Rheological Properties and Inkjet Printability of a Green Silver-Based Conductive Ink for Wearable Flexible Textile Antennas
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
2.2. Formulation of Silver-Based Ink
2.3. Rheological Properties of Conductive Inks
2.4. Computational Methodology
2.5. Construction of Conductive Pattern on the PET Fabric
2.6. Characterization
3. Results and Discussion
3.1. Characterization of AgNPs and Suspension Matrices
3.2. Impact of Ink Composition on Rheological Characteristics
3.3. Effect of PEG Content on the Dynamic Viscoelasticity of Suspension Matrices
3.4. DFT Study of Na–alginate and PEG Interaction
3.4.1. HOMO-LUMO Analysis
3.4.2. MEP Mapping
3.5. Effect of AgNPs on Suspension Matrices Viscosity
- Additional Resistance: The presence of solid nanoparticles introduces additional resistance to the movement of the ink formulation, contributing to higher viscosity.
- Flocculation: AgNPs have a high Hamaker constant, representing van der Waals interactions between nanoparticles, which can lead to a phenomenon called flocculation. As the silver content increases, the flocculation effect becomes more noticeable, explaining the increase in viscosity at a zero shear rate.
3.6. Effect of AgNPs on the Flow Capacity of Vehicles
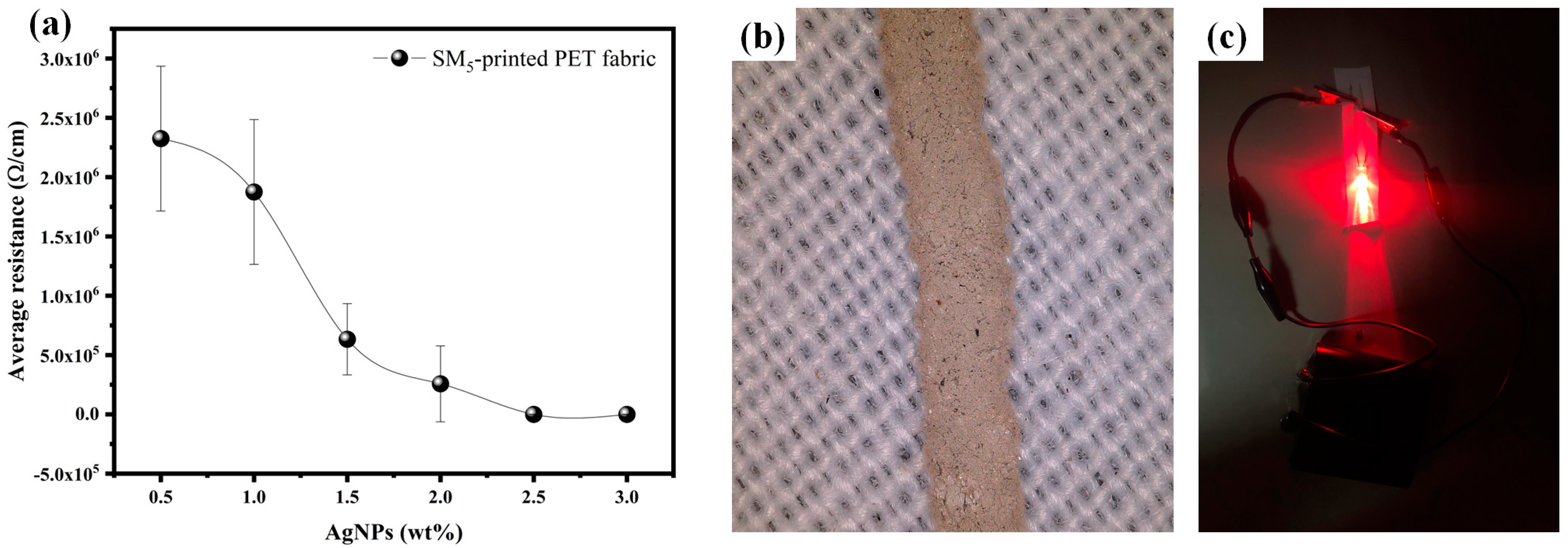
3.7. Electrical Properties of Conductive Ink and Printed Conductive Lines
3.8. Potential Application of Silver-Based Ink in Wearable Textile Antennas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Xiao, X.; Zhao, X.; Tat, T.; Bick, M. Electronic Textiles for Wearable Point-of-Care Systems. Chem. Rev. 2021, 122, 3259–3291. [Google Scholar] [CrossRef]
- Rossi, R.M. 15—High-Performance Sportswear. In High-Performance Apparel; McLoughlin, J., Sabir, T., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2018; pp. 341–356. ISBN 978-0-08-100904-8. [Google Scholar]
- Aali Mohammadi, R.; Shirazi, M.; Moaref, R.; Jamalpour, S.; Tamsilian, Y.; Kiasat, A. 11—Protective Smart Textiles for Sportswear. In Protective Textiles from Natural Resources; Mondal, M.I.H., Ed.; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2022; pp. 317–345. ISBN 978-0-323-90477-3. [Google Scholar]
- Smart Personal Protective Equipment (PPE): Current PPE Needs, Opportunities for Nanotechnology and e-Textiles—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/2058-8585/ac32a9/meta (accessed on 26 August 2023).
