counts with vascular complications in essential thrombocythemia: Analysis of the prospective PT1 cohort. Blood 2012, 120, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Budde, U.; van Genderen, P.J. Acquired von Willebrand disease in patients with high platelet counts. Semin. Thromb. Hemost. 1997, 23, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Ikeda, A.; Layana, M.C.; Bartlett, J.D. ADAM10: Possible functions in enamel development. Front. Physiol. 2022, 13, 1032383. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.; Harper, K.; Grondin, F.; Pelmus, M.; McDonald, P.P.; Dubois, C.M. Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor alpha-induced increases in tumor necrosis factor-alpha converting enzyme/ADAM17 expression by synovial cells. J. Biol. Chem. 2007, 282, 33714–33724. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, S.; Dragani, A.; Ranalli, P.; Petrucci, G.; Basso, M.; Tartaglione, R.; Rocca, B.; De Cristofaro, R. Qualitative and quantitative modifications of von Willebrand factor in patients with essential thrombocythemia and controlled platelet count. J. Thromb. Haemost. 2015, 13, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Sakai, K.; Hayakawa, M.; Kashiwagi, H.; Yagi, H.; Seki, Y.; Hasegawa, A.; Tanaka, H.; Amano, I.; Tomiyama, Y.; et al. Increased cleavage of von Willebrand factor by ADAMTS13 may contribute strongly to acquired von Willebrand syndrome development in patients with essential thrombocythemia. J. Thromb. Haemost. 2022, 20, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Asakura, H. Coagulopathy and Fibrinolytic Pathophysiology in COVID-19 and SARS-CoV-2 Vaccination. Int. J. Mol. Sci. 2022, 23, 3338. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Miyakawa, Y.; Kokame, K.; Ueda, Y.; Wada, H.; Higasa, S.; Yagi, H.; Ogawa, Y.; Sakai, K.; Miyata, T.; et al. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) in Japan 2023. Int J Hematol 2023, 118, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Amiral, J.; Bridey, F.; Dreyfus, M.; Vissoc, A.M.; Fressinaud, E.; Wolf, M.; Meyer, D. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb. Haemost. 1992, 68, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, M.; Podd, A.; Yuan, L.; Newman, D.K.; Wen, R.; Arepally, G.; Wang, D. Critical role for mouse marginal zone B cells in PF4/heparin antibody production. Blood 2013, 121, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Taylor, S.M.; Hartman, N.K.; Arepally, G.M.; Sachais, B.S.; Cines, D.B.; Poncz, M.; McKenzie, S.E. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcgammaRIIA. Blood 2001, 98, 2442–2447. [Google Scholar] [CrossRef]
- Kelton, J.G.; Sheridan, D.; Santos, A.; Smith, J.; Steeves, K.; Smith, C.; Brown, C.; Murphy, W.G. Heparin-induced thrombocytopenia: Laboratory studies. Blood 1988, 72, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.M.; Mayer, I.M. Antibodies from patients with heparin-induced thrombocytopenia stimulate monocytic cells to express tissue factor and secrete interleukin-8. Blood 2001, 98, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Tutwiler, V.; Madeeva, D.; Ahn, H.S.; Andrianova, I.; Hayes, V.; Zheng, X.L.; Cines, D.B.; McKenzie, S.E.; Poncz, M.; Rauova, L. Platelet transactivation by monocytes promotes thrombosis in heparin-induced thrombocytopenia. Blood 2016, 127, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.M.; Padmanabhan, A. Heparin-Induced Thrombocytopenia: A Focus on Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Basciano, P.A.; Knopman, J.; Bernstein, R.A. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood 2014, 123, 3651–3654. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Linkins, L.A. Non-necrotizing heparin-induced skin lesions and the 4T’s score. J. Thromb. Haemost. 2010, 8, 1483–1485. [Google Scholar] [CrossRef]
- Cuker, A.; Gimotty, P.A.; Crowther, M.A.; Warkentin, T.E. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood 2012, 120, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Nagler, M.; Bachmann, L.M.; ten Cate, H.; ten Cate-Hoek, A. Diagnostic value of immunoassays for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood 2016, 127, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Arnold, D.M.; Nazi, I.; Kelton, J.G. The platelet serotonin-release assay. Am. J. Hematol. 2015, 90, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Kinoshita, H.; Sugano, M.; Kurobe, H.; Kanbara, T.; Fujimoto, E.; Kitaichi, T.; Fujita, H.; Sogabe, H.; Kitagawa, T. Delayed-onset severe heparin-induced thrombocytopenia after total arch replacement under cardiopulmonary bypass. J. Med. Investig. 2013, 60, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Linkins, L.A.; Dans, A.L.; Moores, L.K.; Bona, R.; Davidson, B.L.; Schulman, S.; Crowther, M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. 2), e495S–e530S. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Arepally, G.M.; Chong, B.H.; Cines, D.B.; Greinacher, A.; Gruel, Y.; Linkins, L.A.; Rodner, S.B.; Selleng, S.; Warkentin, T.E.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood Adv. 2018, 2, 3360–3392. [Google Scholar] [CrossRef] [PubMed]
- Gruel, Y.; De Maistre, E.; Pouplard, C.; Mullier, F.; Susen, S.; Roullet, S.; Blais, N.; Le Gal, G.; Vincentelli, A.; Lasne, D.; et al. Diagnosis and management of heparin-induced thrombocytopenia. Anaesth. Crit. Care Pain. Med. 2020, 39, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Jones, C.G.; Pechauer, S.M.; Curtis, B.R.; Bougie, D.W.; Irani, M.S.; Bryant, B.J.; Alperin, J.B.; Deloughery, T.G.; Mulvey, K.P.; et al. IVIg for Treatment of Severe Refractory Heparin-Induced Thrombocytopenia. Chest 2017, 152, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.E.; Wallis, D.E.; Berkowitz, S.D.; Matthai, W.H.; Fareed, J.; Walenga, J.M.; Bartholomew, J.; Sham, R.; Lerner, R.G.; Zeigler, Z.R.; et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation 2001, 103, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.E.; Wallis, D.E.; Leya, F.; Hursting, M.J.; Kelton, J.G. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch. Intern. Med. 2003, 163, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Alahmadi, M.; Sawh, S.; Kovacs, M.J.; Lazo-Langner, A. Fondaparinux for the treatment of suspected heparin-induced thrombocytopenia: A propensity score-matched study. Blood 2015, 125, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf, M.; Steindl, J.; Beyer-Westendorf, J.; Schellong, S.; Dohmen, P.M.; Brachmann, J.; Madlener, K.; Pötzsch, B.; Klamroth, R.; Hankowitz, J.; et al. Use of Fondaparinux Off-Label or Approved Anticoagulants for Management of Heparin-Induced Thrombocytopenia. J. Am. Coll. Cardiol. 