Pioneering Enhanced Corrosion Resistance along the Normal Plane of an Ultra-Light Mg-Li Extruded Sheet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation and Microstructural Analysis
2.2. Hydrogen Evolution, Weight Loss, and Immersion Testing
2.3. Electrochemical Testing
3. Results and Discussion
3.1. Microstructure
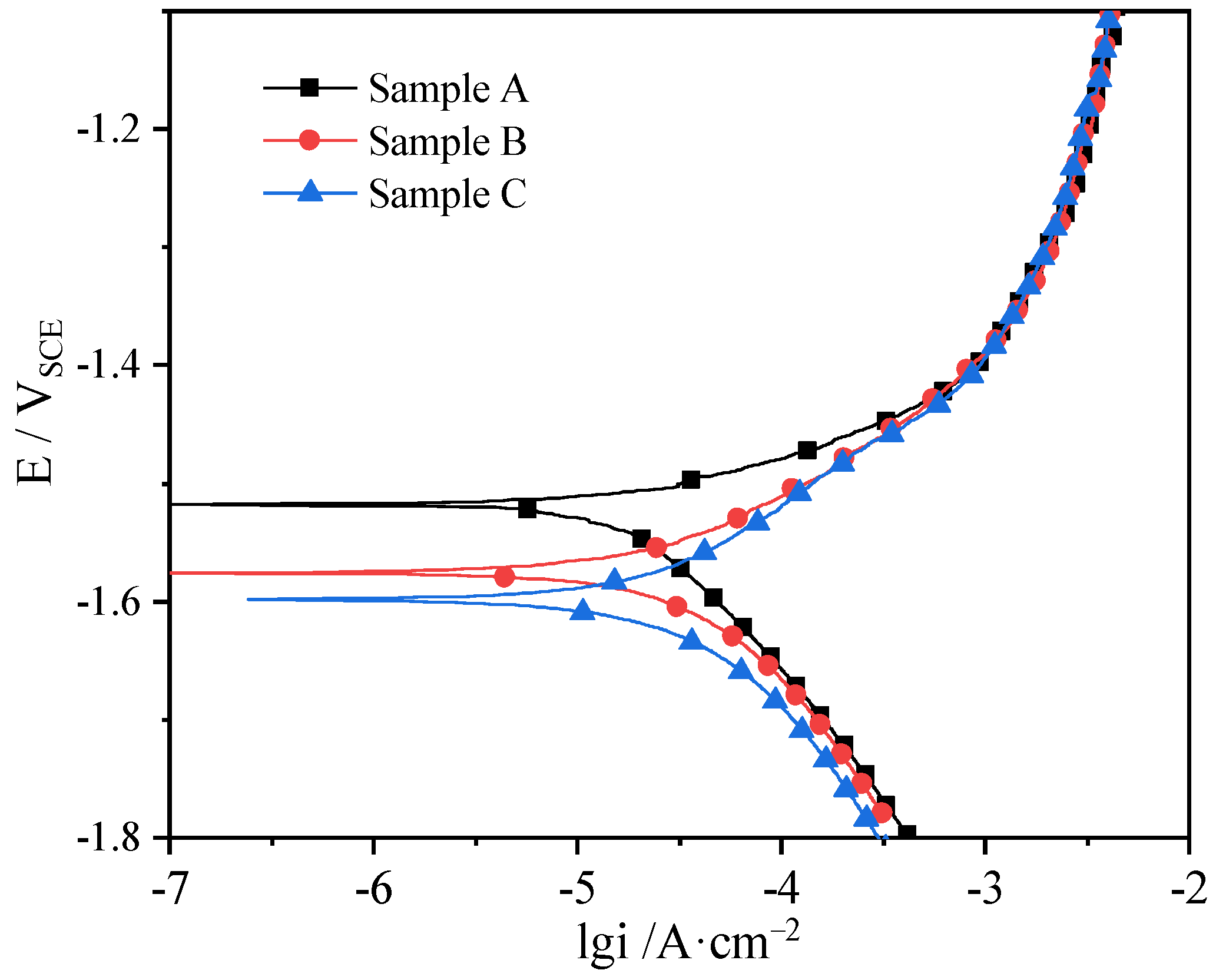
3.2. Corrosion Resistance
3.3. Corrosion Morphologies
4. Conclusions
- (1)
- The corrosion resistance of different sectional samples cut from as-extruded Mg-5Li alloy reduces in the order of sample A (ND plane) > sample B (ED plane) > sample C (TD plane), primarily due to the various effects of texture and grain size.
- (2)
- All the different sectional samples in as-extruded Mg-5Li alloys exhibit the typical filiform corrosion at the initial corrosion stage, but the large corrosion pits gradually emerge, prolonging the corrosion time, with sample C displaying pronounced susceptibility.