- Chen, D.; Lawo, M. Smart Textiles and Smart Personnel Protective Equipment. In Smart Textiles: Fundamentals, Design, and Interaction; Schneegass, S., Amft, O., Eds.; Human–Computer Interaction Series; Springer International Publishing: Cham, Switzerland, 2017; pp. 333–357. ISBN 978-3-319-50124-6. [Google Scholar]
- Joo, H.G.; Jang, Y.H.; Choi, H.S. Electrical Contact Resistance for a Conductive Velcro System. Tribol. Int. 2014, 80, 115–121. [Google Scholar] [CrossRef]
- Barman, J.; Tirkey, A.; Batra, S.; Paul, A.A.; Panda, K.; Deka, R.; Babu, P.J. The Role of Nanotechnology Based Wearable Electronic Textiles in Biomedical and Healthcare Applications. Mater. Today Commun. 2022, 32, 104055. [Google Scholar] [CrossRef]
- Smart Textiles for Personalized Healthcare|Nature Electronics. Available online: https://www.nature.com/articles/s41928-022-00723-z (accessed on 26 August 2023).
- Chen, G.; Li, Y.; Bick, M.; Chen, J. Smart Textiles for Electricity Generation. Chem. Rev. 2020, 120, 3668–3720. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Minutillo, S.; Tamburrano, A.; Sarto, M.S. Production and Characterization of Graphene Nanoplatelet-Based Ink for Smart Textile Strain Sensors via Screen Printing Technique. Mater. Des. 2021, 198, 109306. [Google Scholar] [CrossRef]
- Large-Scale Waterproof and Stretchable Textile-Integrated Laser-Printed Graphene Energy Storages|Scientific Reports. Available online: https://www.nature.com/articles/s41598-019-48320-z (accessed on 26 August 2023).