2017, 70, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- van Es, N.; Coppens, M.; Schulman, S.; Middeldorp, S.; Büller, H.R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: Evidence from phase 3 trials. Blood 2014, 124, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Kelton, J.G. Temporal aspects of heparin-induced thrombocytopenia. N. Engl. J. Med. 2001, 344, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Cadroy, Y.; Amiral, J.; Raynaud, H.; Brunel, P.; Mazaleyrat, A.; Sauer, M.; Sié, P. Evolution of antibodies anti-PF4/heparin in a patient with a history of heparin-induced thrombocytopenia reexposed to heparin. Thromb. Haemost. 1994, 72, 783–784. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Sheppard, J.A. Serological investigation of patients with a previous history of heparin-induced thrombocytopenia who are reexposed to heparin. Blood 2014, 123, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Gruel, Y.; Lang, M.; Darnige, L.; Pacouret, G.; Dreyfus, X.; Leroy, J.; Charbonnier, B. Fatal effect of re-exposure to heparin after previous heparin-associated thrombocytopenia and thrombosis. Lancet 1990, 336, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P.; Giri, S.; Pathak, R.; Bhatt, V.R. Heparin Reexposure in Patients With a History of Heparin-Induced Thrombocytopenia. Clin. Appl. Thromb. Hemost. 2015, 21, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A. HIT on dialysis—When is it safe to re-challenge? Nephron Clin. Pract. 2006, 104, c149–c150. [Google Scholar] [CrossRef]
- Hartman, V.; Malbrain, M.; Daelemans, R.; Meersman, P.; Zachée, P. Pseudo-pulmonary embolism as a sign of acute heparin-induced thrombocytopenia in hemodialysis patients: Safety of resuming heparin after disappearance of HIT antibodies. Nephron Clin. Pract. 2006, 104, c143–c148. [Google Scholar] [CrossRef]
- Wanaka, K.; Matsuo, T.; Matsuo, M.; Kaneko, C.; Miyashita, K.; Asada, R.; Matsushima, H.; Nakajima, Y. Re-exposure to heparin in uremic patients requiring hemodialysis with heparin-induced thrombocytopenia. J. Thromb. Haemost. 2010, 8, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Tiede, A.; Sachs, U.J.; Czwalinna, A.; Werwitzke, S.; Bikker, R.; Krauss, J.K.; Donnerstag, F.; Weißenborn, K.; Höglinger, G.; Maasoumy, B.; et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood 2021, 138, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int. J. Lab. Hematol. 2021, 43, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Langerak, T.; Bakker, G.J.; Porcelijn, L.; Lauw, M.N.; van de Laar, R.J.; Eefting, M. Vaccine-induced immune thrombocytopenia and thrombosis after mRNA-1273 booster vaccination. Thromb. Res. 2022, 214, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Sangli, S.; Virani, A.; Cheronis, N.; Vannatter, B.; Minich, C.; Noronha, S.; Bhagavatula, R.; Speredelozzi, D.; Sareen, M.; Kaplan, R.B. Thrombosis With Thrombocytopenia After the Messenger RNA-1273 Vaccine. Ann. Intern. Med. 2021, 174, 1480–1482. [Google Scholar] [CrossRef] [PubMed]
- Su, P.H.; Yu, Y.C.; Chen, W.H.; Lin, H.C.; Chen, Y.T.; Cheng, M.H.; Huang, Y.M. Case Report: Vaccine-Induced Immune Thrombotic Thrombocytopenia in a Pancreatic Cancer Patient After Vaccination With Messenger RNA-1273. Front. Med. 2021, 8, 772424. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Kanack, A.J.; Kaplan, R.B.; Sangli, S. COVID-19 mRNA-1273 vaccine induces production of vaccine-induced immune thrombotic thrombocytopenia antibodies. Am. J. Hematol. 2022, 97, E223–E225. [Google Scholar] [CrossRef]
- Rodríguez, C.; Pérez-Nieva, A.; Máiz, L.; Meijón, M.D.M.; Llamas, P.; Monreal, M.; Bikdeli, B.; Jiménez, D. Vaccine-induced immune thrombotic thrombocytopenia after the BNT162b2 mRNA Covid-19 vaccine: A case study. Thromb. Res. 2021, 208, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Fu, P.A.; Hsu, Y.T.; Chen, T.Y. Vaccine-Induced Immune Thrombotic Thrombocytopenia following BNT162b2 mRNA COVID-19 Booster: A Case Report. Vaccines 2023, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Johansen, S.; Laegreid, I.J.; Ernstsen, S.L.; Azrakhsh, N.A.; Kittang, A.O.; Lindås, R.; Gjertsen, B.T.; Vetti, N.; Mørtberg, T.V.; Sørvoll, I.H.; et al. Thrombosis and thrombocytopenia after HPV vaccination. J. Thromb. Haemost. 2022, 20, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Marchandot, B.; Carmona, A.; Trimaille, A.; Curtiaud, A.; Morel, O. Procoagulant microparticles: A possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis. J. Thromb. Thrombolysis 2021, 52, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Hwang, J.; Park, S.H.; Lee, S.W.; Lee, S.B.; Lee, M.H.; Jeong, G.H.; Kim, M.S.; Kim, J.Y.; Koyanagi, A.; Jacob, L.; et al. Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: The FAPIC score. Eur. Heart J. 2021, 42, 4053–4063. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Asakura, H. Vaccine-induced immune thrombotic thrombocytopenia: Update on diagnosis and management considering different resources: Comment from Yamada et al. J. Thromb. Haemost. 2022, 20, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Asakura, H. Management of disseminated intravascular coagulation associated with aortic aneurysm and vascular malformations. Int. J. Hematol. 2021, 113, 15–23. [Google Scholar] [CrossRef]
- Yamada, S.; Asakura, H. Therapeutic Strategies for Disseminated Intravascular Coagulation Associated with Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 1296. [Google Scholar] [CrossRef]
- Asakura, H. Classifying types of disseminated intravascular coagulation: Clinical and animal models. J. Intensive Care 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.; Narwal, A.; West, E.; Martin, J.; Bagot, C.N.; Page, A.R.; Watson, H.G.; Whyte, C.S.; Mutch, N.J. Fibrinogenolysis and fibrinolysis in vaccine-induced immune thrombocytopenia and thrombosis. J. Thromb. Haemost. 2023, 21, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Uzun, G.; Althaus, K.; Singh, A.; Möller, P.; Ziemann, U.; Mengel, A.; Rosenberger, P.; Guthoff, M.; Petzold, G.C.; Müller, J.; et al. The use of IV immunoglobulin in the treatment of vaccine-induced immune thrombotic thrombocytopenia. Blood 2021, 138, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, A.; Arnold, D.M.; Warkentin, T.E.; Smith, J.W.; Pannu, T.; Shrum, J.M.; Al Maqrashi, Z.A.A.; Shroff, A.; Lessard, M.C.; Blais, N.; et al. Adjunct Immune Globulin for Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 385, 720–728. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Sharma, P.; Moon, M.J.; Noonan, J.; Goodall, E.; Tran, H.A.; Peter, K. Activation of circulating platelets in vaccine-induced thrombotic thrombocytopenia and its reversal by intravenous immunoglobulin. Br. J. Haematol. 2022, 196, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.U.; Ikram, M.; Shafiq, Z.; Sarfraz, A.; Sarfraz, Z.; Jaiswal, V.; Sarfraz, M.; Chérrez-Ojeda, I. COVID-19 Vaccine-Associated Thrombosis With Thrombocytopenia Syndrome (TTS): A Systematic Review and Post Hoc Analysis. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211048815. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Pötzsch, B.; Greinacher, A. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie 2021, 41, 184–189. [Google Scholar] [PubMed]
