An Assessment of the Effectiveness and Safety of Chimeric Antigen Receptor T-Cell Therapy in Multiple Myeloma Patients with Relapsed or Refractory Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection Process
2.4. Data Extraction
2.5. Risk of Bias
2.6. Data Synthesis and Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics and Initial Qualities of the Enrolled Patients
3.3. Meta-Analysis Results
3.3.1. Efficacy Outcomes
3.3.2. Safety Outcomes
3.3.3. Subgroup Analysis
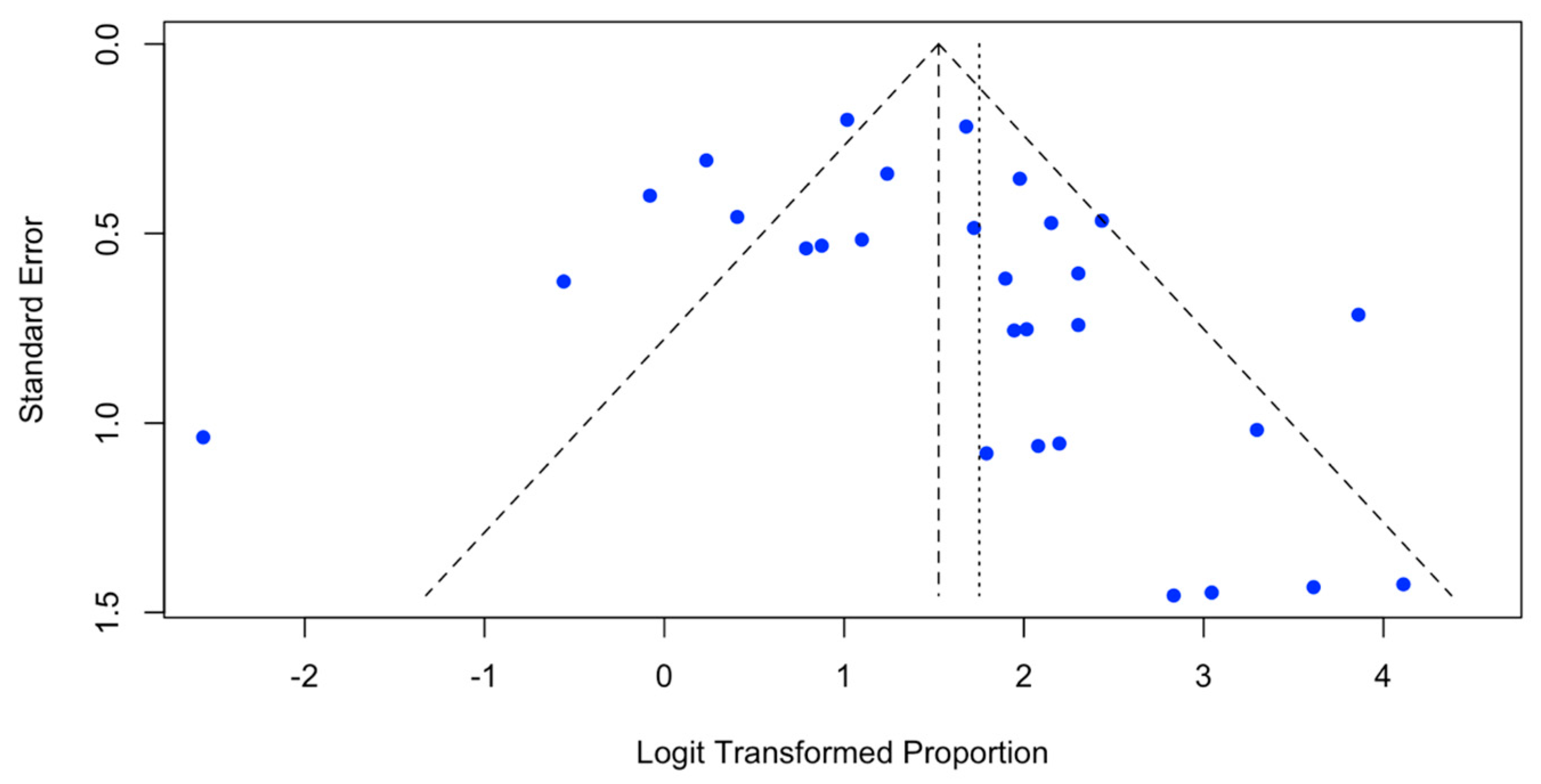
3.4. Risk of Bias in the Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyle, R.A. Multiple myeloma: How did it begin? Mayo Clin. Proc. 1994, 69, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, C.; Fuhrmann, S.; Strifler, S.; Schmidt-Hieber, M.; Einsele, H.; Knop, S. The Diagnosis and Treatment of Multiple Myeloma. Dtsch. Arztebl. Int. 2016, 113, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Firth, J. Haematology: Multiple myeloma. Clin. Med. 2019, 19, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Hosen, N. Chimeric antigen receptor T-cell therapy for multiple myeloma. Int. J. Hematol. 2020, 111, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, J.; Tuchman, S.; Grant, S.J. What Is Multiple Myeloma? JAMA 2022, 327, 497. [Google Scholar] [CrossRef] [PubMed]
- Brigle, K.; Rogers, B. Pathobiology and Diagnosis of Multiple Myeloma. Semin. Oncol. Nurs. 2017, 33, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Dima, D.; Ullah, F.; Mazzoni, S.; Williams, L.; Faiman, B.; Kurkowski, A.; Chaulagain, C.; Raza, S.; Samaras, C.; Valent, J.; et al. Management of Relapsed-Refractory Multiple Myeloma in the Era of Advanced Therapies: Evidence-Based Recommendations for Routine Clinical Practice. Cancers 2023, 15, 2160. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Wang, L.; Jia, Y.; Qin, Y. Chimeric antigen receptor T-cell therapy for multiple myeloma. Front. Immunol. 2022, 13, 1050522. [Google Scholar] [CrossRef]
- Sadelain, M.; Rivière, I.; Riddell, S. Therapeutic T cell engineering. Nature 2017, 545, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.H.; Lonial, S. Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice. CA Cancer J. Clin. 2023, 73, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh Kozani, P.; Naseri, A.; Mirarefin, S.M.J.; Salem, F.; Nikbakht, M.; Evazi Bakhshi, S. Nanobody-based CAR-T cells for cancer immunotherapy. Biomark Res. 2022, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw Dejenie, T.; Tiruneh G/Medhin, M.; Dessie Terefe, G.; Tadele Admasu, F.; Wale Tesega, W.; Chekol Abebe, E. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum. Vaccin Immunother. 2022, 18, 2114254. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Dotti, G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin. Biol. Ther. 2011, 11, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering CAR-T cells. Biomark Res. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Riddell, S.R. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015, 36, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, W.; Li, D.; Niu, T.; Wang, W. BCMA-targeting chimeric antigen receptor T-cell therapy for multiple myeloma. Cancer Lett. 2023, 553, 215949. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef]
- Adkins, S. CAR T-Cell Therapy: Adverse Events and Management. J. Adv. Pract. Oncol. 2019, 10, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Hu, G.; Wang, W.; Xiao, Y.; Cai, H.; Jiang, L.; Meng, L.; Yang, Y.; Zhou, X.; et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood 2021, 137, 2890–2901. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.J.; Yang, S.S.; Sun, Y.; Wu, W.; Liu, Y.F.; Zhuang, Y.; Zhang, W.; Weng, X.Q.; Wu, J.; et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc. Natl. Acad. Sci. USA 2019, 116, 9543–9551. [Google Scholar] [CrossRef]