- (3)
- Corrosion anisotropy indeed exists in the as-extruded Mg-5Li alloy testing in 0.1 mol/L NaCl solution, indicating the best corrosion performance of the normal plane and itsl possible application in practice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, J.; Frost, P.; Loonam, A.; Eastwood, L.; Lorig, C. Magnesium-lithium base alloys—Preparation, fabrication, and general characteristics. JOM 1949, 1, 149–168. [Google Scholar] [CrossRef]
- Cao, F.; Xiang, C.; Kong, S.; Guo, N.; Shang, H. Room Temperature Strengthening and High-Temperature Superplasticity of Mg-Li-Al-Sr-Y Alloy Fabricated by Asymmetric Rolling and Friction Stir Processing. Materials 2023, 16, 2345. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jin, S.; Wu, R. Corrosion behavior of Mg−Li alloys: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 3228–3254. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Xiao, D.; Huang, L.; Liu, W.; Tang, S. Effect of Rolling Temperature on Microstructure and Properties of Al-Mg-Li Alloy. Materials 2022, 15, 7517. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xiong, Q.; Arens, A. Influence of crystallographic texture and grain size on the corrosion behaviour of as-extruded Mg alloy AZ31 sheets. Corros. Sci. 2017, 126, 374–380. [Google Scholar] [CrossRef]
- Fang, Z.; He, L.; Wang, J.; Ma, X.; Wang, G.; Wu, R.; Jin, S.; Wang, J.; Lu, Z.; Yang, Z.; et al. Effect of I-Phase on Microstructure and Corrosion Resistance of Mg-8.5Li-6.5Zn-1.2Y Alloy. Materials 2023, 16, 3007. [Google Scholar] [CrossRef]
- Maurya, R.; Siddiqui, A.R.; Balani, K. An environment-friendly phosphate chemical conversion coating on novel Mg-9Li-7Al-1Sn and Mg-9Li-5Al-3Sn-1Zn alloys with remarkable corrosion protection. Appl. Surf. Sci. 2018, 443, 429–440. [Google Scholar] [CrossRef]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.; Xiao, Y. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef]
- Li, C.; Deng, B.; Dong, L.; Shi, B.; Dong, Y.; Peng, F. Effect of Zn addition on the corrosion behaviours of as-cast BCC Mg-11Li based alloys in NaCl solution. Mater. Des. 2022, 221, 111019. [Google Scholar] [CrossRef]
- Li, C.; Deng, B.; Dong, L.; Liu, X.; Du, K.; Shi, B. Effect of Zn addition on the microstructure and mechanical properties of as-cast BCC Mg-11Li based alloys. J. Alloys Compd. 2022, 895, 162718. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Q.; Wang, H. Progress in corrosion and protection of extruded magnesium Alloys. Surf. Technol. 2020, 49, 112–119. [Google Scholar]
- Meng, S.; Yu, H.; Fan, S. Recent progress and development in extrusion of rare earth free Mg alloys. Acta Metall. Sin. Engl. 2019, 32, 145–168. [Google Scholar] [CrossRef]
- Bland, L.; Gusieva, K.; Scully, J. Effect of crystallographic orientation on the corrosion of magnesium: Comparison of film forming and bare crystal facets using electrochemical impedance and Raman spectroscopy. Electrochim. Acta 2016, 227, 136–151. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Wang, C.; Ju, J.; Wang, G.; Jiang, J.; Ma, A.; Jing, B.; Xue, F.; Xin, Y. Anisotropy investigation of an ECAP-processed Mg-Al-Ca-Mn alloy with synergistically enhanced mechanical properties and corrosion resistance. J. Alloys Compd. 2022, 911, 165046. [Google Scholar] [CrossRef]
- Liu, X.; Bian, L.; Tian, F.; Han, S.; Tao, W.; Liang, W. Microstructural evolution and mechanical response of duplex Mg-Li alloy containing particles during ECAP processing. Mater. Charact. 2022, 188, 11910. [Google Scholar] [CrossRef]
- Li, C.; Liang, D.; Lin, Y.; Dong, Y.; Shi, B.; Yan, C.; Zhang, Z. Effect of Li Content on the Surface Film Formed on the Binary Mg–Li Alloys in NaCl Solution. Met. Mater. Int. 2023. [Google Scholar] [CrossRef]
- Guo, Z.; Ji, Q.; Wu, R.; Jia, H.; An, D.; Ma, X.; Jin, S.; Li, J.; Liu, J.; Wu, H.; et al. High-Strength β-Phase Magnesium–Lithium Alloy Prepared by Multidirectional Rolling. Materials 2023, 16, 3227. [Google Scholar] [CrossRef]