- Tao, X.; Koncar, V. 25—Textile Electronic Circuits Based on Organic Fibrous Transistors. In Smart Textiles and Their Applications; Koncar, V., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Oxford, UK, 2016; pp. 569–598. ISBN 978-0-08-100574-3. [Google Scholar]
- He, W.; Ma, R.; Kang, D.J. High-Performance, Flexible Planar Microsupercapacitors Based on Crosslinked Polyaniline Using Laser Printing Lithography. Carbon 2020, 161, 117–122. [Google Scholar] [CrossRef]
- Mo, L.; Ran, J.; Yang, L.; Fang, Y.; Zhai, Q.; Li, L. Flexible Transparent Conductive Films Combining Flexographic Printed Silver Grids with CNT Coating. Nanotechnology 2016, 27, 065202. [Google Scholar] [CrossRef]
- Liu, L.; Shen, Z.; Zhang, X.; Ma, H. Highly Conductive Graphene/Carbon Black Screen Printing Inks for Flexible Electronics. J. Colloid Interface Sci. 2021, 582, 12–21. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, H.; Wang, Y.; Yin, J.; Huang, Y. Drop-on-Demand (DOD) Inkjet Dynamics of Printing Viscoelastic Conductive Ink. Addit. Manuf. 2021, 48, 102451. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y. Printing Conductive Nanomaterials for Flexible and Stretchable Electronics: A Review of Materials, Processes, and Applications. Adv. Mater. Technol. 2019, 4, 1800546. [Google Scholar] [CrossRef]
- Cummins, G.; Desmulliez, M.P.Y. Inkjet Printing of Conductive Materials: A Review. Circuit World 2012, 38, 193–213. [Google Scholar] [CrossRef]
- Boumegnane, A.; Nadi, A.; Cochrane, C.; Boussu, F.; Cherkaoui, O.; Tahiri, M. Formulation of Conductive Inks Printable on Textiles for Electronic Applications: A Review. Text. Prog. 2022, 54, 103–200. [Google Scholar] [CrossRef]
- Kela, L.; Auvinen, A.; Tapper, U.; Hokkinen, J. Metallic Nanoparticles for Conductive Inks: International Congress of Metallurgy and Materials, SAM-CONAMET/IBEROMAT 2014. In SAM-CONAMET 2014 Pendrive Content. 2014. Available online: https://publications.vtt.fi/julkaisut/muut/2014/OA-Metallic_nanoparticles.pdf (accessed on 27 March 2024).
- Zhao, B.; Sivasankar, V.S.; Subudhi, S.K.; Sinha, S.; Dasgupta, A.; Das, S. Applications, Fluid Mechanics, and Colloidal Science of Carbon-Nanotube-Based 3D Printable Inks. Nanoscale 2022, 14, 14858–14894. [Google Scholar] [CrossRef] [PubMed]
- Saidina, D.S.; Eawwiboonthanakit, N.; Mariatti, M.; Fontana, S.; Hérold, C. Recent Development of Graphene-Based Ink and Other Conductive Material-Based Inks for Flexible Electronics. J. Electron. Mater. 2019, 48, 3428–3450. [Google Scholar] [CrossRef]
- Polymers|Free Full-Text Recent Developments and Implementations of Conductive Polymer-Based Flexible Devices in Sensing Applications. Available online: https://www.mdpi.com/2073-4360/14/18/3730 (accessed on 26 August 2023).
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent Development in Silver-Based Ink for Flexible Electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Ibanez-Labiano, I.; Ergoktas, M.S.; Kocabas, C.; Toomey, A.; Alomainy, A.; Ozden-Yenigun, E. Graphene-Based Soft Wearable Antennas. Appl. Mater. Today 2020, 20, 100727. [Google Scholar] [CrossRef]
- Tighezza, M.; Rahim, S.K.A.; Islam, M.T. Flexible Wideband Antenna for 5G Applications. Microw. Opt. Technol. Lett. 2018, 60, 38–44. [Google Scholar] [CrossRef]
- AL-Haddad, M.A.S.M.; Jamel, N.; Nordin, A.N. Flexible Antenna: A Review of Design, Materials, Fabrication, and Applications. J. Phys. Conf. Ser. 2021, 1878, 012068. [Google Scholar] [CrossRef]