- Yan, L.; Qu, S.; Shang, J.; Shi, X.; Kang, L.; Xu, N.; Zhu, M.; Zhou, J.; Jin, S.; Yao, W.; et al. Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma. Cancer Med. 2021, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Qi, K.; Shi, M.; Cao, J.; Bhansali, R.; Wang, X.; Liu, Y.; Li, H.; Zhang, H.; et al. Efficacy and Safety of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Immunoglobulin D Multiple Myeloma. Transplant. Cell. Ther. 2021, 27, 273.e1–273.e5. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Xiao, X.; Lv, H.; Jiang, Y.; Li, X.; Yuan, T.; Zhao, M. A combination of humanized anti-BCMA and murine anti-CD38 CAR-T cell therapy in patients with relapsed or refractory multiple myeloma. Leuk. Lymphoma 2022, 63, 1418–1427. [Google Scholar] [CrossRef]
- Du, J.; Wei, R.; Jiang, S.; Jiang, H.; Li, L.; Qiang, W.; He, H.; Shi, L.; Ma, Q.; Yu, K.; et al. CAR-T cell therapy targeting B cell maturation antigen is effective for relapsed/refractory multiple myeloma, including cases with poor performance status. Am. J. Hematol. 2022, 97, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.; Gu, W.; Shi, M.; Lan, J.; Yan, Z.; Jin, L.; Xia, J.; Ma, S.; Liu, Y.; et al. Long-Term Follow-Up of Combination of B-Cell Maturation Antigen and CD19 Chimeric Antigen Receptor T Cells in Multiple Myeloma. J. Clin. Oncol. 2022, 40, 2246–2256. [Google Scholar] [CrossRef]
- Zhao, W.H.; Wang, B.Y.; Chen, L.J.; Fu, W.J.; Xu, J.; Liu, J.; Jin, S.W.; Chen, Y.X.; Cao, X.M.; Yang, Y.; et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: A phase 1, single-arm, open-label, multicenter study in China (LEGEND-2). J. Hematol. Oncol. 2022, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, S.; Devlin, S.M.; Landa, J.; Nath, K.; Diamonte, C.; Carstens, E.J.; Russo, D.; Auclair, R.; Fitzgerald, L.; Cadzin, B.; et al. GPRC5D-Targeted CAR T Cells for Myeloma. N. Engl. J. Med. 2022, 387, 1196–1206. [Google Scholar] [CrossRef]
- Qu, X.; An, G.; Sui, W.; Wang, T.; Zhang, X.; Yang, J.; Zhang, Y.; Zhang, L.; Zhu, D.; Huang, J.; et al. Phase 1 study of C-CAR088, a novel humanized anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. J. Immunother. Cancer 2022, 10, e005145. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, H.; Zhao, X.; Jin, D.; Liang, Y.; Xiong, T.; Li, L.; Tang, W.; Zhang, J.; Liu, M.; et al. High efficacy and safety of CD38 and BCMA bispecific CAR-T in relapsed or refractory multiple myeloma. J. Exp. Clin. Cancer Res. 2022, 41, 2. [Google Scholar] [CrossRef]
- Ri, M.; Suzuki, K.; Ishida, T.; Kuroda, J.; Tsukamoto, T.; Teshima, T.; Goto, H.; Jackson, C.C.; Sun, H.; Pacaud, L.; et al. Ciltacabtagene autoleucel in patients with relapsed/refractory multiple myeloma: CARTITUDE-1 (phase 2) Japanese cohort. Cancer Sci. 2022, 113, 4267–4276. [Google Scholar] [CrossRef]
- Cohen, A.D.; Mateos, M.V.; Cohen, Y.C.; Rodriguez-Otero, P.; Paiva, B.; van de Donk, N.W.C.J.; Martin, T.; Suvannasankha, A.; De Braganca, K.C.; Corsale, C.; et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood 2023, 141, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Minakata, D.; Ishida, T.; Ando, K.; Suzuki, R.; Tanaka, J.; Hagiwara, S.; Ananthakrishnan, R.; Kuwayama, S.; Nishio, M.; Kanda, Y.; et al. Phase 2 results of idecabtagene vicleucel (ide-cel, bb2121) in Japanese patients with relapsed and refractory multiple myeloma. Int. J. Hematol. 2023, 117, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, H.; Yan, Z.; Zhou, D.; Wang, Y.; Qi, Y.; Cao, J.; Li, D.; Cheng, H.; Sang, W.; et al. Anti-G Protein-Coupled Receptor, Class C Group 5 Member D Chimeric Antigen Receptor T Cells in Patients with Relapsed or Refractory Multiple Myeloma: A Single-Arm, Phase II Trial. J. Clin. Oncol. 2023, 41, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.F.; Bishop, M.R.; Kumar, S.; Giralt, S.A.; Nooka, A.K.; Larson, S.M.; Locke, F.L.; Raje, N.S.; Lei, L.; Dong, J.; et al. A phase 1, multicenter study evaluating the safety and efficacy of KITE-585, an autologous anti-BCMA CAR T-cell therapy, in patients with relapsed/refractory multiple myeloma. Am. J. Cancer Res. 2021, 11, 3285–3293. [Google Scholar] [PubMed]
