Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation Attenuates Abnormalities in Glucose Metabolism in Streptozotocin-Induced Mildly Diabetic Rats Fed a High-Fat Diet
Abstract
:1. Introduction
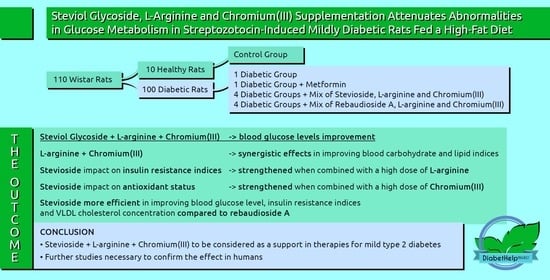
2. Results
2.1. Effects of the Diet and Combined Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation on Overall Growth Indices and the Relative Organ Weights
2.2. Effects of the Diet and Combined Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation on Glucose-Related Indices
2.3. Effects of the Diet and Combined Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation on Lipid Metabolism Indices
2.4. Effects of the Diet and Combined Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation on Other Biochemical Indices
2.5. Histopathological Analyses
3. Discussion
4. Materials and Methods
4.1. Test Supplements
4.2. Animals and Diets
4.3. Experimental Protocol
4.4. Histopathological and Serum Biochemical Analysis
4.5. Chromium Determination in Diets and Organs
4.6. Formulas for Calculation of EI, FER, GTT, HOMA-IR, HOMA-β, and QUICKI
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas Ninth Edition 2019. Available online: Https://Www.Diabetesatlas.Org/Upload/Resources/Material/20200302_133351_IDFATLAS9e-Final-Web.Pdf (accessed on 24 July 2022).
- Akiyode, O.F.; Adesoye, A.A. Adverse Effects Associated with Newer Diabetes Therapies. J. Pharm. Pract. 2017, 30, 238–244. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-Associated Lactic Acidosis: Current Perspectives on Causes and Risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Nutraceuticals and Diet-Based Phytochemicals in Type 2 Diabetes Mellitus: From Whole Food to Components with Defined Roles and Mechanisms. Curr. Diabetes Rev. 2019, 16, 12–25. [Google Scholar] [CrossRef]
- Ciocoiu, M.; Mirón, A.; Mares, L.; Tutunaru, D.; Pohaci, C.; Groza, M.; Badescu, M. The Effects of Sambucus Nigra Polyphenols on Oxidative Stress and Metabolic Disorders in Experimental Diabetes Mellitus. J. Physiol. Biochem. 2009, 65, 297–304. [Google Scholar] [CrossRef]
- Raman, B.V.; Krishna, A.N.V.; Rao, B.N.; Saradhi, M.P.; Rao, M.V.B. Plants with Antidiabetic Activities and Their Medicinal Values. Int. Res. J. Pharm. 2012, 3, 11–15. [Google Scholar]
- Watson, A.A.; Fleet, G.W.J.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated Alkaloids—Natural Occurrence and Therapeutic Applications. Phytochemistry 2001, 56, 265–295. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.-Y.; Fleet, G.W. Iminosugars as Therapeutic Agents: Recent Advances and Promising Trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Kamalakkannan, N. Rutin Improves Glucose Homeostasis in Streptozotocin Diabetic Tissues by Altering Glycolytic and Gluconeogenic Enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef]
- Chen, J.; Hou, X.; Wang, G.; Zhong, Q.; Liu, Y.; Qiu, H.; Yang, N.; Gu, J.; Wang, C.; Zhang, L.; et al. Terpene Glycoside Component from Moutan Cortex Ameliorates Diabetic Nephropathy by Regulating Endoplasmic Reticulum Stress-Related Inflammatory Responses. J. Ethnopharmacol. 2016, 193, 433–444. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Yashiro, K.; Matsuda, H. Kotalanol, a Potent a-Glucosidase Inhibitor with Thiosugar Sulfonium Sulfate Structure, from Antidiabetic Ayurvedic Medicine Salacia Reticulata. Chem. Pharm. Bull. 1998, 46, 1339–1340. [Google Scholar] [CrossRef] [Green Version]
- Kazmi, I.; Rahman, M.; Afzal, M.; Gupta, G.; Saleem, S.; Afzal, O.; Shaharyar, M.A.; Nautiyal, U.; Ahmed, S.; Anwar, F. Anti-Diabetic Potential of Ursolic Acid Stearoyl Glucoside: A New Triterpenic Gycosidic Ester from Lantana Camara. Fitoterapia 2012, 83, 142–146. [Google Scholar] [CrossRef]
- I. Sweidan, N.; H. Abu Zarga, M. Acylated Flavonoid Glucoside from Marrubium Vulgare. Lett. Org. Chem. 2016, 13, 277–282. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; VAnil Kumar, N.; Sharopov, F.; Ramirez-Alarcon, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Yang, L.-F.; Shimadate, Y.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis and Glycosidase Inhibition of N -Substituted Derivatives of 1,4-Dideoxy-1,4-Imino- d -Mannitol (DIM). Org. Biomol. Chem. 2020, 18, 999–1011. [Google Scholar] [CrossRef]
- Chennaiah, A.; Dahiya, A.; Dubbu, S.; Vankar, Y.D. A Stereoselective Synthesis of an Imino Glycal: Application in the Synthesis of (-)-1- Epi -Adenophorine and a Homoimindosugar: A Stereoselective Synthesis of an Imino Glycal: Application in the Synthesis of (-)-1- Epi -Adenophorine and a Homoimindosugar. Eur. J. Org. Chem. 2018, 2018, 6574–6581. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of Glycals into Vicinal-1,2-Diazides and 1,2-(or 2,1)-Azidoacetates Using Hypervalent Iodine Reagents and Me 3 SiN 3. Application in the Synthesis of N-Glycopeptides, Pseudo-Trisaccharides and an Iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-Oxa-Oxa Annulated Sugars as Glycosidase Inhibitors from 2-Formyl Galactal Using Iodocyclization as a Key Step. Arkivoc 2022, 2022, 5–23. [Google Scholar] [CrossRef]