- Huynh, A.; Kelton, J.G.; Arnold, D.M.; Daka, M.; Nazy, I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021, 596, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Langer, F.; Makris, M.; Pai, M.; Pavord, S.; Tran, H.; Warkentin, T.E. Vaccine-induced immune thrombotic thrombocytopenia (VITT)—Update on diagnosis and management considering different resources: Response to Comment from Yamada et al. J. Thromb. Haemost. 2022, 20, 542–543. [Google Scholar] [CrossRef]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef] [PubMed]
- Kadohira, Y.; Yamada, S.; Matsuura, E.; Hayashi, T.; Morishita, E.; Nakao, S.; Asakura, H. Aortic Aneurysm-associated Disseminated Intravascular Coagulation that Responded Well to a Switch from Warfarin to Rivaroxaban. Intern. Med. 2017, 56, 2913–2917. [Google Scholar] [CrossRef] [PubMed]
- Munter, G.; Hershko, C. Increased warfarin sensitivity as an early manifestation of occult prostate cancer with chronic disseminated intravascular coagulation. Acta Haematol. 2001, 105, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Craven, B.; Lester, W.; Boyce, S.; Thomas, W.; Kanny, A.; Davies, C.; Pavord, S.; Hermans, J.; Makris, M.; Bart-Smith, E.; et al. Natural history of PF4 antibodies in vaccine-induced immune thrombocytopenia and thrombosis. Blood 2022, 139, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, C.J.; Laroche, V.; Selby, R.; Pendergrast, J.; Barth, D.; Côté, B.; Gagnon, N.; Roberge, G.; Carrier, M.; Castellucci, L.A.; et al. Therapeutic Plasma Exchange in Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 385, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Major, A.; Carll, T.; Chan, C.W.; Christenson, C.; Aldarweesh, F.; Wool, G.D.; Cohen, K.S. Refractory vaccine-induced immune thrombotic thrombocytopenia (VITT) managed with delayed therapeutic plasma exchange (TPE). J. Clin. Apher. 2022, 37, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Panagiota, V.; Dobbelstein, C.; Werwitzke, S.; Ganser, A.; Cooper, N.; Sachs, U.J.; Tiede, A. Long-Term Outcomes after Vaccine-Induced Thrombotic Thrombocytopenia. Viruses 2022, 14, 1702. [Google Scholar] [CrossRef] [PubMed]
- von Hundelshausen, P.; Lorenz, R.; Siess, W.; Weber, C. Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT): Targeting Pathomechanisms with Bruton Tyrosine Kinase Inhibitors. Thromb. Haemost. 2021, 121, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Asakura, H. SARS-CoV-2 vaccination associated coagulopathy. J. Jpn. Soc. Lab. Hematol. 2023, 24, 11–23. [Google Scholar]
- Ham, T.H.; Dingle, J.H. Studies on destruction of red blood cells. II. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria: Certain immunological aspects of the hemolytic mechanism with special reference to serum complement. J. Clin. Investig. 1939, 18, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Crosby, W.H. Paroxysmal nocturnal hemoglobinuria: Relation of the clinical manifestations to underlying pathogenic mechanisms. Blood 1953, 8, 769–812. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Kawakita, M.; Koito, A.; Kawaguchi, T.; Nakakuma, H.; Kagimoto, T.; Schichishima, T.; Terasawa, T.; Akagaki, Y.; Inai, S. Paroxysmal nocturnal haemoglobinuria with coexisting deficiency of the ninth component of complement: Lack of massive haemolytic attack. Br. J. Haematol. 1990, 74, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Elebute, M.; Kelly, R.; Urbano-Ispizua, A.; Hill, A.; Rother, R.P.; Khursigara, G.; Fu, C.L.; Omine, M.; Browne, P.; et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am. J. Hematol. 2010, 85, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Butler, S.A.; Braren, V.; Hartmann, R.C.; Jenkins, D.E., Jr. The kidneys in paroxysmal nocturnal hemoglobinuria. Blood 1981, 57, 83–89. [Google Scholar] [CrossRef]
- Hugel, B.; Socié, G.; Vu, T.; Toti, F.; Gluckman, E.; Freyssinet, J.M.; Scrobohaci, M.L. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood 1999, 93, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Kanakura, Y.; Ohyashiki, K.; Shichishima, T.; Okamoto, S.; Ando, K.; Ninomiya, H.; Kawaguchi, T.; Nakao, S.; Nakakuma, H.; Nishimura, J.; et al. Safety and efficacy of the terminal complement inhibitor eculizumab in Japanese patients with paroxysmal nocturnal hemoglobinuria: The AEGIS clinical trial. Int. J. Hematol. 2011, 93, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Muus, P.; Dührsen, U.; Risitano, A.M.; Schubert, J.; Luzzatto, L.; Schrezenmeier, H.; Szer, J.; Brodsky, R.A.; Hill, A.; et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007, 110, 4123–4128. [Google Scholar] [CrossRef]
- Hillmen, P.; Lewis, S.M.; Bessler, M.; Luzzatto, L.; Dacie, J.V. Natural history of paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 1995, 333, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Mary, J.Y.; de Gramont, A.; Rio, B.; Leporrier, M.; Rose, C.; Heudier, P.; Rochant, H.; Cahn, J.Y.; Gluckman, E. Paroxysmal nocturnal haemoglobinuria: Long-term follow-up and prognostic factors. French Society of Haematology. Lancet 1996, 348, 573–577. [Google Scholar] [CrossRef]
- de Latour, R.P.; Mary, J.Y.; Salanoubat, C.; Terriou, L.; Etienne, G.; Mohty, M.; Roth, S.; de Guibert, S.; Maury, S.; Cahn, J.Y.; et al. Paroxysmal nocturnal hemoglobinuria: Natural history of disease subcategories. Blood 2008, 112, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, H.; Muus, P.; Socié, G.; Szer, J.; Urbano-Ispizua, A.; Maciejewski, J.P.; Brodsky, R.A.; Bessler, M.; Kanakura, Y.; Rosse, W.; et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica 2014, 99, 922–929. [Google Scholar] [CrossRef]
- Alayash, A.I. Haptoglobin: Old protein with new functions. Clin. Chim. Acta 2011, 412, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Richards, S.; Hillmen, P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 2003, 102, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Muus, P.; Röth, A.; Elebute, M.O.; Risitano, A.M.; Schrezenmeier, H.; Szer, J.; Browne, P.; Maciejewski, J.P.; Schubert, J.; et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2013, 162, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J.; de Groot, P.G.; de Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. J. Thromb. Haemost. 2020, 18, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V. Interaction between Antiphospholipid Antibodies and Protein C Anticoagulant Pathway: A Narrative Review. Semin. Thromb. Hemost. 