- Mei, H.; Li, C.; Jiang, H.; Zhao, X.; Huang, Z.; Jin, D.; Guo, T.; Kou, H.; Liu, L.; Tang, L.; et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2021, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, S.; Matous, J.V.; Chhabra, S.; Liedtke, M.; Sidana, S.; Oluwole, O.O.; Malik, S.; Nath, R.; Anwer, F.; Cruz, J.C.; et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: Phase 1 UNIVERSAL trial interim results. Nat. Med. 2023, 29, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2023, 41, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Lim, W.C.; Galas-Filipowicz, D.; Fung, K.; Taylor, J.; Patel, D.; Akbar, Z.; Alvarez Mediavilla, E.; Wawrzyniecka, P.; Shome, D.; et al. Limited efficacy of APRIL CAR in patients with multiple myeloma indicate challenges in the use of natural ligands for CAR T-cell therapy. J. Immunother. Cancer 2023, 11, e006699. [Google Scholar] [CrossRef]
- Oliver-Caldés, A.; González-Calle, V.; Cabañas, V.; Español-Rego, M.; Rodríguez-Otero, P.; Reguera, J.L.; López-Corral, L.; Martin-Antonio, B.; Zabaleta, A.; Inogés, S.; et al. Fractionated initial infusion and booster dose of ARI0002h, a humanised, BCMA-directed CAR T-cell therapy, for patients with relapsed or refractory multiple myeloma (CARTBCMA-HCB-01): A single-arm, multicentre, academic pilot study. Lancet Oncol. 2023, 24, 913–924. [Google Scholar] [CrossRef]
- Asherie, N.; Kfir-Erenfeld, S.; Avni, B.; Assayag, M.; Dubnikov, T.; Zalcman, N.; Lebel, E.; Zimran, E.; Shaulov, A.; Pick, M.; et al. Development and manufacture of novel locally produced anti-BCMA CAR T cells for the treatment of relapsed/refractory multiple myeloma: Results from a phase I clinical trial. Haematologica 2023, 108, 1827–1839. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.Q.; Zhao, W.; Jing, H.; Fu, W.; Hu, J.; Chen, L.; Zhang, Y.; Yao, D.; Chen, D.; Schecter, J.M.; et al. Phase II, Open-Label Study of Ciltacabtagene Autoleucel, an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor-T-Cell Therapy, in Chinese Patients With Relapsed/Refractory Multiple Myeloma (CARTIFAN-1). J. Clin. Oncol. 2023, 41, 1275–1284. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, G.; Zhou, L.; Zhou, J.; Chen, S.; Zhang, W.; Wang, D.; Luo, X.; Cui, J.; Huang, S.; et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): A first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol. 2023, 10, e107–e116. [Google Scholar] [CrossRef] [PubMed]
- Sanoyan, D.A.; Seipel, K.; Bacher, U.; Kronig, M.N.; Porret, N.; Wiedemann, G.; Daskalakis, M.; Pabst, T. Real-life experiences with CAR T-cell therapy with idecabtagene vicleucel (ide-cel) for triple-class exposed relapsed/refractory multiple myeloma patients. BMC Cancer 2023, 23, 345. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; Sidana, S.; Peres, L.C.; Colin Leitzinger, C.; Shune, L.; Shrewsbury, A.; Gonzalez, R.; Sborov, D.W.; Wagner, C.; Dima, D.; et al. Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma: Real-World Experience From the Myeloma CAR T Consortium. J. Clin. Oncol. 2023, 41, 2087–2097. [Google Scholar] [CrossRef]
- Tanenbaum, B.; Miett, T.; Patel, S.A. The emerging therapeutic landscape of relapsed/refractory multiple myeloma. Ann. Hematol. 2023, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef]