- Typek, R.; Dawidowicz, A.L.; Bernacik, K. Aqueous and Alcoholic Adducts of Steviol and Steviol Glycosides in Food Products Containing Stevia. Food Chem. 2020, 317, 126359. [Google Scholar] [CrossRef]
- Hellfritsch, C.; Brockhoff, A.; Stähler, F.; Meyerhof, W.; Hofmann, T. Human Psychometric and Taste Receptor Responses to Steviol Glycosides. J. Agric. Food Chem. 2012, 60, 6782–6793. [Google Scholar] [CrossRef]
- The European Commission. Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with Regard to Steviol Glycosides. L 295; OJEU: Brussels, Belgium, 2011. [Google Scholar]
- FDA. Agency Response Letter GRAS Notice No. GRN 000395; FDA: Silver Spring, MD, USA, 2012. [Google Scholar]
- Kurek, J.M.; Krejpcio, Z. The Functional and Health-Promoting Properties of Stevia Rebaudiana Bertoni and Its Glycosides with Special Focus on the Antidiabetic Potential–A Review. J. Funct. Foods 2019, 61, 103465. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gregersen, S.; Poulsen, C.R.; Hermansen, K. Stevioside Acts Directly on Pancreatic β Cells to Secrete Insulin: Actions Independent of Cyclic Adenosine Monophosphate and Adenosine Triphosphate—Sensitivie K+-Channel Activity. Metabolism 2000, 49, 208–214. [Google Scholar] [CrossRef]
- Abudula, R.; Jeppesen, P.B.; Rolfsen, S.E.D.; Xiao, J.; Hermansen, K. Rebaudioside A Potently Stimulates Insulin Secretion from Isolated Mouse Islets: Studies on the Dose-, Glucose-, and Calcium-Dependency. Metabolism 2004, 53, 1378–1381. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gregersen, S.; Rolfsen, S.E.D.; Jepsen, M.; Colombo, M.; Agger, A.; Xiao, J.; Kruhøffer, M.; Ørntoft, T.; Hermansen, K. Antihyperglycemic and Blood Pressure-Reducing Effects of Stevioside in the Diabetic Goto-Kakizaki Rat. Metabolism 2003, 52, 372–378. [Google Scholar] [CrossRef]
- Philippaert, K.; Pironet, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Segal, A.; Antoine, N.; Gysemans, C.; et al. Steviol Glycosides Enhance Pancreatic Beta-Cell Function and Taste Sensation by Potentiation of TRPM5 Channel Activity. Nat. Commun. 2017, 8, 14733. [Google Scholar] [CrossRef]
- Chen, J.; Jeppesen, P.B.; Nordentoft, I.; Hermansen, K. Stevioside Counteracts Beta-Cell Lipotoxicity without Affecting Acetyl CoA Carboxylase. Rev. Diabet. Stud. 2006, 3, 178. [Google Scholar] [CrossRef]
- Chen, J.; Jeppesen, P.B.; Abudula, R.; Dyrskog, S.E.U.; Colombo, M.; Hermansen, K. Stevioside Does Not Cause Increased Basal Insulin Secretion or β-Cell Desensitization as Does the Sulphonylurea, Glibenclamide: Studies in Vitro. Life Sci. 2006, 78, 1748–1753. [Google Scholar] [CrossRef]
- Myint, K.Z.; Chen, J.; Zhou, Z.; Xia, Y.; Lin, J.; Zhang, J. Structural Dependence of Antidiabetic Effect of Steviol Glycosides and Their Metabolites on Streptozotocin-Induced Diabetic Mice. J. Sci. Food Agric. 2020, 100, 3841–3849. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, S.-C.; Chan, P.; Chu, Y.-L.; Yang, H.-Y.; Cheng, J.-T. Mechanism of the Hypoglycemic Effect of Stevioside, a Glycoside of Stevia Rebaudiana. Planta Med. 2005, 71, 108–113. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gregersen, S.; Alstrup, K.K.; Hermansen, K. Stevioside Induces Antihyperglycaemic, Insulinotropic and Glucagonostatic Effects in Vivo: Studies in the Diabetic Goto-Kakizaki (GK) Rats. Phytomedicine 2002, 9, 9–14. [Google Scholar] [CrossRef]
- Chang, J.-C.; Wu, M.C.; Liu, I.-M.; Cheng, J.-T. Increase of Insulin Sensitivity by Stevioside in Fructose-rich Chow-fed Rats. Horm. Metab. Res. 2005, 37, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Kurek, J.M.; Król, E.; Krejpcio, Z. Steviol Glycosides Supplementation Affects Lipid Metabolism in High-Fat Fed STZ-Induced Diabetic Rats. Nutrients 2020, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Kurek, J.M.; Zielińska-Wasielica, J.; Kowalska, K.; Krejpcio, Z.; Olejnik, A. Modulating Effects of Steviol and Steviol Glycosides on Adipogenesis, Lipogenesis, Glucose Uptake and Insulin Resistance in 3T3-L1 Adipocyte Model. J. Funct. Foods 2022, 94, 105141. [Google Scholar] [CrossRef]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef]
- Szlas, A.; Kurek, J.M.; Krejpcio, Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review. Nutrients 2022, 14, 961. [Google Scholar] [CrossRef]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.E.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary L-Arginine Supplementation Reduces Fat Mass in Zucker Diabetic Fatty Rats. J. Nutr. 2005, 135, 714–721. [Google Scholar] [CrossRef]