2022, 48, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Urbanus, R.T.; de Laat, B. Antiphospholipid antibodies and the protein C pathway. Lupus 2010, 19, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kremers, R.M.W.; Zuily, S.; Kelchtermans, H.; Peters, T.C.; Bloemen, S.; Regnault, V.; Hemker, H.C.; de Groot, P.G.; Wahl, D.; de Laat, B. Prothrombin conversion is accelerated in the antiphospholipid syndrome and insensitive to thrombomodulin. Blood Adv. 2018, 2, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Navaz, S.; Tsodikov, A.; Kmetova, K.; Kluge, L.; Ambati, A.; Hoy, C.K.; Yalavarthi, S.; de Andrade, D.; Tektonidou, M.G.; et al. Anti-Neutrophil Extracellular Trap Antibodies in Antiphospholipid Antibody-Positive Patients: Results From the Antiphospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking Clinical Database and Repository. Arthritis Rheumatol. 2023, 75, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Triplett, D.A. Antiphospholipid antibodies: Proposed mechanisms of action. Am. J. Reprod. Immunol. 1992, 28, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Guariso, G.; Ruffatti, A.; Casonato, A.; Drigo, P.; Ghirardello, A.; Zancan, L. Antiphospholipid syndrome in a child with trisomy 21: The relationship between anticardiolipin G antibodies and the von Willebrand factor. Clin. Exp. Rheumatol. 1992, 10, 613–616. [Google Scholar] [PubMed]
- Mukai, M.; Ieko, M.; Atsumi, T.; Notoya, A.; Kohno, M. Multiple thromboses in major arteries in a patient with antiphospholipid syndrome associated with excess of a large multimer of von Willebrand factor. Lupus 2001, 10, 895–896. [Google Scholar] [CrossRef]
- Shirai, J.; Ida, A.; Jiang, Y.; Sanokawa-Akakura, R.; Miura, Y.; Yan, K.; Hamano, Y.; Hirose, S.; Shirai, T. Genetic polymorphism of murine tissue plasminogen activator associated with antiphospholipid syndrome. Genes. Immun. 1999, 1, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Boffa, M.C.; Khamashta, M.A.; Hughes, G.R. The Euro-Phospholipid project: Epidemiology of the antiphospholipid syndrome in Europe. Lupus 2009, 18, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; PG, D.E.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.R.J.; Bucci, T.; Iannaccone, L.; Marottoli, V.; Arcaro, A.; Gentile, F.; Ciampa, A. Validity of Coagulation Activation Markers in Antiphospholipid Syndrome: A Systematic Review and Meta-analysis with a Short Data Report. Semin. Thromb. Hemost. 2019, 45, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Ieko, M.; Naito, S.; Yoshida, M.; Ohmura, K.; Takahashi, N.; Sugawara, N.; Kiyohara, K.; Shimosegawa, K.; Ichinose, A. Lupus anticoagulant-hypoproaccelerin (factor V) syndrome (LAHPS-V): A new hemorrhagic condition associated with lupus anticoagulant. Int. J. Hematol. 2022, 116, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Ambati, A.; Knight, J.S.; Zuo, Y. Antiphospholipid syndrome management: A 2023 update and practical algorithm-based approach. Curr. Opin. Rheumatol. 2023, 35, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; McCrae, K.R. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 2017, 31, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Khairani, C.D.; Bejjani, A.; Piazza, G.; Jimenez, D.; Monreal, M.; Chatterjee, S.; Pengo, V.; Woller, S.C.; Cortes-Hernandez, J.; Connors, J.M.; et al. Direct Oral Anticoagulants vs Vitamin K Antagonists in Patients with Antiphospholipid Syndromes: Meta-Analysis of Randomized Trials. J. Am. Coll. Cardiol. 2023, 81, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- George, J.N.; Nester, C.M. Syndromes of thrombotic microangiopathy. N. Engl. J. Med. 2014, 371, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Amorosi, E.L. Thrombotic thrombocytopenic purpura: Report of 16 cases and review of the literature. Medicine 1966, 45, 139–159. [Google Scholar] [CrossRef]
- Chiasakul, T.; Cuker, A. Clinical and laboratory diagnosis of TTP: An integrated approach. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E. Pathophysiology of thrombotic thrombocytopenic purpura. Blood 2017, 130, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.; Coppo, P.; de la Rubia, J.; Friedman, K.D.; Kremer Hovinga, J.; Lämmle, B.; Matsumoto, M.; Pavenski, K.; Sadler, E.; et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J. Thromb. Haemost. 2017, 15, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Kokame, K.; Matsumoto, M.; Soejima, K.; Yagi, H.; Ishizashi, H.; Funato, M.; Tamai, H.; Konno, M.; Kamide, K.; Kawano, Y.; et al. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc. Natl. Acad. Sci. USA 2002, 99, 11902–11907. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kokame, K.; Soejima, K.; Miura, M.; Hayashi, S.; Fujii, Y.; Iwai, A.; Ito, E.; Tsuji, Y.; Takeda-Shitaka, M.; et al. Molecular characterization of ADAMTS13 gene mutations in Japanese patients with Upshaw-Schulman syndrome. Blood 2004, 103, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Wada, H.; Nakatsuka, Y.; Kubo, M.; Hayakawa, M.; Matsumoto, M. Characteristics Behaviors of Coagulation and Fibrinolysis Markers in Acquired Thrombotic Thrombocytopenic Purpura. J. Intensive Care Med. 2021, 36, 436–442. [Google Scholar] [CrossRef] [PubMed]
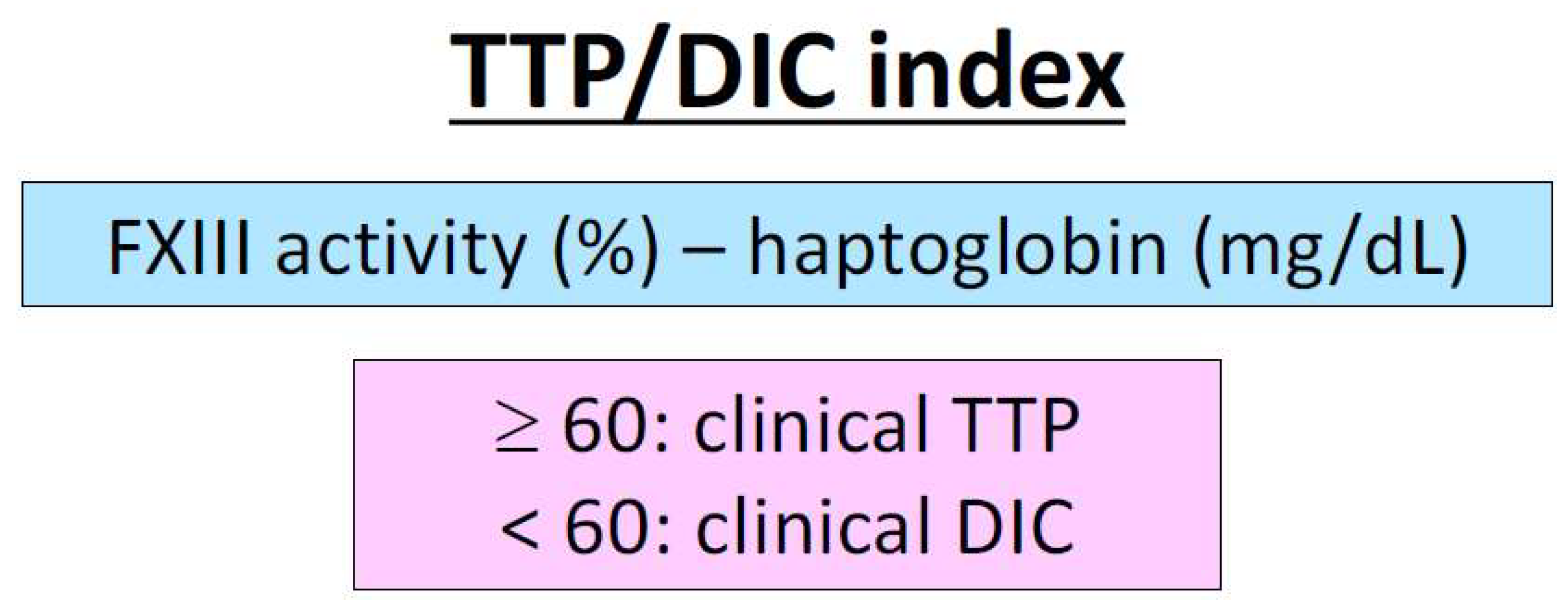
- Yamada, S.; Asakura, H.; Kubo, M.; Sakai, K.; Miyamoto, T.; Matsumoto, M. Distinguishing immune-mediated thrombotic thrombocytopenic purpura from septic disseminated intravascular coagulation using plasma levels of haptoglobin and factor XIII activity. Res. Pract. Thromb. Haemost. 2023, 7, 100076. [Google Scholar] [CrossRef] [PubMed]
- Rock, G.A.; Shumak, K.H.; Buskard, N.A.; Blanchette, V.S.; Kelton, J.G.; Nair, R.C.; Spasoff, R.A. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N. Engl. J. Med. 1991, 325, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Mazzara, R.; Monteagudo, J.; Sanz, C.; Puig, L.; Martínez, A.; Ordinas, A.; Castillo, R. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: A multivariate analysis of factors predicting the response to plasma exchange. Ann. Hematol. 1995, 70, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, M.; Sakai, K.; Harada, K.; Kanetake, J.; Kubo, M.; Hamada, E.; Hayakawa, M.; Hatakeyama, K.; Matsumoto, M. Strong association between insufficient plasma exchange and fatal outcomes in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura. Int. J. Hematol. 2021, 114, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Balduini, C.L.; Gugliotta, L.; Luppi, M.; Laurenti, L.; Klersy, C.; Pieresca, C.; Quintini, G.; Iuliano, F.; Re, R.; Spedini, P.; et al. High versus standard dose methylprednisolone in the acute phase of idiopathic thrombotic thrombocytopenic purpura: A randomized study. Ann. Hematol. 2010, 89, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Yagi, H.; Ishizashi, H.; Wada, H.; Fujimura, Y. The Japanese experience with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Semin. Hematol. 2004, 41, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Isonishi, A.; Bennett, C.L.; Plaimauer, B.; Scheiflinger, F.; Matsumoto, M.; Fujimura, Y. Poor responder to plasma exchange therapy in acquired thrombotic thrombocytopenic purpura is associated with ADAMTS13 inhibitor boosting: Visualization of an ADAMTS13 inhibitor complex and its proteolytic clearance from plasma. Transfusion 2015, 55, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Kremer Hovinga, J.A.; Vesely, S.K.; Terrell, D.R.; Lämmle, B.; George, J.N. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood 2010, 115, 1500–1511, quiz 1662. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Bennett, C.L.; Isonishi, A.; Qureshi, Z.; Hori, Y.; Hayakawa, M.; Yoshida, Y.; Yagi, H.; Fujimura, Y. Acquired idiopathic ADAMTS13 activity deficient thrombotic thrombocytopenic purpura in a population from Japan. PLoS ONE 2012, 7, e33029. [Google Scholar] [CrossRef] [PubMed]