- Garfall, A.L.; Cohen, A.D.; Susanibar-Adaniya, S.P.; Hwang, W.T.; Vogl, D.T.; Waxman, A.J.; Lacey, S.F.; Gonzalez, V.E.; Fraietta, J.A.; Gupta, M.; et al. Anti-BCMA/CD19 CAR T Cells with Early Immunomodulatory Maintenance for Multiple Myeloma Responding to Initial or Later-Line Therapy. Blood Cancer Discov. 2023, 4, 118–133. [Google Scholar] [CrossRef]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.V.; Fernández de Larrea, C.; Martínez-López, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef]
- Boccadoro, M.; San-Miguel, J.; Suzuki, K.; Van De Donk, N.W.C.J.; Cook, G.; Jakubowiak, A.; Madduri, D.; Afifi, S.; Stevens, A.-S.; Schecter, J.M.; et al. DVRd Followed By Ciltacabtagene Autoleucel Versus DVRd Followed By ASCT in Patients with Newly Diagnosed Multiple Myeloma Who Are Transplant Eligible: A Randomized Phase 3 Study (EMagine/CARTITUDE-6). Blood 2022, 140, 4630–4632. [Google Scholar] [CrossRef]
- Dytfeld, D.; Dhakal, B.; Agha, M.; Manier, S.; Delforge, M.; Kuppens, S.; Afifi, S.; Deraedt, W.; Taraseviciute-Morris, A.; Schecter, J.M.; et al. Bortezomib, Lenalidomide and Dexamethasone (VRd) Followed By Ciltacabtagene Autoleucel Versus Vrd Followed By Lenalidomide and Dexamethasone (Rd) Maintenance in Patients with Newly Diagnosed Multiple Myeloma Not Intended for Transplant: A Randomized, Phase 3 Study (CARTITUDE-5). Blood 2021, 138, 1835. [Google Scholar] [CrossRef]
- Anderson, L.D.; Dhakal, B.; Jain, T.; Oluwole, O.O.; Shah, G.L.; Sidana, S.; Perales, M.A.; Pasquini, M.C. Chimeric Antigen Receptor T Cell Therapy for Myeloma: Where Are We Now and What Is Needed to Move Chimeric Antigen Receptor T Cells Forward to Earlier Lines of Therapy? Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transplant. Cell. Ther. 2024, 30, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Avet-Loiseau, H.; Anderson, K.C.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.; Iqbal, M.; Chavez, J.C.; Kharfan-Dabaja, M.A. Cytokine Release Syndrome: Current Perspectives. Immunotargets Ther. 2019, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, V.E.; Wong, C.; Huang, C.Y.; Kambhampati, S.; Wolf, J.; Martin, T.G.; Shah, N.; Wong, S.W. Macrophage activation syndrome-like (MAS-L) manifestations following BCMA-directed CAR T cells in multiple myeloma. Blood Adv. 2021, 5, 5344–5348. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B.; Danish, H.H.; Ali, A.B.; Li, K.; LaRose, S.; Monk, A.D.; Cote, D.J.; Spendley, L.; Kim, A.H.; Robertson, M.S.; et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain 2019, 142, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Chohan, K.L.; Siegler, E.L.; Kenderian, S.S. CAR-T Cell Therapy: The Efficacy and Toxicity Balance. Curr. Hematol. Malig. Rep. 2023, 18, 9–18. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Reactions Related to CAR-T Cell Therapy. Front. Immunol. 2021, 12, 663201. [Google Scholar] [CrossRef]
- Mohan, M.; Chakraborty, R.; Bal, S.; Nellore, A.; Baljevic, M.; D’Souza, A.; Pappas, P.G.; Berdeja, J.G.; Callander, N.; Costa, L.J. Recommendations on prevention of infections during chimeric antigen receptor T-cell and bispecific antibody therapy in multiple myeloma. Br. J. Haematol. 2023, 203, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Porter, D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2019, 25, e123–e127. [Google Scholar] [CrossRef] [PubMed]