- Krykpayev, B.; Farooqui, M.F.; Bilal, R.M.; Vaseem, M.; Shamim, A. A Wearable Tracking Device Inkjet-Printed on Textile. Microelectron. J. 2017, 65, 40–48. [Google Scholar] [CrossRef]
- Salmerón, J.F.; Molina-Lopez, F.; Briand, D.; Ruan, J.J.; Rivadeneyra, A.; Carvajal, M.A.; Capitán-Vallvey, L.F.; de Rooij, N.F.; Palma, A.J. Properties and Printability of Inkjet and Screen-Printed Silver Patterns for RFID Antennas. J. Electron. Mater. 2014, 43, 604–617. [Google Scholar] [CrossRef]
- Nie, X.; Wang, H.; Zou, J. Inkjet Printing of Silver Citrate Conductive Ink on PET Substrate. Appl. Surf. Sci. 2012, 261, 554–560. [Google Scholar] [CrossRef]
- Zhang, J.; Ahmadi, M.; Fargas, G.; Perinka, N.; Reguera, J.; Lanceros-Méndez, S.; Llanes, L.; Jiménez-Piqué, E. Silver Nanoparticles for Conductive Inks: From Synthesis and Ink Formulation to Their Use in Printing Technologies. Metals 2022, 12, 234. [Google Scholar] [CrossRef]
- Kamyshny, A.; Sowade, E.; Magdassi, S. Inkjet Ink Formulations: Overview and Fundamentals. In Inkjet Printing in Industry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 93–124. ISBN 978-3-527-82807-4. [Google Scholar]
- Enríquez, E.; Reinosa, J.J.; Fuertes, V.; Fernández, J.F. Advances and Challenges of Ceramic Pigments for Inkjet Printing. Ceram. Int. 2022, 48, 31080–31101. [Google Scholar] [CrossRef]
- Shahariar, H.; Kim, I.; Soewardiman, H.; Jur, J.S. Inkjet Printing of Reactive Silver Ink on Textiles. ACS Appl. Mater. Interfaces 2019, 11, 6208–6216. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T.L.A.C. Silver Nanoparticle Conductive Inks: Synthesis, Characterization, and Fabrication of Inkjet-Printed Flexible Electrodes. Sci. Rep. 2020, 10, 8878. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Mariatti, M. Effect of Natural Surfactant on the Performance of Reduced Graphene Oxide Conductive Ink. J. Clean. Prod. 2022, 376, 134254. [Google Scholar] [CrossRef]
- Rajan, K.; Roppolo, I.; Chiappone, A.; Bocchini, S.; Perrone, D.; Chiolerio, A. Silver Nanoparticle Ink Technology: State of the Art. Nanotechnol. Sci. Appl. 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chemistry of Solid Metal-Based Inks and Pastes for Printed Electronics—A Review—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2352940718306802 (accessed on 26 August 2023).
- Zhao, Y.; Zhang, X.; Wang, Y.; Wu, Z.; An, J.; Lu, Z.; Mei, L.; Li, C. In Situ Cross-Linked Polysaccharide Hydrogel as Extracellular Matrix Mimics for Antibiotics Delivery. Carbohydr. Polym. 2014, 105, 63–69. [Google Scholar] [CrossRef]
- Glasser, A.; Cloutet, É.; Hadziioannou, G.; Kellay, H. Tuning the Rheology of Conducting Polymer Inks for Various Deposition Processes. Chem. Mater. 2019, 31, 6936–6944. [Google Scholar] [CrossRef]
- An, F.; Fang, K.; Liu, X.; Li, C.; Liang, Y.; Liu, H. Rheological Properties of Carboxymethyl Hydroxypropyl Cellulose and Its Application in High Quality Reactive Dye Inkjet Printing on Wool Fabrics. Int. J. Biol. Macromol. 2020, 164, 4173–4182. [Google Scholar] [CrossRef]
- Boumegnane, A.; Nadi, A.; Dahrouch, A.; Stambouli, A.; Cherkaoui, O.; Tahiri, M. Investigation of Silver Conductive Ink Printable on Textiles for Wearable Electronics Applications: Effect of Silver Concentration and Polymer Matrix. Fibers Polym. 2023, 24, 2977–2993. [Google Scholar] [CrossRef]
- Synthesis of New Set of Imidazo[1,2-a]Pyridine-Schiff Bases Derivatives as Potential Antimicrobial Agents: Experimental and Theoretical Approaches. J. Mol. Struct. 2023, 1292, 136186. [CrossRef]
- Gaussian 09 Citation|Gaussian.Com. Available online: https://gaussian.com/g09citation/ (accessed on 28 April 2024).