- Vesely, S.K.; George, J.N.; Lämmle, B.; Studt, J.D.; Alberio, L.; El-Harake, M.A.; Raskob, G.E. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: Relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood 2003, 102, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Kaufman, R.M.; Goodnough, L.T.; Sadler, J.E. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood 2004, 103, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; McDonald, V.; Cavenagh, J.; Hunt, B.J.; Longair, I.; Cohen, H.; Machin, S.J. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood 2011, 118, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Froissart, A.; Buffet, M.; Veyradier, A.; Poullin, P.; Provôt, F.; Malot, S.; Schwarzinger, M.; Galicier, L.; Vanhille, P.; Vernant, J.P.; et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit. Care Med. 2012, 40, 104–111. [Google Scholar] [CrossRef]
- Miyakawa, Y.; Imada, K.; Ichinohe, T.; Nishio, K.; Abe, T.; Murata, M.; Ueda, Y.; Fujimura, Y.; Matsumoto, M.; Okamoto, S. Efficacy and safety of rituximab in Japanese patients with acquired thrombotic thrombocytopenic purpura refractory to conventional therapy. Int. J. Hematol. 2016, 104, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Cataland, S.; Scully, M.; Coppo, P.; Knoebl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Minkue Mi Edou, J.; et al. Caplacizumab prevents refractoriness and mortality in acquired thrombotic thrombocytopenic purpura: Integrated analysis. Blood Adv. 2021, 5, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, Y.; Imada, K.; Ichikawa, S.; Uchiyama, H.; Ueda, Y.; Yonezawa, A.; Fujitani, S.; Ogawa, Y.; Matsushita, T.; Asakura, H.; et al. The efficacy and safety of caplacizumab in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura: An open-label phase 2/3 study. Int. J. Hematol. 2023, 117, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sakai, K.; Kubo, M.; Azumi, H.; Hamamura, A.; Ochi, S.; Amagase, H.; Kunieda, H.; Ogawa, Y.; Yagi, H.; et al. Persistent ADAMTS13 Inhibitor Delays Recovery of ADAMTS13 activity in Caplacizumab-Treated Japanese iTTP Patients. Blood Adv. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Scully, M.; Hunt, B.J.; Benjamin, S.; Liesner, R.; Rose, P.; Peyvandi, F.; Cheung, B.; Machin, S.J. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br. J. Haematol. 2012, 158, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, Y.; Baudel, J.L.; Wynckel, A.; Galicier, L.; Azoulay, E.; Provôt, F.; Pène, F.; Mira, J.P.; Presne, C.; Poullin, P.; et al. Are platelet transfusions harmful in acquired thrombotic thrombocytopenic purpura at the acute phase? Experience of the French thrombotic microangiopathies reference center. Am. J. Hematol. 2015, 90, E127–E129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mhaskar, R.; Grossman, B.J.; Kaufman, R.M.; Tobian, A.A.; Kleinman, S.; Gernsheimer, T.; Tinmouth, A.T.; Djulbegovic, B. Platelet transfusion: A systematic review of the clinical evidence. Transfusion 2015, 55, 1116–1127, quiz 1115. [Google Scholar] [CrossRef] [PubMed]
- Beloncle, F.; Buffet, M.; Coindre, J.P.; Munoz-Bongrand, N.; Malot, S.; Pène, F.; Mira, J.P.; Galicier, L.; Guidet, B.; Baudel, J.L.; et al. Splenectomy and/or cyclophosphamide as salvage therapies in thrombotic thrombocytopenic purpura: The French TMA Reference Center experience. Transfusion 2012, 52, 2436–2444. [Google Scholar] [CrossRef] [PubMed]
- Ziman, A.; Mitri, M.; Klapper, E.; Pepkowitz, S.H.; Goldfinger, D. Combination vincristine and plasma exchange as initial therapy in patients with thrombotic thrombocytopenic purpura: One institution’s experience and review of the literature. Transfusion 2005, 45, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nosari, A.; Redaelli, R.; Caimi, T.M.; Mostarda, G.; Morra, E. Cyclosporine therapy in refractory/relapsed patients with thrombotic thrombocytopenic purpura. Am. J. Hematol. 2009, 84, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Kappers-Klunne, M.C.; Wijermans, P.; Fijnheer, R.; Croockewit, A.J.; van der Holt, B.; de Wolf, J.T.; Löwenberg, B.; Brand, A. Splenectomy for the treatment of thrombotic thrombocytopenic purpura. Br. J. Haematol. 2005, 130, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Kawano, N.; Yokota-Ikeda, N.; Yoshida, S.; Kuriyama, T.; Yamashita, K.; Sugio, Y.; Makino, S.; Ono, N.; Inoue, Y.; Himeji, D.; et al. Therapeutic modality of 11 patients with TTP in a single institution in Miyazaki from 2000 to 2011. Intern. Med. 2013, 52, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio, A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020, 18, 2496–2502. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Matsumoto, M.; Isonishi, A.; Yagi, H.; Kokame, K.; Soejima, K.; Murata, M.; Miyata, T. Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 283–301. [Google Scholar] [CrossRef] [PubMed]
- Kremer Hovinga, J.A.; George, J.N. Hereditary Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 381, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.J.; Kendall, G.; Scully, M. Recombinant ADAMTS13 in Severe Neonatal Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2022, 387, 2391–2392. [Google Scholar] [CrossRef]