- Frigault, M.; Rotte, A.; Ansari, A.; Gliner, B.; Heery, C.; Shah, B. Dose fractionation of CAR-T cells. A systematic review of clinical outcomes. J. Exp. Clin. Cancer Res. 2023, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, S.; Chen, S.; Wang, Y.; Sun, Q.; Xu, X.; Li, Y. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J. Exp. Clin. Cancer Res. 2021, 40, 367. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Maldonado, V.; Rives, S.; Español-Rego, M.; Alonso-Saladrigues, A.; Montoro, M.; Magnano, L.; Giné, E.; Pascal, M.; Díaz-Beyá, M.; Castella, M.; et al. Factors associated with the clinical outcome of patients with relapsed/refractory CD19. J. Immunother. Cancer 2021, 9, e003644. [Google Scholar] [CrossRef]
- Afrough, A.; Hashmi, H.; Hansen, D.K.; Sidana, S.; Ahn, C.; Dima, D.; Freeman, C.L.; Puglianini, O.A.C.; Kocoglu, M.H.; Atrash, S.; et al. Impact of bridging therapy (BT) on outcome of relapsed refractory multiple myeloma (RRMM) with Ide-cel CAR T-cell therapy: Real-world experience from the US myeloma CAR T consortium. J. Clin. Oncol. 2023, 41, 8013. [Google Scholar] [CrossRef]
- Ferreri, C.J.; Hildebrandt, M.A.T.; Hashmi, H.; Shune, L.O.; McGuirk, J.P.; Sborov, D.W.; Wagner, C.B.; Kocoglu, M.H.; Rapoport, A.; Atrash, S.; et al. Real-world experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted therapy. Blood Cancer J. 2023, 13, 117. [Google Scholar] [CrossRef]








| First Author, Year | CAR-T Production Name | Bridging Therapy, Number (%) | Lymphodepletion Regimen | CAR-T Cell Dose | Median Follow-Up (Range), Years or Months |
|---|---|---|---|---|---|
| Raje 2019 [29] | Ide-cel | 14 (42.4) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 50–800 × 106 CAR-T cells Dose escalation—50 × 106 (n = 3), 150 × 106 (n = 6), 450 × 106 (n = 9), 800 × 106 (n = 3) Dose expansion—150 × 106 (n = 2), 450 × 106 (n = 10) | 11 m (6–23) |
| Xu 2019 [30] | LCAR-B38M | NR | fludarabine 25 mg/m2 + cyclophosphamide 250 mg/m2 daily for 3 days, or cyclophosphamide 300 mg/m2 daily for 3 days | 0.21–1.52 × 106 CAR-T cells/Kg | 417 d (12–535) |
| Cohen 2019 [52] | CART-BCMA | NR | Cohort 1—No LD Cohort 2—Cyclophosphamide 1.5 g/m2 Cohort 3—Cyclophosphamide 1.5 g/m2 | 10–500 × 106 CAR-T cells Cohort 1—100–500 × 106 Cohort 2—10–50 × 106 Cohort 3—100–500 × 106 | NR |
| Yan 2020 [31] | CD19 and BCMA CAR-T | NR | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | Anti-BCMA—2.5–6.8 × 107 CAR-T cells/Kg Anti-CD19—1 × 107 CAR-T cells/Kg | 20 m |
| Chen 2020 [32] | CD19 and BCMA CAR-T | NR | fludarabine 30mg/m2 daily for 3 days + cyclophosphamide 750mg/m2 for 1 day | Anti-BCMA—1–2 × 106 CAR-T cells/Kg Anti-CD19—1 × 106 CAR-T cells/Kg | 433 d (230–742) |
| Wang 2021 [28] | CT103A | 1 (5.55) | fludarabine 25 mg/m2 + cyclophosphamide 20 mg/Kg daily for 3 days | 1–6 × 106 CAR-T cells/Kg 1 × 106 (9), 3 × 106 (6), 6 × 106 (3) | 394 d |
| Cornell 2021 [44] | KITE-585 | 5 (35.7) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 3–100 × 107 CAR-T cells | 12 m (8.7–14) |
| Mei 2021 [45] | BM38 | NR | fludarabine 25 mg/m2 + cyclophosphamide 250 mg/m2 daily for 3 days | 0.5—4.0 × 106 CAR-T cells/Kg | 9 m (0.5–18.5) |
| Munshi 2021 [46] | Ide-cel | 112 (87.5) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 150–450 × 106 CAR-T cells 150 × 106 (n = 4), 300 × 106 (n = 70), 450 × 106 (n = 54) | 13.3 m (0.20–21.2) |
| Zhang 2022 [33] | BCMA and CD38 CAR-T | NR | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | Anti-BCMA—2.0 × 106 CAR-T cells/Kg Anti-CD38—2.0 × 106 CAR-T cells/Kg | 24 m (0.5–33) |
| Du 2022 [34] | HDS269B | NR | fludarabine 30mg/m2 + cyclophosphamide 300mg/m2 daily for 3 days | 9 × 106 CAR-T cells/Kg | 14 m (1–42.5) |
| Wang 2022 [35] | CD19 and BCMA CAR-T | NR | fludarabine 30mg/m2 daily for 3 days + cyclophosphamide 750 mg/m2 for 1 day | 1 × 106 CAR-T cells/Kg | 21.3 m |
| Zhao 2022 [36] | LCAR-B38M | 0 | fludarabine 25 mg/m2 + cyclophosphamide 250 mg/m2, or cyclophosphamide 300 mg/m2 daily for 4 days | 0.07–2.10 × 106 cells/kg | 47.8 m (0.4–60.7) |