- Szabo, A.; Ostlund, N.S. Modern Quantum Chemistry: Introduction to Advanced Electronic Structure Theory; Courier Corporation: North Chelmsford, MA, USA, 2012; ISBN 978-0-486-13459-8. [Google Scholar]
- Nair, R.M.; Bindhu, B.; Reena, V.L. A Polymer Blend from Gum Arabic and Sodium Alginate—Preparation and Characterization. J. Polym. Res. 2020, 27, 154. [Google Scholar] [CrossRef]
- Swamy, T.M.M.; Ramaraj, B.; Siddaramaiah. Sodium Alginate and Poly(Ethylene Glycol) Blends: Thermal and Morphological Behaviors. J. Macromol. Sci. Part A 2010, 47, 877–881. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Poli, A.; Gomez d’Ayala, G.; Nicolaus, B.; Malinconinco, M.; Santagata, G. Vegetable Wastes Derived Polysaccharides as Natural Eco-Friendly Plasticizers of Sodium Alginate. Carbohydr. Polym. 2020, 229, 115427. [Google Scholar] [CrossRef] [PubMed]
- Materials|Free Full-Text|Salicylic Acid Co-Precipitation with Alginate via Supercritical Atomization for Cosmetic Applications. Available online: https://www.mdpi.com/1996-1944/15/21/7634 (accessed on 26 August 2023).
- Jin, J.; Lin, F.; Liu, R.; Xiao, T.; Zheng, J.; Qian, G.; Liu, H.; Wen, P. Preparation and Thermal Properties of Mineral-Supported Polyethylene Glycol as Form-Stable Composite Phase Change Materials (CPCMs) Used in Asphalt Pavements. Sci. Rep. 2017, 7, 16998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, H.; Liu, Y.; Zou, X.; Shi, J.; Zhao, Y.; Ye, Y.; Yu, Y.; Guo, J. Preparation of Calcium Alginate/Polyethylene Glycol Acrylate Double Network Fiber with Excellent Properties by Dynamic Molding Method. Carbohydr. Polym. 2019, 226, 115277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, H.; Liu, Y.; Zou, X.; Shi, J.; Zhao, Y.; Ye, Y.; Yu, Y.; Guo, J. Sodium Alginate-Polyethylene Glycol Diacrylate Based Double Network Fiber: Rheological Properties of Fiber Forming Solution with Semi-Interpenetrating Network Structure. Int. J. Biol. Macromol. 2020, 142, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow Behavior, Thixotropy and Dynamical Viscoelasticity of Sodium Alginate Aqueous Solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Ma, Y.; Geng, Y.; Tan, J. Rheological and Mechanical Properties of Ultrahigh Molecular Weight Polyethylene/High Density Polyethylene/Polyethylene Glycol Blends. Adv. Ind. Eng. Polym. Res. 2019, 2, 51–60. [Google Scholar] [CrossRef]
- Dynamic Formation of Calcium Alginate/Polyethylene Glycol Acrylate Dual Network Fibers Enhanced by Polyvinyl Alcohol Microcrystalline Cross-Linking—New Journal of Chemistry (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2020/nj/d0nj03538f/unauth (accessed on 26 August 2023).