- Clemmensen, C.; Smajilovic, S.; Smith, E.P.; Woods, S.C.; Bräuner-Osborne, H.; Seeley, R.J.; D’Alessio, D.A.; Ryan, K.K. Oral L-Arginine Stimulates GLP-1 Secretion to Improve Glucose Tolerance in Male Mice. Endocrinology 2013, 154, 3978–3983. [Google Scholar] [CrossRef]
- Bogdanski, P.; Szulinska, M.; Suliburska, J.; Pupek-Musialik, D.; Jablecka, A.; Witmanowski, H. Supplementation with L-Arginine Favorably Influences Plasminogen Activator Inhibitor Type 1 Concentration in Obese Patients. A Randomized, Double Blind Trial. J. Endocrinol. Investig. 2013, 36, 221–226. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Pupek-Musialik, D.; Jablecka, A. Changes in Mineral Status Are Associated with Improvements in Insulin Sensitivity in Obese Patients Following L-Arginine Supplementation. Eur. J. Nutr. 2014, 53, 387–393. [Google Scholar] [CrossRef]
- Krejpcio, Z. Chromium and Insulin Signaling. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 611–616. ISBN 978-1-4614-1532-9. [Google Scholar]
- Di Bona, K.R.; Love, S.; Rhodes, N.R.; McAdory, D.; Sinha, S.H.; Kern, N.; Kent, J.; Strickland, J.; Wilson, A.; Beaird, J.; et al. Chromium Is Not an Essential Trace Element for Mammals: Effects of a “Low-Chromium” Diet. JBIC J. Biol. Inorg. Chem. 2011, 16, 381–390. [Google Scholar] [CrossRef]
- Vincent, J.B. Is the Pharmacological Mode of Action of Chromium(III) as a Second Messenger? Biol. Trace Elem. Res. 2015, 166, 7–12. [Google Scholar] [CrossRef]
- Deshmukh, N.S.; Bagchi, M.; Lau, F.C.; Bagchi, D. Safety of a Novel Oxygen-Coordinated Niacin-Bound Chromium(III) Complex (NBC): I. Two-Generation Reproduction Toxicity Study. J. Inorg. Biochem. 2009, 103, 1748–1754. [Google Scholar] [CrossRef]
- Maret, W. Chromium Supplementation in Human Health, Metabolic Syndrome, And Diabetes. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Carver, P.L., Ed.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2019; pp. 231–252. ISBN 978-3-11-052787-2. [Google Scholar]
- Staniek, H.; Krejpcio, Z.; Iwanik, K. Evaluation of the Acute Oral Toxicity Class of Tricentric Chromium(III) Propionate Complex in Rat. Food Chem. Toxicol. 2010, 48, 859–864. [Google Scholar] [CrossRef]
- Staniek, H.; Krejpcio, Z. The Effects of Tricentric Chromium(III) Propionate Complex Supplementation on Pregnancy Outcome and Maternal and Foetal Mineral Status in Rat. Food Chem. Toxicol. 2009, 47, 2673–2678. [Google Scholar] [CrossRef]
- Clodfelder, B.J.; Chang, C.; Vincent, J.B. Absorption of the Biomimetic Chromium Cation Triaqua-Μ3-Oxo-μ-Hexapropionatotrichromium(III) in Rats. Biol. Trace Elem. Res. 2004, 98, 159–170. [Google Scholar] [CrossRef]
- Clodfelder, B.J.; Upchurch, R.G.; Vincent, J.B. A Comparison of the Insulin-Sensitive Transport of Chromium in Healthy and Model Diabetic Rats. J. Inorg. Biochem. 2004, 98, 522–533. [Google Scholar] [CrossRef]
- Anker, C.C.B.; Rafiq, S.; Jeppesen, P.B. Effect of Steviol Glycosides on Human Health with Emphasis on Type 2 Diabetic Biomarkers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 1965. [Google Scholar] [CrossRef]
- Kohli, R.; Meininger, C.J.; Haynes, T.E.; Yan, W.; Self, J.T.; Wu, G. Dietary L-Arginine Supplementation Enhances Endothelial Nitric Oxide Synthesis in Streptozotocin-Induced Diabetic Rats. J. Nutr. 2004, 134, 600–608. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory Role for the Arginine-Nitric Oxide Pathway in Metabolism of Energy Substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z.; Azizi, F. Habitual Intake of Dietary L-Arginine in Relation to Risk of Type 2 Diabetes: A Prospective Study. BMC Endocr. Disord. 2021, 21, 113. [Google Scholar] [CrossRef]
- Król, E.; Krejpcio, Z. Evaluation of Anti-Diabetic Potential of Chromium(III) Propionate Complex in High-Fat Diet Fed and STZ Injected Rats. Food Chem. Toxicol. 2011, 49, 3217–3223. [Google Scholar] [CrossRef]
- Król, E.; Krejpcio, Z. Chromium(III) Propionate Complex Supplementation Improves Carbohydrate Metabolism in Insulin-Resistance Rat Model. Food Chem. Toxicol. 2010, 48, 2791–2796. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Orhan, C.; Sahin, N.; Kucuk, O.; Ozercan, I.H.; Juturu, V.; Komorowski, J.R. Anti-Diabetic Activity of Chromium Picolinate and Biotin in Rats with Type 2 Diabetes Induced by High-Fat Diet and Streptozotocin. Br. J. Nutr. 2013, 110, 197–205. [Google Scholar] [CrossRef]
- Pandey, K.B.; Mishra, N.; Rizvi, S.I. Protein Oxidation Biomarkers in Plasma of Type 2 Diabetic Patients. Clin. Biochem. 2010, 43, 508–511. [Google Scholar] [CrossRef]
- Holvoet, P.; Rull, A.; García-Heredia, A.; López-Sanromà, S.; Geeraert, B.; Joven, J.; Camps, J. Stevia-Derived Compounds Attenuate the Toxic Effects of Ectopic Lipid Accumulation in the Liver of Obese Mice: A Transcriptomic and Metabolomic Study. Food Chem. Toxicol. 2015, 77, 22–33. [Google Scholar] [CrossRef]