| Mailankody 2022 [37] | MCARH109 | 16 (94.1) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 25–450 × 106 CAR-T cells 25 × 106 (n = 3), 50 × 106 (n = 3), 150 × 106 (n = 6), 450 × 106 (n = 5) | 10.1 m |
| Qu 2022 [38] | C-CAR088 | 7 (22.6) | fludarabine 30mg/m2 + cyclophosphamide 300mg/m2 daily for 3 days | 1.0–6.0 × 106 CAR-T cells/Kg 1.0 × 106 (n = 4), 3.0 × 106 (n = 13), 4.5–6.0 × 106 (n = 14) | 9.4 m (1.9–24.2) |
| Tang 2022 [39] | BCMA and CD38 CAR-T | NR | fludarabine 25 mg/m2 + cyclophosphamide 250 mg/m2 daily for 3 days | 0.5–10.0 × 106 CAR-T cells/Kg | 11.5 m (6.0–26.0) |
| Ri 2022 [40] | Cilta-cel | 9 (100) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 0.41–0.72 × 106 CAR-T cells/Kg | 8.5 m |
| Martin 2022 [48] | Cilta-cel | 73 (75.3) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 0.51–0.95 × 106 CAR-T cells/Kg | 27.7 m |
| Mi 2022 [53] | Cilta-cel | NR | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 0.42–0.84 × 106 CAR-T cells/Kg | 18 m (0.20–28.0) |
| Asherie 2022 [51] | HBI0101 | 3 (15.0) | fludarabine 25 mg/m2 + cyclophosphamide 250 mg/m2 daily for 3 days | 150–800 × 106 CAR-T cells 150 × 106 (n = 6), 450 × 106 (n = 7), 800 × 106 (n = 7) | 136 d |
| Cohen 2023 [41] | Cilta-cel | 18 (90.0) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 0.21–1.11 × 106 CAR-T cells/Kg | 11.3 m (0.60–16.0) |
| Xia 2023 [43] | anti-GPRC5D CAR T | NR | fludarabine 30 mg/m2 daily for 3 days + cyclophosphamide 750 mg/m2 for 1 day | 2 × 106 CAR-T cells/Kg | 5.2 m (3.2–8.9) |
| Mailankody 2023 [47] | ALLO-715 | 0 | fludarabine 90 mg/m2 + cyclophosphamide 900 mg/m2 daily for 3 days, or cyclophosphamide 900 mg/m2 daily for 3 days | 40–480 × 106 CAR-T cells 40 × 106 (n = 3), 160 × 106 (n = 7), 320 × 106 (n = 27), 480 × 106 (n = 6) | 10.2 m (3.8-NE) |
| Lee 2023 [49] | APRIL CAR-T | 4 (36.4) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 15–900 × 106 CAR-T cells | NR |
| Oliver-Caldés 2023 [50] | ARI0002h | 14 (46.7) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 0.3–3 × 106 CAR-T cells/Kg | 18 m (15–20) |
| Zhang 2023 [54] | OriCAR-017 | 2 (20.0) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 1–6 × 106 CAR-T cells/Kg 1 × 106 (n = 3), 3 × 106 (n = 4), 6 × 106 (n = 3) | 238 d (182–307) |
| Minakata 2023 [42] | Ide-cel | 8 (88.9) | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 450 × 106 CAR-T cells | 12.9 m (3.30–17.8) |
| Sanoyan 2023 * [55] | Ide-cel | NR | fludarabine 30 mg/m2 + cyclophosphamide 300 mg/m2 daily for 3 days | 450 × 106 CAR-T cells | 5.7 m (0.6–9.0) |
| Hansen 2023 * [56] | Ide-cel | 123 (77.4) | fludarabine (dose adjustment based on creatinine clearance) + cyclophosphamide 300 mg/m2 daily for 3 days | NR | 6.1m (0.0–13.1) |
| First Author, Year | Extramedullary Disease, Number (%) | High-Risk Cytogenetic Profile, Number (%) | Median No. of Previous Antimyeloma Regimens | Prior Treatment Class | Previous CAR-T Cell Therapy, Number (%) | Previous BCMA Therapy, Number (%) | ASCT before CAR-T, Number (%) |
|---|---|---|---|---|---|---|---|
| Raje 2019 [29] | 9 (27) | 15 (46) del(17p), t(4;14), t(14;16) | 7 (3–23) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 32 (97) |
| Xu 2019 [30] | NR | 6(35) t(4;14), del(17p) | 5 (3–11) | ASCT, proteasome inhibitor, immunomodulatory drug | 0 | 0 | 8 (47) |
| Cohen 2019 [52] | 7 (28) | 24 (96) Defined as del(17p), t(14;16), t(4;14), gain 1q | 7 (3–13) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 23 (92) |
| Yan 2020 [31] | NR | 5 (50) t(4;14), 1q21 amp | 4 (2–7) | ASCT, proteasome inhibitor, immunomodulatory drug | 0 | 0 | 6 (60) |
| Chen 2020 [32] | 1 (14) | 2 (28) Defined as del(17p), t(4;14) or t(14;16) | 5 (2–9) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 2 (28) |