- Allangawi, A.; Aziz Aljar, M.A.; Ayub, K.; El-Fattah, A.A.; Mahmood, T. Removal of Methylene Blue by Using Sodium Alginate-Based Hydrogel; Validation of Experimental Findings via DFT Calculations. J. Mol. Graph. Model. 2023, 122, 108468. [Google Scholar] [CrossRef] [PubMed]
- Khnifira, M.; El Hamidi, S.; Mahsoune, A.; Sadiq, M.; Serdaroğlu, G.; Kaya, S.; Qourzal, S.; Barka, N.; Abdennouri, M. Adsorption of Methylene Blue Cationic Dye onto Brookite and Rutile Phases of Titanium Dioxide: Quantum Chemical and Molecular Dynamic Simulation Studies. Inorg. Chem. Commun. 2021, 129, 108659. [Google Scholar] [CrossRef]
- Woo, K.; Jang, D.; Kim, Y.; Moon, J. Relationship between Printability and Rheological Behavior of Ink-Jet Conductive Inks. Ceram. Int. 2013, 39, 7015–7021. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Mia, S.; Xing, T.; Chen, G. Development of Inkjet Printing Ink Based on Component Solubility Parameters and Its Properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125676. [Google Scholar] [CrossRef]
- Dzisah, P.; Ravindra, N.M. Modeling of Rheological Properties of Metal Nanoparticle Conductive Inks for Printed Electronics. In TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Cham, Switzerland, 2021; pp. 964–979. [Google Scholar]
- Yeates, S.G.; Xu, D.; Madec, M.-B.; Caras-Quintero, D.; Alamry, K.A.; Malandraki, A.; Sanchez-Romaguera, V. Fluids for Inkjet Printing. In Inkjet Technology for Digital Fabrication; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 87–112. ISBN 978-1-118-45294-3. [Google Scholar]
- Boumeganane, A.; Nadi, A.; Cherkaoui, O.; Tahiri, M. Inkjet Printing of Silver Conductive Ink on Textiles for Wearable Electronic Applications. Mater. Today Proc. 2022, 58, 1235–1241. [Google Scholar] [CrossRef]
- Seifert, D.B.; Phillips, J.A. Porous Alginate–Poly(Ethylene Glycol) Entrapment System for the Cultivation of Mammalian Cells. Biotechnol. Prog. 1997, 13, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Inkjet Printing of Silver Conductive Ink on Textiles for Electronic Applications: Impact of Ink Formulation on Electrical Performances of the Ink—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/1757-899X/1266/1/012006/meta (accessed on 26 August 2023).
- Wagih, M.; Hilton, G.S.; Weddell, A.S.; Beeby, S. Broadband Millimeter-Wave Textile-Based Flexible Rectenna for Wearable Energy Harvesting. IEEE Trans. Microw. Theory Tech. 2020, 68, 4960–4972. [Google Scholar] [CrossRef]
- Aghoutane, B.; Das, S.; EL Ghzaoui, M.; Madhav, B.T.P.; El Faylali, H. A Novel Dual Band High Gain 4-Port Millimeter Wave MIMO Antenna Array for 28/37 GHz 5G Applications. AEU—Int. J. Electron. Commun. 2022, 145, 154071. [Google Scholar] [CrossRef]
- Douhi, S.; Islam, T.; Saravanan, R.A.; Eddiai, A.; Das, S.; Cherkaoui, O. Design of a Flexible Rectangular Antenna Array with High Gain for RF Energy Harvesting and Wearable Devices. J. Nano-Electron. Phys. 2023, 15, 03010. [Google Scholar] [CrossRef]
- Singh, J.; Kalra, P.; Singh, S.; Sidhu, E. High Gain Textile Rectangular Microstrip Patch Antenna Design Employing Denim Substrate for Satellite Space to Earth Downlink Applications. In Proceedings of the 2016 International Conference on Global Trends in Signal Processing, Information Computing and Communication (ICGTSPICC), Jalgaon, India, 22–24 December 2016; pp. 338–343. [Google Scholar]
- Chowdhury, S.R.; Ali, K. Effects of Human Body on Antenna Performance: A Quantitative Study. In Proceedings of the 2016 19th International Conference on Computer and Information Technology (ICCIT), Dhaka, Bangladesh, 18–20 December 2016; pp. 108–112. [Google Scholar]
- Takimoto, T.; Onishi, T.; Saito, K.; Takahashi, M.; Uebayashi, S.; Ito, K. Characteristics of Biological Tissue Equivalent Phantoms Applied to UWB Communications. Electron. Commun. Jpn. (Part I Commun.) 2007, 90, 48–55. [Google Scholar] [CrossRef]
- Zu, H.-R.; Wu, B.; Zhang, Y.-H.; Zhao, Y.-T.; Song, R.-G.; He, D.-P. Circularly Polarized Wearable Antenna with Low Profile and Low Specific Absorption Rate Using Highly Conductive Graphene Film. IEEE Antennas Wirel. Propag. Lett. 2020, 19, 2354–2358. [Google Scholar] [CrossRef]
- Arif, A.; Zubair, M.; Ali, M.; Khan, M.U.; Mehmood, M.Q. A Compact, Low-Profile Fractal Antenna for Wearable On-Body WBAN Applications. IEEE Antennas Wirel. Propag. Lett. 2019, 18, 981–985. [Google Scholar] [CrossRef]
- Mahapatra, S.; Mohanty, M.N. A Review on State of Art Techniques of Antennas for Body Area Networks. Int. J. Sens. Wirel. Commun. and Control 2021, 11, 604–618. [Google Scholar] [CrossRef]
- Ultra-Wide Band Flexible Antenna Applicable for Dual-Band on-Body Communications|International Journal of Microwave and Wireless Technologies|Cambridge Core. Available online: https://www.cambridge.org/core/journals/international-journal-of-microwave-and-wireless-technologies/article/abs/ultrawide-band-flexible-antenna-applicable-for-dualband-onbody-communications/93642163AB15AE1685025739DB57486C (accessed on 27 August 2023).