- Lian, C.-Y.; Zhai, Z.-Z.; Li, Z.-F.; Wang, L. High Fat Diet-Triggered Non-Alcoholic Fatty Liver Disease: A Review of Proposed Mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Rizwan, F.; Yesmine, S.; Banu, S.G.; Chowdhury, I.A.; Hasan, R.; Chatterjee, T.K. Renoprotective Effects of Stevia (Stevia Rebaudiana Bertoni), Amlodipine, Valsartan, and Losartan in Gentamycin-Induced Nephrotoxicity in the Rat Model: Biochemical, Hematological and Histological Approaches. Toxicol. Rep. 2019, 6, 683–691. [Google Scholar] [CrossRef]
- Elsaid, F.H.; Khalil, A.A.; Ibrahim, E.M.; Mansour, A.; Hussein, A.M. Effects of Exercise and Stevia on Renal Ischemia/Reperfusion Injury in Rats. Acta Sci. Pol. Technol. Aliment. 2019, 18, 317–332. [Google Scholar] [CrossRef]
- Ozbayer, C.; Kurt, H.; Kalender, S.; Ozden, H.; Gunes, H.V.; Basaran, A.; Cakmak, E.A.; Civi, K.; Kalender, Y.; Degirmenci, I. Effects of Stevia Rebaudiana (Bertoni) Extract and N-Nitro-L-Arginine on Renal Function and Ultrastructure of Kidney Cells in Experimental Type 2 Diabetes. J. Med. Food 2011, 14, 1215–1222. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Singh, P.P.; Mahadi, F.; Roy, A.; Sharma, P. Reactive Oxygen Species, Reactive Nitrogen Species and Antioxidants in Etiopathogenesis of Diabetes Mellitus Type-2. Ind. J. Clin. Biochem. 2009, 24, 324–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opara, E.C. Oxidative Stress, Micronutrients, Diabetes Mellitus and Its Complications. J. R. Soc. Promot. Health 2002, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Geeraert, B.; Crombé, F.; Hulsmans, M.; Benhabilès, N.; Geuns, J.M.; Holvoet, P. Stevioside Inhibits Atherosclerosis by Improving Insulin Signaling and Antioxidant Defense in Obese Insulin-Resistant Mice. Int. J. Obes. 2010, 34, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, J.C.; Moguel-Ordoñez, Y.B.; Segura-Campos, M.R. Biological Activity of Stevia Rebaudiana Bertoni and Their Relationship to Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; López, M.D.; Martínez-López, S.; Victoriano, M.; Sharifi-Rad, J.; Martorell, M.; Rodrigues, C.F.; Martins, N. Stevia Rebaudiana Bertoni Bioactive Effects: From in Vivo to Clinical Trials towards Future Therapeutic Approaches. Phytother. Res. PTR 2019, 33, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Misra, M.K. Therapeutic Role of L-Arginine on Free Radical Scavenging System in Ischemic Heart Diseases. Indian J. Biochem. Biophys. 2009, 46, 498–502. [Google Scholar] [PubMed]
- Lucotti, P.; Setola, E.; Monti, L.D.; Galluccio, E.; Costa, S.; Sandoli, E.P.; Fermo, I.; Rabaiotti, G.; Gatti, R.; Piatti, P. Beneficial Effects of a Long-Term Oral l -Arginine Treatment Added to a Hypocaloric Diet and Exercise Training Program in Obese, Insulin-Resistant Type 2 Diabetic Patients. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E906–E912. [Google Scholar] [CrossRef]
- Jabecka, A.; Ast, J.; Bogdaski, P.; Drozdowski, M.; Pawlak-Lemaska, K.; Cielewicz, A.R.; Pupek-Musialik, D. Oral L-Arginine Supplementation in Patients with Mild Arterial Hypertension and Its Effect on Plasma Level of Asymmetric Dimethylarginine, L-Citruline, L-Arginine and Antioxidant Status. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1665–1674. [Google Scholar]
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of Dietary Arginine Supplementation on Reproductive Performance of Mice with Porcine Circovirus Type 2 Infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef]
- Sundaram, B.; Aggarwal, A.; Sandhir, R. Chromium Picolinate Attenuates Hyperglycemia-Induced Oxidative Stress in Streptozotocin-Induced Diabetic Rats. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2013, 27, 117–121. [Google Scholar] [CrossRef]
- Kolahian, S.; Sadri, H.; Shahbazfar, A.A.; Amani, M.; Mazadeh, A.; Mirani, M. The Effects of Leucine, Zinc, and Chromium Supplements on Inflammatory Events of the Respiratory System in Type 2 Diabetic Rats. PLoS ONE 2015, 10, e0133374. [Google Scholar] [CrossRef]
- Kooshki, F.; Tutunchi, H.; Vajdi, M.; Karimi, A.; Niazkar, H.R.; Shoorei, H.; Pourghassem Gargari, B. A Comprehensive Insight into the Effect of Chromium Supplementation on Oxidative Stress Indices in Diabetes Mellitus: A Systematic Review. Clin. Exp. Pharmacol. Physiol. 2021, 48, 291–309. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; Amer, M.A. L-Arginine Ameliorates Oxidative Stress in Alloxan-Induced Experimental Diabetes Mellitus. J. Appl. Toxicol. 2004, 24, 93–97. [Google Scholar] [CrossRef]
- Pai, M.-H.; Huang, K.-H.; Wu, C.-H.; Yeh, S.-L. Effects of Dietary Arginine on Inflammatory Mediator and Receptor of Advanced Glycation Endproducts (RAGE) Expression in Rats with Streptozotocin-Induced Type 2 Diabetes. Br. J. Nutr. 2010, 104, 686–692. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, C.-J.; Liu, C.-H.; Mao, F.C. Chromium Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in KK/HlJ Mice. Biochem. Biophys. Res. Commun. 2010, 397, 459–464. [Google Scholar] [CrossRef]