| Wang 2021 [28] | 5 (27.8) | 7 (38.9) | 4 (3–6) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb, murine BCMA CART | 4 (22.2) | 4 (22.2), Murine BCMA CART | 6 (33.3) |
| Cornell 2021 [44] | NR | 2 (12) | 5.5 (3–8) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 16 (94) |
| Mei 2021 [45] | 9 (39) | 17 (74) Includes amplification 1q21, del(17p), del(13q), t(4;14), t(11;14) and t(14;16) | 4 (2–9) | ASCT, proteasome inhibitor, immunomodulatory drug | NR | NR | 3 (13) |
| Munshi 2021 [46] | 50 (39) | 45 (35) Defined as del(17p), t(14;16), t(4;14) | 6 (3–16) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 120 (94) |
| Zhang 2022 [33] | 3 (13.6) | 19 (86.4) Defined as del(17p), t(14;16), t(4;14 | 8 (4–12) | Proteasome inhibitor, immunomodulatory drugs, anthracyclines/cyclophosphamide, ASCT | 0 | 0 | 19 (86.4) |
| Du 2022 [34] | 11 (22.45) | 21 (42.86) Defined as del(17p), t(14;16), t(4;14) | 4 (2–12) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 14 (28.57) |
| Wang 2022 [35] | 15 (24) | 18 (29) Defined as del(17p), t(14;16), t(4;14) | 4 (2–17) | ASCT, CAR-T cell infusion, proteasome inhibitor, immunomodulatory drug, anti-CD38 monoclonal antibody | 4 (7) | 0 | 17 (27) |
| Zhao 2022 [36] | 22 (29.7) | 15 (35.7) Defined as del(17p), t(14;16), t(4;14) | 3 (1–9) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 18 (24.3) |
| Mailankody 2022 [37] | 8 (47) | 13 (76) Defined as del(17p), t(14;16), t(4;14) and 1q gain | 6 (4–14) | ASCT, CAR-T cell infusion, proteasome inhibitor, immunomodulatory drug, anti-CD38 monoclonal antibody, BCMA targeted therapies | 8 (47), BCMA CAR-T cell | 10 (59) | 17 (100) |
| Qu 2022 [38] | 3 (9.7) | 15 (48) Defined as del(17p), p53 mutation, t(14;16), t(4;14), t(14;20) and 1q gain | 4 (2–13) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | NR | NR | 7 (22.6) |
| Tang 2022 [39] | 8 (50) | 11 (68.8) Including 1q21, del17p | 3 (2–3) | ASCT, proteasome inhibitor, immunomodulatory drug | 0 | 0 | 3 (18.8) |
| Ri 2022 [40] | 3 (33.3) | 5 (55.6) Defined as del(17p), t(14;16), t(4;14) | 5 (3–7) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 7 (77.8) |
| Martin 2022 [48] | 13 (13) | 23 (24) Defined as del(17p), t(14;16), t(4;14) | 6 (4–8) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 87 (90) |
| Mi 2022 [53] | 5 (10.4) | 21 (43.8) Defined as del(17p), t(14;16), t(4;14) | 4 (3–9) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 17 (35.4) |
| Asherie 2022 [51] | 6 (30) | 10 (50) Defined as del(17p), t(14;16), t(4;14) | 6 (3–13) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb, anti-BCMA conjugated antibody | NR | 9 (45), Anti-BCMA conjugated antibody | 17 (85) |
| Cohen 2023 [41] | 5 (25) | 3 (15), all del17p | 8 (4–13) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb, noncellular BCMA-directed therapy (ADC or BsAb) | 0 | 20 (100) | 20 (100) |
| Xia 2023 [43] | 11 (33) | 13 (39) Defined as del(17p), t(14;16), t(4;14) and amp(1q) | 4 (2–12) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb, BCMA CAR-T cell therapy | 9 (27), BCMA CAR-T cell | 9 (27), BCMA CAR-T cell | 6 (18) |
| Mailankody 2023 [47] | 9 (20.9) | 16 (37.2) Defined as del(17p), t(14;16), t(4;14) | 5 (3–11) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb, BCMA-directed therapy | 0 | 3 (7) | 39 (90.7) |
| Lee 2023 [49] | 3 (27.3) | 4 (36.4) Defined as t(4;14), t(14;20) and t(14;16), del(17p), 1q gain, 1p loss | 5 (3–6) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 6 (54.5) |
| Oliver-Caldés 2023 [50] | 6 (20) | 10 (33) Defined as TP53 alterations, t(14;16), t(4;14) | 3.5 (2.8–5.0) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 28 (93) |