- Asmis, L.M.; Serra, A.; Krafft, A.; Licht, A.; Leisinger, E.; Henschkowski-Serra, J.; Ganter, M.T.; Hauptmann, S.; Tinguely, M.; Kremer Hovinga, J.A. Recombinant ADAMTS13 for Hereditary Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2022, 387, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Knöbl, P.; Kentouche, K.; Rice, L.; Windyga, J.; Schneppenheim, R.; Kremer Hovinga, J.A.; Kajiwara, M.; Fujimura, Y.; Maggiore, C.; et al. Recombinant ADAMTS-13: First-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood 2017, 130, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello-Pellegrini, T.N.; Marsden, P.A. Shiga toxin-associated hemolytic uremic syndrome: Advances in pathogenesis and therapeutics. Curr. Opin. Nephrol. Hypertens. 2012, 21, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yada, N.; Fujioka, M.; Bennett, C.L.; Inoki, K.; Miki, T.; Watanabe, A.; Yoshida, T.; Hayakawa, M.; Matsumoto, M.; Fujimura, Y. STEC:O111-HUS complicated by acute encephalopathy in a young girl was successfully treated with a set of hemodiafiltration, steroid pulse, and soluble thrombomodulin under plasma exchange. Clin. Case Rep. 2015, 3, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Yesilbas, O.; Yozgat, C.Y.; Akinci, N.; Sonmez, S.; Tekin, E.; Talebazadeh, F.; Jafarov, U.; Temur, H.O.; Yozgat, Y. Acute Myocarditis and Eculizumab Caused Severe Cholestasis in a 17-Month-Old Child Who Has Hemolytic Uremic Syndrome Associated with Shiga Toxin-Producing Escherichia coli. J. Pediatr. Intensive Care 2021, 10, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Takase, Y.; Hatachi, K.; Hashimoto, K.; Shirakawa, T.; Nakashima, Y.; Funakoshi, Y.; Moriuchi, H. A case of Shiga toxin-producing Escherichia coli O157-assiciated hemolytic uremic syndrome lacking diarrhea or bloody stool. J. Pediatr. Infect. Dis. Immunol. 2021, 33, 359–365. [Google Scholar]
- Ake, J.A.; Jelacic, S.; Ciol, M.A.; Watkins, S.L.; Murray, K.F.; Christie, D.L.; Klein, E.J.; Tarr, P.I. Relative nephroprotection during Escherichia coli O157:H7 infections: Association with intravenous volume expansion. Pediatrics 2005, 115, e673–e680. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.A.; Beattie, T.J.; Cowieson, J.; Miyashita, Y.; Strife, C.F.; Frem, J.C.; Peterson, J.M.; Butani, L.; Jones, D.P.; Havens, P.L.; et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch. Pediatr. Adolesc. Med. 2011, 165, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.; Ahlenstiel, T.; Kreuzer, M.; Drube, J.; Froede, K.; Franke, D.; Ehrich, J.H.; Haubitz, M. Early erythropoietin reduced the need for red blood cell transfusion in childhood hemolytic uremic syndrome: A randomized prospective pilot trial. Pediatr. Nephrol. 2009, 24, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Andreoli, S.P.; Billmire, D.F. Bleeding risk for surgical dialysis procedures in children with hemolytic uremic syndrome. Pediatr. Nephrol. 2010, 25, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Ito, S.; Sako, M.; Saitoh, A.; Hataya, H.; Mizuguchi, M.; Morishima, T.; Ohnishi, K.; Kawamura, N.; Kitayama, H.; et al. Guidelines for the management and investigation of hemolytic uremic syndrome. Clin. Exp. Nephrol. 2014, 18, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Byrne, L.; Vishram, B.; Sawyer, C.; Balasegaram, S.; Ahyow, L.; Johnson, S. Shiga toxin-producing Escherichia coli haemolytic uraemic syndrome (STEC-HUS): Diagnosis, surveillance and public-health management in England. J. Med. Microbiol. 2020, 69, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.P.; Griffin, P.M.; Lozano, P.; Christie, D.L.; Kobayashi, J.M.; Tarr, P.I. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. Pediatrics 1997, 100, E12. [Google Scholar] [CrossRef] [PubMed]
- Cimolai, N.; Basalyga, S.; Mah, D.G.; Morrison, B.J.; Carter, J.E. A continuing assessment of risk factors for the development of Escherichia coli O157:H7-associated hemolytic uremic syndrome. Clin. Nephrol. 1994, 42, 85–89. [Google Scholar] [PubMed]
- Cimolai, N.; Morrison, B.J.; Carter, J.E. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome. Pediatrics 1992, 90, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Sawai, T.; Nangaku, M.; Ashida, A.; Fujimaru, R.; Hataya, H.; Hidaka, Y.; Kaname, S.; Okada, H.; Sato, W.; Yasuda, T.; et al. Diagnostic criteria for atypical hemolytic uremic syndrome proposed by the Joint Committee of the Japanese Society of Nephrology and the Japan Pediatric Society. Clin. Exp. Nephrol. 2014, 18, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nangaku, M.; Hataya, H.; Sawai, T.; Ashida, A.; Fujimaru, R.; Hidaka, Y.; Kaname, S.; Maruyama, S.; Yasuda, T.; et al. Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin. Exp. Nephrol. 2016, 20, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009, 361, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Redecha, P.; Tilley, R.; Tencati, M.; Salmon, J.E.; Kirchhofer, D.; Mackman, N.; Girardi, G. Tissue factor: A link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood 2007, 110, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.K.; Hattori, R.; Esmon, C.T.; Sims, P.J. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J. Biol. Chem. 1990, 265, 3809–3814. [Google Scholar] [CrossRef] [PubMed]
- Loirat, C.; Frémeaux-Bacchi, V. Atypical hemolytic uremic syndrome. Orphanet J. Rare Dis. 2011, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Kato, H.; Yoshida, Y.; Usui, T.; Takata, M.; Fujimoto, M.; Wada, H.; Uchida, Y.; Kokame, K.; Matsumoto, M.; et al. Clinical characteristics and genetic backgrounds of Japanese patients with atypical hemolytic uremic syndrome. Clin. Exp. Nephrol. 2018, 22, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Caprioli, J.; Bresin, E.; Mossali, C.; Pianetti, G.; Gamba, S.; Daina, E.; Fenili, C.; Castelletti, F.; Sorosina, A.; et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. 2010, 5, 1844–1859. [Google Scholar] [CrossRef]
- Fakhouri, F.; Hourmant, M.; Campistol, J.M.; Cataland, S.R.; Espinosa, M.; Gaber, A.O.; Menne, J.; Minetti, E.E.; Provôt, F.; Rondeau, E.; et al. Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial. Am. J. Kidney Dis. 2016, 68, 84–93. [Google Scholar] [CrossRef]
- Greenbaum, L.A.; Fila, M.; Ardissino, G.; Al-Akash, S.I.; Evans, J.; Henning, P.; Lieberman, K.V.; Maringhini, S.; Pape, L.; Rees, L.; et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016, 89, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Legendre, C.M.; Licht, C.; Muus, P.; Greenbaum, L.A.; Babu, S.; Bedrosian, C.; Bingham, C.; Cohen, D.J.; Delmas, Y.; Douglas, K.; et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2013, 368, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.; Greenbaum, L.A.; Muus, P.; Babu, S.; Bedrosian, C.L.; Cohen, D.J.; Delmas, Y.; Douglas, K.; Furman, R.R.; Gaber, O.A.; et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015, 87, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, E.; Scully, M.; Ariceta, G.; Barbour, T.; Cataland, S.; Heyne, N.; Miyakawa, Y.; Ortiz, S.; Swenson, E.; Vallee, M.; et al. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020, 97, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Ariceta, G.; Dixon, B.P.; Kim, S.H.; Kapur, G.; Mauch, T.; Ortiz, S.; Vallee, M.; Denker, A.E.; Kang, H.G.; Greenbaum, L.A. The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2021, 100, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Goodship, T.H.; Cook, H.T.; Fakhouri, F.; Fervenza, F.C.; Frémeaux-Bacchi, V.; Kavanagh, D.; Nester, C.M.; Noris, M.; Pickering, M.C.; Rodríguez de Córdoba, S.; et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Campistol, J.M.; Arias, M.; Ariceta, G.; Blasco, M.; Espinosa, L.; Espinosa, M.; Grinyó, J.M.; Macía, M.; Mendizábal, S.; Praga, M.; et al. An update for atypical haemolytic uraemic syndrome: Diagnosis and treatment. A consensus document. Nefrologia 2015, 35, 421–447. [Google Scholar] [CrossRef]