- Selcuk, M.Y.; Aygen, B.; Dogukan, A.; Tuzcu, Z.; Akdemir, F.; Komorowski, J.R.; Atalay, M.; Sahin, K. Chromium Picolinate and Chromium Histidinate Protects against Renal Dysfunction by Modulation of NF-ΚB Pathway in High-Fat Diet Fed and Streptozotocin-Induced Diabetic Rats. Nutr. Metab. 2012, 9, 30. [Google Scholar] [CrossRef]
- Sahin, K.; Onderci, M.; Tuzcu, M.; Ustundag, B.; Cikim, G.; Ozercan, İ.H.; Sriramoju, V.; Juturu, V.; Komorowski, J.R. Effect of Chromium on Carbohydrate and Lipid Metabolism in a Rat Model of Type 2 Diabetes Mellitus: The Fat-Fed, Streptozotocin-Treated Rat. Metabolism 2007, 56, 1233–1240. [Google Scholar] [CrossRef]
- Ognik, K.; Dworzański, W.; Sembratowicz, I.; Fotschki, B.; Cholewińska, E.; Listos, P.; Juśkiewicz, J. The Effect of the High-Fat Diet Supplemented with Various Forms of Chromium on Rats Body Composition, Liver Metabolism and Organ Histology Cr in Liver Metabolism and Histology of Selected Organs. J. Trace Elem. Med. Biol. 2021, 64, 126705. [Google Scholar] [CrossRef]
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, Anti-Diabetic and Renal Protective Properties of Stevia Rebaudiana. J. Diabetes Complicat. 2013, 27, 103–113. [Google Scholar] [CrossRef]
- Casas-Grajales, S.; Ramos-Tovar, E.; Chávez-Estrada, E.; Alvarez-Suarez, D.; Hernández-Aquino, E.; Reyes-Gordillo, K.; Cerda-García-Rojas, C.M.; Camacho, J.; Tsutsumi, V.; Lakshman, M.R.; et al. Antioxidant and Immunomodulatory Activity Induced by Stevioside in Liver Damage: In Vivo, in Vitro and in Silico Assays. Life Sci. 2019, 224, 187–196. [Google Scholar] [CrossRef]
- Latha, S.; Sheetal, C.; Ray, R.S. Hydroalcoholic Extract of Stevia Rebaudiana Bert. Leaves and Stevioside Ameliorates Lipopolysaccharide Induced Acute Liver Injury in Rats. Biomed. Pharmacother. 2017, 95, 1040–1050. [Google Scholar] [CrossRef]
- Potočnjak, I.; Broznić, D.; Kindl, M.; Kropek, M.; Vladimir-Knežević, S.; Domitrović, R. Stevia and Stevioside Protect against Cisplatin Nephrotoxicity through Inhibition of ERK1/2, STAT3, and NF-ΚB Activation. Food Chem. Toxicol. 2017, 107, 215–225. [Google Scholar] [CrossRef]
- Kaur, T.; Singh, D.; Singh, A.P.; Pathak, D.; Arora, S.; Singh, B.; Kaur, S.; Singh, B. Stevioside Protects against Rhabdomyolysis-induced Acute Kidney Injury through PPAR -γ Agonism in Rats. Drug Dev. Res. 2021, 82, 59–67. [Google Scholar] [CrossRef]
- Shen, W.; Fan, K.; Zhao, Y.; Zhang, J.; Xie, M. Stevioside Inhibits Unilateral Ureteral Obstruction-induced Kidney Fibrosis and Upregulates Renal PPARγ Expression in Mice. J. Food Biochem. 2020, 44, e13520. [Google Scholar] [CrossRef]
- Rotimi, S.O.; Rotimi, O.A.; Adelani, I.B.; Onuzulu, C.; Obi, P.; Okungbaye, R. Stevioside Modulates Oxidative Damage in the Liver and Kidney of High Fat/Low Streptozocin Diabetic Rats. Heliyon 2018, 4, e00640. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Karimzadeh, I.; Sagheb, M.M.; Khalili, H. The Renal Safety of L-Carnitine, L-Arginine, and Glutamine in Athletes and Bodybuilders. J. Ren. Nutr. 2019, 29, 221–234. [Google Scholar] [CrossRef]
- Reyes, A.A.; Karl, I.E.; Klahr, S. Role of Arginine in Health and in Renal Disease. Am. J. Physiol. Ren. Physiol. 1994, 267, F331–F346. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Golzarand, M.; Davudabadi-Farahani, R.; Azizi, F. Dietary Animal-Derived L-Arginine Intakes and Risk of Chronic Kidney Disease: A 6-Year Follow-up of Tehran Lipid and Glucose Study. Iran. J. Kidney Dis. 2017, 11, 352–359. [Google Scholar]
- Suksomboon, N.; Poolsup, N.; Yuwanakorn, A. Systematic Review and Meta-Analysis of the Efficacy and Safety of Chromium Supplementation in Diabetes. J. Clin. Pharm. Ther. 2014, 39, 292–306. [Google Scholar] [CrossRef]
- Ryan, G.J.; Wanko, N.S.; Redman, A.R.; Cook, C.B. Chromium as Adjunctive Treatment for Type 2 Diabetes. Ann. Pharmacother. 2003, 37, 876–885. [Google Scholar] [CrossRef]
- Chen, J.; Jeppesen, P.B.; Nordentoft, I.; Hermansen, K. Stevioside Improves Pancreatic β-Cell Function during Glucotoxicity via Regulation of Acetyl-CoA Carboxylase. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1906–E1916. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B. Protective and Therapeutic Effects of Natural Products against Diabetes Mellitus via Regenerating Pancreatic β -cells and Restoring Their Dysfunction. Phytother. Res. 2021, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Bhattacharjee, J.; Salazar-Gonzalez, R.-M.; Park, S.; Jang, A.; Warren, M.; Merritt, R.; Michail, S.; Bouret, S.; Kohli, R. Rebaudioside Affords Hepatoprotection Ameliorating Sugar Sweetened Beverage- Induced Nonalcoholic Steatohepatitis. Sci. Rep. 2020, 10, 6689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, R.; Vengatash Babu, K.; Ramachandran, V. Effect of Rebaudioside A, a Diterpenoid on Glucose Homeostasis in STZ-Induced Diabetic Rats. J. Physiol. Biochem. 2012, 68, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Hernández-Aquino, E.; Casas-Grajales, S.; Buendia-Montaño, L.D.; Galindo-Gómez, S.; Camacho, J.; Tsutsumi, V.; Muriel, P. Stevia Prevents Acute and Chronic Liver Injury Induced by Carbon Tetrachloride by Blocking Oxidative Stress through Nrf2 Upregulation. Oxid. Med. Cell. Longev. 2018, 2018, 3823426. [Google Scholar] [CrossRef] [PubMed]