- Mishra, S.; Khan, F.; Panigrahi, S. A crystal plasticity based approach to establish role of grain size and crystallographic texture in the Tension–Compression yield asymmetry and strain hardening behavior of a Magnesium–Silver–Rare Earth alloy. J. Magnes. Alloys 2022, 10, 2546–2562. [Google Scholar] [CrossRef]
- Li, R.; Pan, F.; Jiang, B.; Dong, H.; Yang, Q. Effect of Li addition on the mechanical behavior and texture of the as-extruded AZ31 magnesium alloy. Mater. Sci. Eng. A 2013, 562, 33–38. [Google Scholar] [CrossRef]
- Tian, G.; Wang, J.; Xue, C.; Wang, S.; Yang, X.; Su, H.; Li, Q.; Li, X.; Yan, C.; Yang, Z. Improving corrosion resistance of Mg–Li alloys by Sn microalloying. J. Mater. Res. Technol. 2023, 26, 199–217. [Google Scholar] [CrossRef]
- Wiese, B.; Harmuth, J.; Willumeit-Römer, R.; Bohlen, J. Property Variation of Extruded Mg-Gd Alloys by Mn Addition and Processing. Crystals 2022, 12, 1036. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, B.; Dai, Z. Microstructure, texture, and tensile properties of Mg-3Li alloy extruded at different temperatures. J. Mater. Eng. Perform. 2022, 31, 5782–5789. [Google Scholar] [CrossRef]
- Li, C.; He, Y.; Huang, H. Effect of lithium content on the mechanical and corrosion behaviors of HCP binary Mg–Li alloys. J. Magnes. Alloys 2021, 9, 569–580. [Google Scholar] [CrossRef]
- Azzeddine, H.; Hanna, A.; Dakhouche, A.; Baudin, T.; Brisset, F.; Huang, Y.; Langdon, T.G. Evaluation of Thermal Stability and Its Effect on the Corrosion Behaviour of Mg-RE Alloys Processed by High-Pressure Torsion. Crystals 2023, 13, 662. [Google Scholar] [CrossRef]
- Xin, R.; Li, B.; Li, L.; Liu, Q. Influence of texture on corrosion rate of AZ31 Mg alloy in 3. 5wt.% NaCl, Mater. Des. 2011, 32, 8–9. [Google Scholar]
- Qin, J.; Li, Z.; Ma, M. Diversity of intergranular corrosion and stress corrosion cracking for 5083 Al alloy with different grain sizes. Trans. Nonferrous Met. Soc. China 2022, 32, 765–777. [Google Scholar] [CrossRef]
- Cui, Q.; Yi, D.; Wang, H. Effects of grain size and secondary phase on corrosion behavior and electrochemical performance of Mg-3Al-5Pb-1Ga-Y sacrificial anode. J. Rare Earth 2019, 37, 1341–1350. [Google Scholar] [CrossRef]
- Williams, G.; Birbilis, N.; Mcmurray, H. The source of hydrogen evolved from a magnesium anode. Electrochem. Commun. 2013, 36, 1–5. [Google Scholar] [CrossRef]
- Cano, Z.; Danaie, M.; Kish, J. Physical characterization of cathodically-activated corrosion filaments on magnesium alloy AZ31B. Corrosion 2015, 71, 146–159. [Google Scholar] [CrossRef]
- Schmutz, P.; Guillanumin, V.; Lillard, R. Influence of dichromate ions on corrosion processes on pure magnesium. J. Electrochem. Soc. 2003, 150, B99. [Google Scholar] [CrossRef]
- Mccall, C.; Hill, M.; Lillard, R. Crystallographic pitting in magnesium single crystals. Corros. Eng. Sci. Technol. 2005, 40, 337–343. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R. Investigation of surface oxide film on magnesium lithium alloy. J. Alloys Compd. 2009, 484, 585–590. [Google Scholar] [CrossRef]
- Li, C.; Xu, D.; Zhang, Z. Influence of the lithium content on the negative difference effect of Mg-Li alloys. J. Mater. Sci. Technol. 2020, 57, 138–145. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Shan, D. Effects of corrosive media on the localized corrosion forms of Mg-3Zn alloy. Corros. Commun. 2021, 2, 24–32. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Deng, B.; Li, C.; Dong, Y.; Wang, N.; Zhang, Z.; Wang, S. Pioneering Enhanced Corrosion Resistance along the Normal Plane of an Ultra-Light Mg-Li Extruded Sheet. Materials 2023, 16, 6435. https://doi.org/10.3390/ma16196435
Liang J, Deng B, Li C, Dong Y, Wang N, Zhang Z, Wang S. Pioneering Enhanced Corrosion Resistance along the Normal Plane of an Ultra-Light Mg-Li Extruded Sheet. Materials. 2023; 16(19):6435. https://doi.org/10.3390/ma16196435
Chicago/Turabian StyleLiang, Jiexi, Binbin Deng, Chuanqiang Li, Yong Dong, Naiguang Wang, Zhengrong Zhang, and Shidong Wang. 2023. "Pioneering Enhanced Corrosion Resistance along the Normal Plane of an Ultra-Light Mg-Li Extruded Sheet" Materials 16, no. 19: 6435. https://doi.org/10.3390/ma16196435