- Allen, U.; Licht, C. Pandemic H1N1 influenza A infection and (atypical) HUS--more than just another trigger? Pediatr. Nephrol. 2011, 26, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Veesenmeyer, A.F.; Edmonson, M.B. Trends in US hospital stays for Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr. Infect. Dis. J. 2013, 32, 731–735. [Google Scholar] [CrossRef]
- Fan, X.; Yoshida, Y.; Honda, S.; Matsumoto, M.; Sawada, Y.; Hattori, M.; Hisanaga, S.; Hiwa, R.; Nakamura, F.; Tomomori, M.; et al. Analysis of genetic and predisposing factors in Japanese patients with atypical hemolytic uremic syndrome. Mol. Immunol. 2013, 54, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Spinale, J.M.; Ruebner, R.L.; Kaplan, B.S.; Copelovitch, L. Update on Streptococcus pneumoniae associated hemolytic uremic syndrome. Curr. Opin. Pediatr. 2013, 25, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Al-Nouri, Z.L.; Reese, J.A.; Terrell, D.R.; Vesely, S.K.; George, J.N. Drug-induced thrombotic microangiopathy: A systematic review of published reports. Blood 2015, 125, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.; Hosseinzadeh, S.; Burton, K.; Pavord, S.; Dutt, T.; Doree, C.; Lim, W.Y.; Desborough, M.J.R. Drug-induced thrombotic thrombocytopenic purpura: A systematic review and review of European and North American pharmacovigilance data. Br. J. Haematol. 2023, 201, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.N.; Tran, M.H. Cobalamin deficiency presenting with thrombotic microangiopathy (TMA) features: A systematic review. Transfus. Apher. Sci. 2018, 57, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Takahashi, H.; Uchiyama, T.; Eguchi, Y.; Okamoto, K.; Kawasugi, K.; Madoiwa, S.; Wada, H. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb. J. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Obstetrical disseminated intravascular coagulation score. J. Obstet. Gynaecol. Res. 2014, 40, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H. Diversity of disseminated intravascular coagulation and selection of appropriate treatments. Int. J. Hematol. 2021, 113, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Maekawa, T.; Takada, M.; Tanaka, H.; Gonmori, H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl. Haematol. 1983, 49, 265–275. [Google Scholar]
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M.; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Iba, T.; Eguchi, Y.; Ohtomo, Y.; Okamoto, K.; Koseki, K.; Mayumi, T.; Murata, A.; Ikeda, T.; Ishikura, H.; et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit. Care Med. 2006, 34, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Choi, J.R.; Song, K.S.; Rhee, J.H. Plasma factor XIII activity in patients with disseminated intravascular coagulation. Yonsei Med. J. 2006, 47, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Richardson, V.R.; Cordell, P.; Standeven, K.F.; Carter, A.M. Substrates of Factor XIII-A: Roles in thrombosis and wound healing. Clin. Sci. 2013, 124, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Egbring, R.; Kröniger, A.; Seitz, R. Factor XIII deficiency: Pathogenic mechanisms and clinical significance. Semin. Thromb. Hemost. 1996, 22, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Holstein, K.; Spath, B.; Schnabel, C.; Bannas, P.; Bokemeyer, C.; Langer, F. Relevant bleeding diathesis due to acquired factor XIII deficiency. Hamostaseologie 2013, 33 (Suppl. 1), S50–S54. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Okumura, H.; Morishita, E.; Asakura, H. Complete hemostasis achieved by factor XIII concentrate administration in a patient with bleeding after teeth extraction as a complication of aplastic anemia and chronic disseminated intravascular coagulation. Blood Coagul. Fibrinolysis 2020, 31, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Arahata, M.; Morishita, E.; Asakura, H. Blue Rubber Bleb Nevus Syndrome Complicated by Enhanced-Fibrinolytic-Type DIC: A Case Report. Ann. Vasc. Dis. 2021, 14, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Suga, Y.; Morishita, E.; Asakura, H. Effect of Anticoagulant/Antifibrinolytic Combination Therapy on Enhanced Fibrinolytic-Type Disseminated Intravascular Coagulation in End-of-Life Stage Solid Tumor Patients. J. Palliat. Med. 2022, 26, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Nakagawa, N.; Kadohira, Y.; Morishita, E.; Asakura, H. Rivaroxaban in a patient with disseminated intravascular coagulation associated with an aortic aneurysm: A case report. Ann. Intern. Med. 2014, 161, 158–159. [Google Scholar] [CrossRef]
- Yasumoto, A.; Ishiura, R.; Narushima, M.; Yatomi, Y. Successful treatment with dabigatran for consumptive coagulopathy associated with extensive vascular malformations. Blood Coagul. Fibrinolysis 2017, 28, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Ardillon, L.; Lambert, C.; Eeckhoudt, S.; Boon, L.M.; Hermans, C. Dabigatran etexilate versus low-molecular weight heparin to control consumptive coagulopathy secondary to diffuse venous vascular malformations. Blood Coagul. Fibrinolysis 2016, 27, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Binet, Q.; Lambert, C.; Hermans, C. Dabigatran etexilate in the treatment of localized intravascular coagulopathy associated with venous malformations. Thromb. Res. 2018, 168, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, L.; Yang, X.; Xu, Z.; Gu, H.; Chen, H.; Lin, X. Dabigatran etexilate is efficacious in consumptive coagulopathy and pain associated with venous malformations. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 397–403.e1. [Google Scholar] [CrossRef]
- Kawano, H.; Hata, T.; Uda, A.; Maemura, K. Use of rivaroxaban for the effective management of disseminated intravascular coagulation associated with abdominal aortic aneurysm. Intern. Med. 2015, 54, 2625–2628. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Kopp, H.G.; Balletshofer, B.M.; Jaschonek, K.; Kanz, L.; Haering, H.U.; Rittig, K. Management of disseminated intravascular coagulopathy with direct factor Xa inhibitor rivaroxaban in Klippel-Trénaunay syndrome. Blood Coagul. Fibrinolysis 2013, 24, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Vandenbriele, C.; Vanassche, T.; Peetermans, M.; Verhamme, P.; Peerlinck, K. Rivaroxaban for the treatment of consumptive coagulopathy associated with a vascular malformation. J. Thromb. Thrombolysis 2014, 38, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.M.; Richter, G.T.; Crary, S.E. Effectiveness and Safety of Treatment with Direct Oral Anticoagulant Rivaroxaban in Patients with Slow-Flow Vascular Malformations: A Case Series. Lymphat. Res. Biol. 2018, 16, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, S.; Holstein, K.; Dicke, C.; Bokemeyer, C.; Langer, F. Apixaban for the Treatment of Chronic Disseminated Intravascular Coagulation: A Report of Two Cases. Hamostaseologie 2019, 39, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Kobayashi, H.; Seki, Y.; Okoshi, Y.; Sone, H.; Nomoto, N. Successful Treatment with Edoxaban for Disseminated Intravascular Coagulation in a Case of Aortic Dissection Complicated with Immune Thrombocytopenic Purpura. Intern. Med. 2020, 59, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Naeye, R.L. Thrombotic state after a hemorrhagic diathesis, a possible complication of therapy with epsilon-aminocapproic acid. Blood 1962, 19, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Charytan, C.; Purtilo, D. Glomerular capillary thrombosis and acute renal failure after epsilon-amino caproic acid therapy. N. Engl. J. Med. 1969, 280, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Coppo, P.; Schwarzinger, M.; Buffet, M.; Wynckel, A.; Clabault, K.; Presne, C.; Poullin, P.; Malot, S.; Vanhille, P.; Azoulay, E.; et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: The French TMA reference center experience. PLoS ONE 2010, 5, e10208. [Google Scholar] [CrossRef] [PubMed]
- Bendapudi, P.K.; Hurwitz, S.; Fry, A.; Marques, M.B.; Waldo, S.W.; Li, A.; Sun, L.; Upadhyay, V.; Hamdan, A.; Brunner, A.M.; et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: A cohort study. Lancet Haematol. 2017, 4, e157–e164. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Dhaliwal, N.; Upreti, H.; Kasmani, J.; Dane, K.; Moliterno, A.; Braunstein, E.; Brodsky, R.; Chaturvedi, S. Reduced sensitivity of PLASMIC and French scores for the diagnosis of thrombotic thrombocytopenic purpura in older individuals. Transfusion 2020, 61, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Andrès, E.; Affenberger, S.; Zimmer, J.; Vinzio, S.; Grosu, D.; Pistol, G.; Maloisel, F.; Weitten, T.; Kaltenbach, G.; Blicklé, J.F. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin. Lab. Haematol. 2006, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Hayashi, K.; Awai, M. Disseminated intravascular coagulation (DIC). Immunohistochemical study of fibrin-related materials (FRMs) in renal tissues. Acta Pathol. Jpn. 1987, 37, 77–84. [Google Scholar] [PubMed]
- Nishimura, N.; Yoshimoto, K.; Yada, N.; Kakiwaki, A.; Sawa, A.; Senzaki, S.; Kawashima, H.; Yoneima, R.; Ono, S.; Sakai, K.; et al. The Combination of the Lactate Dehydrogenase/Hemoglobin Ratio with the PLASMIC Score Facilitates Differentiation of TTP from Septic DIC without Identification of Schistocytes. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231207629. [Google Scholar] [CrossRef] [PubMed]
- di Masi, A.; De Simone, G.; Ciaccio, C.; D’Orso, S.; Coletta, M.; Ascenzi, P. Haptoglobin: From hemoglobin scavenging to human health. Mol. Aspects Med. 2020, 73, 100851. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Yu, P.; Ni, J.; Zhou, J. Higher serum haptoglobin levels were associated with improved outcomes of patients with septic shock. Crit. Care 2022, 26, 131. [Google Scholar] [CrossRef] [PubMed]

| Classification | Suppressed- Fibrinolytic-Type | Balanced- Fibrinolytic-Type | Enhanced- Fibrinolytic-Type |
|---|---|---|---|
| Underlying disease | Sepsis | Solid tumors | APL, aortic aneurysm, severe COVID-19 |
| Platelet count | Decreased | Decreased | Decreased |
| PT | Prolonged | Prolonged | Normal to prolonged |
| APTT | Prolonged | Prolonged | Mildly shortened to prolonged |
| Fibrinogen | Normal to increased | Decreased | Markedly decreased |
| FDP | Mildly increased | Increased | Markedly increased |
| D-dimer | Mildly increased | Increased | Increased |
| FDP/D-dimer ratio | Approximately 1 | Approximately 1–2 | Approximately 2–5 |
| Antithrombin | Decreased | Decreased to normal | Normal |
| TAT (coagulation activation marker) | Markedly increased | Markedly increased | Markedly increased |
| PIC (fibrinolysis activation marker) | Mildly increased | Increased | Markedly increased |
| α2PI | Normal | Mildly decreased | Markedly decreased |
| Plasminogen | Decreased | Mildly decreased | Decreased |
| PAI | Markedly increased | Mildly increased | Normal to mildly increased |
| Criteria | JMHLW | ISTH | JAAM | JSTH | ||
|---|---|---|---|---|---|---|
| Basic | Hematopoietic Disorder | Infectious | ||||
| Platelet count (×104/μL) | ≤12 + 1 pt ≤8 + 2 pt ≤5 + 3 pt | ≤10 + 1 pt ≤5 + 2 pt | ≤12 + 1 pt ≤8 + 3 pt >30% reduction/24 h + 1 pt >50% reduction/24 h + 3 pt | >8, ≤12 + 1 pt >5, ≤8 + 2 pt ≤5 + 3 pt ≥30% decrease/24 h + 1 pt | - | >8, ≤12 + 1 pt >5, ≤8 + 2 pt ≤5 + 3 pt ≥30% decrease/24 h + 1 pt |
| PT ratio | ≥1.25 + 1 pt ≥1.67 + 2 pt | - | ≥1.2 + 1 pt | ≥1.25, <1.67 + 1 pt ≥1.67 + 2 pt | ||
| PT (s) | - | Prolonged PT ≥3 s + 1 pt ≥6 s + 2 pt | - | - | ||
| Fibrinogen (mg/dL) | ≤150 + 1 pt ≤100 + 2 pt | ≤100 + 1 pt | - | >100, ≤150 + 1 pt ≤100 + 2 pt | - | |
| FDP (μg/mL) | ≥10 + 1 pt ≥20 + 2 pt ≥40 + 3 pt | ≥10 + 2 pt ≥25 + 3 pt | ≥10 + 1 pt ≥25 + 3 pt | ≥10, <20 + 1 pt ≥20, <40 + 2 pt ≥40 + 3 pt | ||
| Antithrombin (%) | - | - | - | ≤70% + 1 pt | ||
| TAT, SF, F1 + 2 | - | - | - | ≥2-fold upper limit of normal + 1 pt | ||
| SIRS | - | - | ≥3 + 1 pt | - | ||
| Underlying diseases | +1 pt | - | - | - | ||
| Bleeding | +1 pt | - | ||||
| Organ failure | +1 pt | |||||
| Liver failure | - | - | - | Yes -3 pt | ||
| Diagnosis of DIC | ≥7 pt | ≥5 pt | ≥4 pt | ≥6 pt | ≥4 pt | ≥5 pt |
| Disease | Important Clinical and Laboratory Findings | ||
|---|---|---|---|
| HIT | History of exposure to heparin, 4Ts score, anti-HIT antibody | ||
| VITT | Vaccination history, thrombosis at unusual sites, anti-HIT antibodies (ELISA assay) | ||
| PNH | Hemolysis, hemoglobinuria, PNH cells (CD55/59-negative) | ||
| APS | Repeated arteriovenous thrombosis, prolonged APTT, positive for at least one of LA/anti-CL antibody/anti-β2GPI antibody | ||
| TMA | TTP | Schizocyte, marked decrease in haptoglobin | Marked decrease in ADAMTS13 activity |
| STEC-HUS | Bloody stool with mucous, EHEC infection, Shiga toxin | ||
| aHUS | Abnormalities in complement-related genes | ||
| Secondary TMA, other TMAs | Underlying disease, other TMAs ruled out | ||
| DIC | PT, APTT, fibrinogen, FDP, D-dimer, antithrombin, TAT, PIC, plasminogen, α2PI | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Asakura, H. How We Interpret Thrombosis with Thrombocytopenia Syndrome? Int. J. Mol. Sci. 2024, 25, 4956. https://doi.org/10.3390/ijms25094956
Yamada S, Asakura H. How We Interpret Thrombosis with Thrombocytopenia Syndrome? International Journal of Molecular Sciences. 2024; 25(9):4956. https://doi.org/10.3390/ijms25094956
Chicago/Turabian StyleYamada, Shinya, and Hidesaku Asakura. 2024. "How We Interpret Thrombosis with Thrombocytopenia Syndrome?" International Journal of Molecular Sciences 25, no. 9: 4956. https://doi.org/10.3390/ijms25094956