- Salil, G.; Nevin, K.G.; Rajamohan, T. Arginine-Rich Coconut Kernel Diet Influences Nitric Oxide Synthase Activity in Alloxandiabetic Rats. J. Sci. Food Agric. 2012, 92, 1903–1908. [Google Scholar] [CrossRef]
- Vasilijević, A.; Buzadžić, B.; Korać, A.; Petrović, V.; Janković, A.; Korać, B. Beneficial Effects of L-Arginine-Nitric Oxide-Producing Pathway in Rats Treated with Alloxan: Role of l -Arginine-NO Pathway in Diabetes. J. Physiol. 2007, 584, 921–933. [Google Scholar] [CrossRef]
- Huang, S.; Peng, W.; Jiang, X.; Shao, K.; Xia, L.; Tang, Y.; Qiu, J. The Effect of Chromium Picolinate Supplementation on the Pancreas and Macroangiopathy in Type II Diabetes Mellitus Rats. J. Diabetes Res. 2014, 2014, 717219. [Google Scholar] [CrossRef]
- Liu, L.; Jin, W.; Lv, J. Oral Administration of the High-Chromium Yeast Improve Blood Plasma Variables and Pancreatic Islet Tissue in Diabetic Mice. Biol. Trace Elem. Res. 2010, 138, 250–264. [Google Scholar] [CrossRef]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural Sweetener Stevia Rebaudiana: Functionalities, Health Benefits and Potential Risks. EXCLI J. 2021, 20, 1412. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J.; McNeal, C.J.; Bazer, F.W.; Rhoads, J.M. Role of L-Arginine in Nitric Oxide Synthesis and Health in Humans. In Amino Acids in Nutrition and Health; Wu, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1332, pp. 167–187. ISBN 978-3-030-74179-2. [Google Scholar]
- Talevi, A. Potential Medicinal Effects and Applications of Stevia Constituents. Phytochem. Rev. 2022, 21, 161–178. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, X.-Y.; Li, J.; Xu, Z.-G.; Chen, L. The Characterization of High-Fat Diet and Multiple Low-Dose Streptozotocin Induced Type 2 Diabetes Rat Model. Exp. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.J.; Rice-Evans, C.A. Factors Influencing the Antioxidant Activity Determined by the ABTS •+ Radical Cation Assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Johansson, L.H.; Håkan Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W. Automated Assays for Superoxide Dismutase, Catalase, Glutathione Peroxidase, and Glutathione Reductase Activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Pruszyńska-Oszmałek, E.; Wojciechowska, M.; Sassek, M.; Krauss, H.; Leciejewska, N.; Szczepankiewicz, D.; Ślósarz, P.; Nogowski, L.; Kołodziejski, P.A. The Long-Term Effects of High-Fat and High-Protein Diets on the Metabolic and Endocrine Activity of Adipocytes in Rats. Biology 2021, 10, 339. [Google Scholar] [CrossRef]




| Parameter | Control (C) | Experimental Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Db | Db + Met | Db + S A1C1 | Db + S A1C2 | Db + S A2C1 | Db + S A2C2 | Db + R A1C1 | Db + R A1C2 | Db + R A2C1 | Db + R A2C2 | ||
| Overall growth and nutritional indices | |||||||||||
| Avg DI (g/day) | 27.92 ± 2.13 f | 24.30 ± 0.93 cde | 23.07 ± 0.55 abcd | 22.35 ± 0.89 ab | 21.97 ± 1.14 a | 22.25 ± 3.24 ab | 23.02 ± 1.90 abcd | 24.75 ± 1.96 de | 22.74 ± 2.24 abc | 25.56 ± 2.12 e | 23.88 ± 2.71 bcd |
| Avg EI (mJ/day) | 0.47 ± 0.04 bcd | 0.50 ± 0.02 d | 0.47 ± 0.01 bcd | 0.44 ± 0.02 ab | 0.43 ± 0.02 a | 0.43 ± 0.06 a | 0.45 ± 0.04 ab | 0.49 ± 0.04 cd | 0.45 ± 0.04 ab | 0.50 ± 0.04 cd | 0.46 ± 0.05 abc |
| WI (1st week) | 28.33 ± 5.33 a | 39.00 ± 10.87 ab | 39.80 ± 13.51 ab | 44.10 ± 12.08 ab | 41.50 ± 5.01 ab | 52.50 ± 27.51 bc | 49.50 ± 8.43 abc | 76.20 ± 37.50 d | 44.80 ± 10.31 ab | 69.60 ± 40.84 cd | 68.30 ± 28.56 cd |
| WI (5th week) | 36.89 ± 6.21 a | 35.70 ± 6.49 a | 36.90 ± 7.20 a | 43.30 ± 8.73 ab | 37.90 ± 2.01 a | 64.10 ± 47.12 bc | 41.63 ± 8.81 ab | 70.80 ± 35.53 c | 46.60 ± 10.22 ab | 71.80 ± 45.21 c | 63.60 ± 28.60 bc |
| BW gain (g) | 55.11 ± 11.65 ab | 77.90 ± 18.35 c | 55.40 ± 13.28 ab | 56.10 ± 25.77 b | 53.50 ± 23.88 ab | 36.30 ± 24.02 ab | 50.00 ± 31.01 ab | 54.10 ± 24.57 ab | 34.10 ± 18.91 a | 49.30 ± 31.77 ab | 46.10 ± 31.03 ab |
| FER | 4.92 ± 0.36 ab | 8.01 ± 0.76 c | 5.98 ± 0.54 bc | 6.26 ± 1.15 bc | 6.02 ± 1.00 bc | 4.15 ± 1.19 ab | 5.55 ± 1.40 ab | 5.57 ± 1.10 ab | 3.69 ± 0.78 a | 4.79 ± 1.35 ab | 4.94 ± 1.32 ab |
| Relative internal organ weight | |||||||||||
| Liver (% BW) | 3.18 ± 0.40 | 3.09 ± 0.34 | 3.17 ± 0.34 | 3.19 ± 0.27 | 3.20 ± 0.42 | 3.20 ± 0.22 | 3.20 ± 0.33 | 3.16 ± 0.33 | 3.33 ± 0.33 | 3.55 ± 0.42 | 3.31 ± 0.50 |