| Zhang 2023 [54] | 4 (40) | 6 (60) Defined as del(17p), t(14;16), t(4;14) | 5.5 (4–10) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb, BCMA CAR-T cell therapy | 5 (50), BCMA CAR-T cell therapy | 5 (50), BCMA CAR-T cell therapy | 2 (20) |
| Minakata 2023 [42] | 5 (56) | 2 (22) Including del(17p), t(4;14) | 4 (3–15) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | 0 | 0 | 7 (78) |
| Sanoyan 2023 [55] | 5 (31) | 6 (38) Defined as del(17p), t(14;16), t(4;14) | 6 (3–12) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb | NR | NR | 16 (100) |
| Hansen 2023 [56] | 76 (48) | 49 (35) Defined as del(17p), t(14;16), t(4;14) | 7 (4–18) | ASCT, proteasome inhibitor, immunomodulatory drug, anti-CD38 mAb, anti-BCMA therapy | NR | 33 (21) | 134 (84) |
| Subgroups | ORR | Grade ≥ 3 CRS |
|---|---|---|
| Prior antimyeloma regimens (<5 vs. ≥5) | <5: 89% (95% CI, 84–92) ≥5: 75% (95% CI, 62–85) p-value for significance: p < 0.01 | <5: 13% (95% CI, 8–21) ≥5: 8% (95% CI, 4–14) p-value for significance: p = 0.18 |
| Prior exposure to BCMA therapy (Yes vs. No) | Yes: 78% (95% CI, 65–87) No: 83% (95% CI, 73–90) p-value for significance: p = 0.44 | Yes: 5% (95% CI, 2–11) No: 13% (95% CI, 8–20) p-value for significance: p = 0.04 |
| Prior ASCT (<78% vs. ≥78%) | <78%: 87% (95% CI, 80–92) ≥78%: 77% (95% CI, 63–87) p-value for significance: p = 0.11 | <78%: 15% (95% CI, 10–23) ≥78%: 6% (95% CI, 1–11) p-value for significance: p = 0.02 |
| High-risk cytogenetics (<39% vs. ≥39%) | <39%: 79% (95% CI, 63–89) ≥39%: 85% (95% CI, 77–90) p-value for significance: p = 0.41 | <39%: 7% (95% CI, 4–12) ≥39%: 15% (95% CI, 10–23) p-value for significance: p = 0.02 |
| ISS stage 3 (<24% vs. ≥24%) | <24%: 86% (95% CI, 75–93) ≥24%: 81% (95% CI, 69–89) p-value for significance: p = 0.45 | <24%: 10% (95% CI, 5–19) ≥24%: 8% (95% CI, 5–13) p-value for significance: p = 0.61 |
| Extramedullary disease (<28% vs. ≥28%) | < 28%: 85% (95% CI, 72–92) ≥ 28%: 81% (95% CI, 73–87) p-value for significance: p = 0.52 | < 28%: 9% (95% CI, 5–16) ≥ 28%: 10% (95% CI, 5–17) p-value for significance: p = 0.91 |
| Bridging therapy (<42% vs. ≥42%) | <42%: 75% (95% CI, 44–92) ≥42%: 81% (95% CI, 73–92) p-value for significance: p = 0.43 | <42%: 9% (95% CI, 5–15) ≥42%: 5% (95% CI, 3–7) p-value for significance: p = 0.07 |
| CAR-T generation (2nd vs. 3rd) | 2nd: 83% (95% CI, 75–88) 3rd: 80% (95% CI, 48–95) p-value for significance: p = 0.86 | 2nd: 10% (95% CI, 6–15) 3rd: 10% (95% CI, 5–18) p-value for significance: p = 0.93 |
| Upper infusion threshold (<490 × 106 cells or 2.05 ×106 cells/kg vs. ≥490 × 106 cells or 2.05 ×106 cells/kg) | <490 × 106 cells or 2.05 ×106 cells/kg: 84% (95% CI, 74–90) ≥490 × 106 cells or 2.05 ×106 cells/kg: 81% (95% CI, 66–90) p-value for significance: p = 0.71 | <490 × 106 cells or 2.05 ×106 cells/kg: 9% (95% CI, 5–16) ≥490 × 106 cells or 2.05 ×106 cells/kg: 12% (95% CI, 7–19) p-value for significance: p = 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.; Bergantim, R. An Assessment of the Effectiveness and Safety of Chimeric Antigen Receptor T-Cell Therapy in Multiple Myeloma Patients with Relapsed or Refractory Disease: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 4996. https://doi.org/10.3390/ijms25094996
Pereira R, Bergantim R. An Assessment of the Effectiveness and Safety of Chimeric Antigen Receptor T-Cell Therapy in Multiple Myeloma Patients with Relapsed or Refractory Disease: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(9):4996. https://doi.org/10.3390/ijms25094996
Chicago/Turabian StylePereira, Rita, and Rui Bergantim. 2024. "An Assessment of the Effectiveness and Safety of Chimeric Antigen Receptor T-Cell Therapy in Multiple Myeloma Patients with Relapsed or Refractory Disease: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 25, no. 9: 4996. https://doi.org/10.3390/ijms25094996