| SM1 | SM2 | SM3 | SM4 | SM5 | SM6 | SM7 | |
|---|---|---|---|---|---|---|---|
| Na–Alginate (%) | 60 | 50 | 40 | 30 | 20 | 10 | 0 |
| PEG (%) | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
| EtOH: DI (%) | 40 | ||||||
| 2θ | hkl | β | D (nm) | D Average (nm) |
|---|---|---|---|---|
| 38.10 | 111 | 0.251 | 34.94 | 30.85 ± 2.15 |
| 44.32 | 200 | 0.288 | 31.06 | |
| 64.45 | 220 | 0.325 | 30.09 | |
| 77.41 | 311 | 0.388 | 2731 |
| Suspension Matrix | Tf of PEG (°C) | Tf of Na–Alginate (°C) | Td of Na–Alginate (°C) |
|---|---|---|---|
| SM1 | -- | 142 | 240 |
| SM2 | 61 | 148 | -- |
| SM3 | 55 | 151 | -- |
| SM4 | 59 | 156 | -- |
| SM5 | 48 | 153 | -- |
| SM6 | 49 | 120 | -- |
| SM7 | 74 | -- | -- |
| Suspension Matrix | τ0 | n | Log k | k |
|---|---|---|---|---|
| SM1 | 0.95 | 0.820 ± 0.003 | −0.848 ± 0.009 | 0.141 |
| SM2 | 0.90 | 0.743 ± 0.002 | −0.995 ± 0.006 | 0.101 |
| SM3 | 0.30 | 0.380 ± 0.018 | −0.572 ± 0.031 | 0.267 |
| SM4 | 0.29 | 0.668 ± 0.114 | −2.914 ± 0.187 | 0.001 |
| SM5 | 0.10 | 0.615 ± 0.020 | −1.925 ± 0.053 | 0.011 |
| SM6 | 0.09 | 0.768 ± 0.012 | −2.219 ± 0.033 | 0.006 |
| SM7 | 0.07 | 0.775 ± 0.010 | −2.520 ± 0.028 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boumegnane, A.; Douhi, S.; Batine, A.; Dormois, T.; Cochrane, C.; Nadi, A.; Cherkaoui, O.; Tahiri, M. Rheological Properties and Inkjet Printability of a Green Silver-Based Conductive Ink for Wearable Flexible Textile Antennas. Sensors 2024, 24, 2938. https://doi.org/10.3390/s24092938
Boumegnane A, Douhi S, Batine A, Dormois T, Cochrane C, Nadi A, Cherkaoui O, Tahiri M. Rheological Properties and Inkjet Printability of a Green Silver-Based Conductive Ink for Wearable Flexible Textile Antennas. Sensors. 2024; 24(9):2938. https://doi.org/10.3390/s24092938
Chicago/Turabian StyleBoumegnane, Abdelkrim, Said Douhi, Assia Batine, Thibault Dormois, Cédric Cochrane, Ayoub Nadi, Omar Cherkaoui, and Mohamed Tahiri. 2024. "Rheological Properties and Inkjet Printability of a Green Silver-Based Conductive Ink for Wearable Flexible Textile Antennas" Sensors 24, no. 9: 2938. https://doi.org/10.3390/s24092938