| Kidneys (% BW) | 0.60 ± 0.08 ab | 0.59 ± 0.05 a | 0.62 ± 0.05 abc | 0.64 ± 0.05 abc | 0.67 ± 0.07 abc | 0.68 ± 0.10 bc | 0.62 ± 0.04 abc | 0.71 ± 0.14 cd | 0.69 ± 0.09 bcd | 0.77 ± 0.16 d | 0.70 ± 0.13 cd |
| Testes (% BW) | 0.81 ± 0.19 | 0.79 ± 0.09 | 0.80 ± 0.14 | 0.78 ± 0.15 | 0.76 ± 0.13 | 0.81 ± 0.10 | 0.76 ± 0.09 | 0.81 ± 0.13 | 0.82 ± 0.07 | 0.74 ± 0.22 | 0.80 ± 0.12 |
| Spleen (% BW) | 0.16 ± 0.03 ab | 0.16 ± 0.02 a | 0.16 ± 0.02 a | 0.17 ± 0.01 ab | 0.16 ± 0.02 a | 0.18 ± 0.02 bc | 0.17 ± 0.01 ab | 0.17 ± 0.02 ab | 0.17 ± 0.01 ab | 0.19 ± 0.03 c | 0.17 ± 0.02 ab |
| Heart (% BW) | 0.26 ± 0.02 | 0.25 ± 0.02 | 0.28 ± 0.03 | 0.26 ± 0.02 | 0.27 ± 0.02 | 0.28 ± 0.02 | 0.27 ± 0.03 | 0.27 ± 0.02 | 0.28 ± 0.03 | 0.29 ± 0.04 | 0.27 ± 0.03 |
| Lungs (% BW) | 0.37 ± 0.07 ab | 0.34 ± 0.05 a | 0.38 ± 0.06 ab | 0.37 ± 0.04 ab | 0.36 ± 0.06 ab | 0.40 ± 0.09 bc | 0.34 ± 0.05 a | 0.41 ± 0.06 bc | 0.45 ± 0.08 c | 0.35 ± 0.05 ab | 0.38 ± 0.05 ab |
| Brain (% BW) | 0.36 ± 0.05 | 0.37 ± 0.04 | 0.38 ± 0.03 | 0.38 ± 0.03 | 0.39 ± 0.04 | 0.39 ± 0.03 | 0.38 ± 0.04 | 0.41 ± 0.03 | 0.40 ± 0.04 | 0.36 ± 0.05 | 0.38 ± 0.04 |
| Index | Main Effects | Interaction Effects | |||||
|---|---|---|---|---|---|---|---|
| Glycoside (Stevioside vs. Rebaudioside A) | L-arginine (2.0% vs. 4.0%) | Chromium(III) (0.001% vs. 0.005%) | Glycoside x L-Arginine | Glycoside x Chromium(III) | L-Arginine x Chromium(III) | Glycoside x L-Arginine x Chromium(III) | |
| Overall growth and nutritional indices | |||||||
| Avg DI (g/day) | 22.40 ± 1.97 | 22.95 ± 1.93 | 23.73 ± 2.59 | NS | * | NS | NS |
| 24.23 ± 2.43 *** | 23.68 ± 2.75 | 22.90 ± 2.11 | |||||
| Avg EI (mJ/day) | 0.44 ± 0.04 | 0.45 ± 0.04 | 0.47 ± 0.05 | NS | * | NS | NS |
| 0.48 ± 0.05 *** | 0.46 ± 0.05 | 0.45 ± 0.04 | |||||
| WI (1st week) | 46.76 ± 16.09 | 51.65 ± 24.41 | 60.60 ± 32.98 | NS | NS | NS | NS |
| 64.73 ± 32.67 ** | 60.53 ± 29.73 | 51.11 ± 18.97 | |||||
| WI (5th week) | 47.00 ± 26.18 | 49.65 ± 22.29 | 62.50 ± 37.79 | NS | NS | NS | NS |
| 63.20 ± 32.87 * | 61.26 ± 36.99 | 47.74 ± 18.51* | |||||
| Relative internal organ weight | |||||||
| Kidneys (% BW) | 0.65 ± 0.07 | 0.68 ± 0.09 | 0.70 ± 0.13 | NS | NS | NS | NS |
| 0.72 ± 0.13 ** | 0.70 ± 0.12 | 0.67 ± 0.09 | |||||
| Spleen (% BW) | 0.17 ± 0.02 | 0.17 ± 0.02 | 0.18 ± 0.02 | NS | NS | NS | NS |
| 0.18 ± 0.02 * | 0.18 ± 0.02 * | 0.17 ± 0.02 | |||||
| Heart (% BW) | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.03 | NS | NS | * | NS |
| 0.28 ± 0.03 | 0.28 ± 0.03 | 0.27 ± 0.03 | |||||
| Lungs (% BW) | 0.37 ± 0.06 | 0.40 ± 0.07 | 0.38 ± 0.06 | * | * | NS | NS |
| 0.40 ± 0.07 * | 0.37 ± 0.06 * | 0.38 ± 0.07 | |||||
| Glucose-related indices | |||||||
| GLU (mg/dL) | 162.11 ± 15.71 | 165.49 ± 14.02 | 165.36 ± 15.97 | NS | NS | NS | NS |
| 170.45 ± 13.25 * | 166.50 ± 16.42 | 166.56 ± 14.44 | |||||
| INS (pmol/L) | 2.52 ± 0.67 | 2.57 ± 0.90 | 2.60 ± 1.10 | * | NS | NS | NS |
| 2.84 ± 1.35 | 2.79 ± 1.23 | 2.77 ± 1.06 | |||||
| HOMA-IR | 9.71 ± 3.10 | 9.60 ± 2.16 | 10.75 ± 5.15 | ** | NS | NS | * |
| 11.84 ± 5.32 * | 11.74 ± 5.58 * | 10.63 ± 3.44 | |||||
| HOMA-β | 88.20 ± 25.04 | 91.43 ± 32.70 | 91.84 ± 33.34 | NS | NS | NS | ** |
| 94.52 ± 40.16 | 90.56 ± 33.03 | 90.13 ± 32.25 | |||||
| QUICKI | 0.28 ± 0.01 | 0.28 ± 0.01 | 0.28 ± 0.01 | * | NS | NS | ** |
| 0.27 ± 0.01 ** | 0.28 ± 0.02 | 0.28 ± 0.01 | |||||
| Lipid metabolism indices | |||||||
| LDL (mg/dL) | 22.45 ± 7.07 | 22.14 ± 7.31 | 22.94 ± 7.65 | * | ** | NS | * |
| 21.86 ± 6.10 | 22.17 ± 5.82 | 21.37 ± 5.25 | |||||
| VLDL-C (mg/dL) | 0.41 ± 0.04 | 0.42 ± 0.03 | 0.43 ± 0.04 | NS | NS | NS | NS |
| 0.44 ± 0.05 ** | 0.44 ± 0.06 | 0.42 ± 0.05 | |||||
| Other biochemical indices | |||||||
| ALT (U/l) | 52.48 ± 12.12 | 46.92 ± 9.34 | 50.94 ± 12.00 | NS | NS | NS | * |
| 48.97 ± 13.65 | 54.34 ± 14.85 ** | 50.53 ± 13.94 | |||||
| AST (U/l) | 230.51 ± 88.94 | 204.54 ± 79.96 | 225.77 ± 88.01 | NS | NS | NS | NS |
| 188.05 ± 67.93* | 214.02 ± 83.72 | 192.79 ± 71.74 | |||||
| UREA (mg/dL) | 40.50 ± 6.73 | 38.38 ± 5.35 | 39.83 ± 6.04 | NS | NS | NS | NS |
| 39.45 ± 5.30 | 41.62 ± 6.35 * | 40.13 ± 6.13 | |||||
| KREA (mg/dL) | 0.27 ± 0.05 | 0.29 ± 0.05 | 0.28 ± 0.06 | NS | NS | NS | NS |
| 0.29 ± 0.06 | 0.27 ± 0.05 * | 0.28 ± 0.05 | |||||
| TP (g/dL) | 6.29 ± 0.38 | 6.36 ± 0.31 | 6.21 ± 0.37 | NS | NS | NS | NS |
| 6.23 ± 0.35 | 6.15 ± 0.39 * | 6.30 ± 0.35 | |||||
| NO (μmol/L) | 3.98 ± 0.33 | 4.05 ± 0.32 | 4.12 ± 0.41 | NS | NS | NS | NS |
| 4.23 ± 0.43 * | 4.18 ± 0.48 | 4.09 ± 0.40 | |||||
| TAC (mM) | 1.30 ± 0.26 | 1.28 ± 0.30 | 1.24 ± 0.29 | * | *** | NS | NS |
| 1.33 ± 0.27 | 1.34 ± 0.21 | 1.37 ± 0.21 | |||||
| CAT | 83.32 ± 19.96 | 82.37 ± 19.08 | 82.14 ± 19.66 | NS | * | NS | * |
| (nmol/min/mL) | 84.08 ± 22.75 | 85.10 ± 23.53 | 85.34 ± 22.99 | ||||
| Kidney Cr(III) conc. (ng/g d.m.) | 3073.75 ± 1383.47 2966.06 ± 1707.92 | 2949.20 ± 1804.11 3090.62 ± 1253.74 | 1696.19 ± 620.46 4343.63 ± 926.08 *** | *** | * | *** | NS |
| Liver Cr(III) conc. | 202.37 ± 63.58 | 319.65 ± 181.23 | 260.54 ± 110.80 | NS | *** | * | ** |
| (ng/g d.m.) | 425.10 ± 152.93 | 316.62 ± 145.76 | 380.27 ± 186.85 | ||||
| Parameter | Control (C) | Experimental Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Db | Db + Met | Db + S A1C1 | Db + S A1C2 | Db + S A2C1 | Db + S A2C2 | Db + R A1C1 | Db + R A1C2 | Db + R A2C1 | Db + R A2C2 | ||
| T-C (mg/dL) | 91.36 ± 21.59 | 102.50 ± 15.62 | 86.70 ± 17.02 | 109.56 ± 10.68 | 89.30 ± 10.15 | 98.28 ± 17.85 | 93.52 ± 21.47 | 93.56 ± 13.18 | 103.03 ± 19.06 | 108.43 ± 31.65 | 101.23 ± 11.53 |
| TG (mg/dL) | 265.10 ± 143.62 | 228.12 ± 62.99 | 188.87 ± 67.54 | 218.13 ± 85.19 | 231.29 ± 85.09 | 256.44 ± 71.46 | 211.36 ± 78.96 | 232.71 ± 106.25 | 227.83 ± 93.45 | 231.94 ± 105.03 | 257.49 ± 134.09 |
| LDL-C (mg/dL) | 16.06 ± 5.84 ab | 19.53 ± 5.01 bcd | 13.92 ± 5.14 a | 29.51 ± 7.06 e | 18.00 ± 3.79 abc | 20.95 ± 6.07 bcd | 21.35 ± 5.84 cd | 18.86 ± 6.43 abcd | 22.18 ± 5.90 cd | 22.44 ± 7.37 cd | 23.96 ± 3.90 d |
| HDL-C (mg/dL) | 65.15 ± 19.29 | 71.25 ± 9.60 | 61.98 ± 13.09 | 68.11 ± 12.16 | 60.21 ± 6.80 | 64.53 ± 15.00 | 59.32 ± 11.15 | 61.80 ± 10.34 | 66.97 ± 13.04 | 70.52 ± 22.91 | 65.53 ± 9.80 |
| VLDL-C (mg/dL) | 0.47 ± 0.05 de | 0.48 ± 0.03 e | 0.44 ± 0.04 abc | 0.41 ± 0.03 a | 0.41 ± 0.04 a | 0.42 ± 0.04 a | 0.41 ± 0.04 a | 0.42 ± 0.02 ab | 0.42 ± 0.03 ab | 0.46 ± 0.06 de | 0.46 ± 0.07 cd |
| Parameter | Control (C) | Experimental Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Db | Db + Met | Db + S A1C1 | Db + S A1C2 | Db + S A2C1 | Db + S A2C2 | Db + R A1C1 | Db + R A1C2 | Db + R A2C1 | Db + R A2C2 | ||
| ALT (U/l) | 31.36 ± 1.88 a | 49.41 ± 9.23 bc | 48.32 ± 11.25 bc | 44.46 ± 5.27 b | 50.84 ± 13.15 bc | 61.37 ± 14.36 d | 52.47 ± 7.83 bcd | 48.94 ± 7.25 bc | 43.39 ± 8.56 ab | 48.13 ± 11.78 bc | 55.4 ± 21.14 cd |
| AST (U/l) | 202.80 ± 71.53 | 216.84 ± 54.14 | 217.77 ± 77.82 | 220.20 ± 82.02 | 213.24 ± 90.10 | 266.15 ± 111.44 | 222.45 ± 71.26 | 224.08 ± 82.32 | 160.65 ± 56.25 | 192.66 ± 67.62 | 174.81 ± 54.68 |
| AST/ALT ratio | 4.63 ± 0.41 | 4.35 ± 0.50 | 4.46 ± 0.84 | 4.44 ± 0.79 | 4.07 ± 0.94 | 4.25 ± 1.12 | 4.22 ± 1.11 | 4.36 ± 1.30 | 3.72 ± 1.13 | 4.01 ± 1.01 | 3.35 ± 0.97 |
| UREA (mg/dL) | 32.41 ± 4.71 ab | 31.47 ± 3.89 a | 32.84 ± 3.15 ab | 38.94 ± 4.74 cd | 38.33 ± 6.62 cd | 42.29 ± 7.38 cd | 42.43 ± 7.74 d | 37.39 ± 5.30 bc | 38.85 ± 5.29 cd | 40.79 ± 6.19 cd | 40.89 ± 4.42 cd |
| KREA (mg/dL) | 0.26 ± 0.05 | 0.26 ± 0.05 | 0.96 ± 2.12 | 0.28 ± 0.04 | 0.27 ± 0.05 | 0.28 ± 0.04 | 0.26 ± 0.05 | 0.32 ± 0.06 | 0.3 ± 0.05 | 0.24 ± 0.05 | 0.29 ± 0.03 |
| TP (g/dl) | 6.47 ± 0.28 | 6.26 ± 0.24 | 6.23 ± 0.10 | 6.30 ± 0.19 | 6.49 ± 0.30 | 6.15 ± 0.47 | 6.21 ± 0.45 | 6.30 ± 0.45 | 6.35 ± 0.25 | 6.09 ± 0.31 | 6.16 ± 0.34 |
| TAC (mM) | 1.02 ± 0.3 c | 0.76 ± 0.3 a | 0.79 ± 0.2 ab | 0.98 ± 0.2 bc | 1.47 ± 0.2 d | 1.32 ± 0.2 d | 1.44 ± 0.1 d | 1.45 ± 0.4 d | 1.31 ± 0.2 d | 1.34 ± 0.2 d | 1.26 ± 0.3 d |
| CAT (nmol/min/mL) | 75.94 ± 23.41 abc | 71.24 ± 17.40 ab | 69.25 ± 14.02 a | 82.72 ± 14.43 abcd | 88.09 ± 19.96 bcd | 90.87 ± 26.40 cd | 70.30 ± 11.62 ab | 75.21 ± 20.55 abc | 83.47 ± 21.30 abcd | 79.78 ± 14.25 abc | 99.39 ± 29.32 d |
| NO (μmol/L) | 3.98 ± 0.28 | 4.13 ± 0.41 | 3.77 ± 0.45 | 4.03 ± 0.47 | 4.00 ± 0.24 | 4.01 ± 0.28 | 3.89 ± 0.39 | 4.07 ± 0.36 | 4.09 ± 0.27 | 4.37 ± 0.49 | 4.43 ± 0.56 |
| Kidney Cr conc. (ng/g d.m.) | 1175.28 ± 193.11 a | 1165.88 ± 250.83 a | 1000.07 ± 215.44 a | 1363.77 ± 281.70 a | 4099.95 ± 549.43 c | 2489.25 ± 596.15 b | 4342.05 ± 985.58 cd | 1233.89 ± 278.40 a | 5099.20 ± 987.83 d | 1697.85 ± 311.23 ab | 3833.31 ± 691.14 c |
| Liver Cr conc. (ng/g d.m.) | 213.79 ± 74.97 bc | 122.62 ± 28.65 a | 213.78 ± 99.19 bc | 188.83 ± 45.17 ab | 178.49 ± 11.50 ab | 200.00 ± 61.72 b | 235.00 ± 91.64 bc | 282.12 ± 56.41 c | 586.80 ± 103.37 f | 371.22 ± 142.32 d | 460.27 ± 104.16 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurek, J.M.; Król, E.; Staniek, H.; Krejpcio, Z. Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation Attenuates Abnormalities in Glucose Metabolism in Streptozotocin-Induced Mildly Diabetic Rats Fed a High-Fat Diet. Pharmaceuticals 2022, 15, 1200. https://doi.org/10.3390/ph15101200
Kurek JM, Król E, Staniek H, Krejpcio Z. Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation Attenuates Abnormalities in Glucose Metabolism in Streptozotocin-Induced Mildly Diabetic Rats Fed a High-Fat Diet. Pharmaceuticals. 2022; 15(10):1200. https://doi.org/10.3390/ph15101200
Chicago/Turabian StyleKurek, Jakub Michał, Ewelina Król, Halina Staniek, and Zbigniew Krejpcio. 2022. "Steviol Glycoside, L-Arginine, and Chromium(III) Supplementation Attenuates Abnormalities in Glucose Metabolism in Streptozotocin-Induced Mildly Diabetic Rats Fed a High-Fat Diet" Pharmaceuticals 15, no. 10: 1200. https://doi.org/10.3390/ph15101200