Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review
Abstract
:1. Introduction
2. Zn-Pb Extraction Technologies
- –
- Sintering unit;
- –
- Shaft furnace unit;
- –
- Lead refining unit;
- –
- Zinc rectification unit.
- –
- upper zone—reduction of lead oxide:
- –
- middle or equilibrium zone—Boudouard reaction:
- –
- lower zone—reduction of zinc oxide:

3. Slags Formed in Zn-Pb Metallurgy
Chemical and Mineral Composition
4. Processes for the Recovery of Metals from Zn-Pb Metallurgical Slags
4.1. Pyrometallurgical Methods
4.1.1. Fuming Process
4.1.2. Isasmelt Process
4.1.3. Kaldo Process
4.1.4. Electric Furnace Process
4.1.5. Thermal Electrolysis
4.1.6. Black Sea Copper Works Process
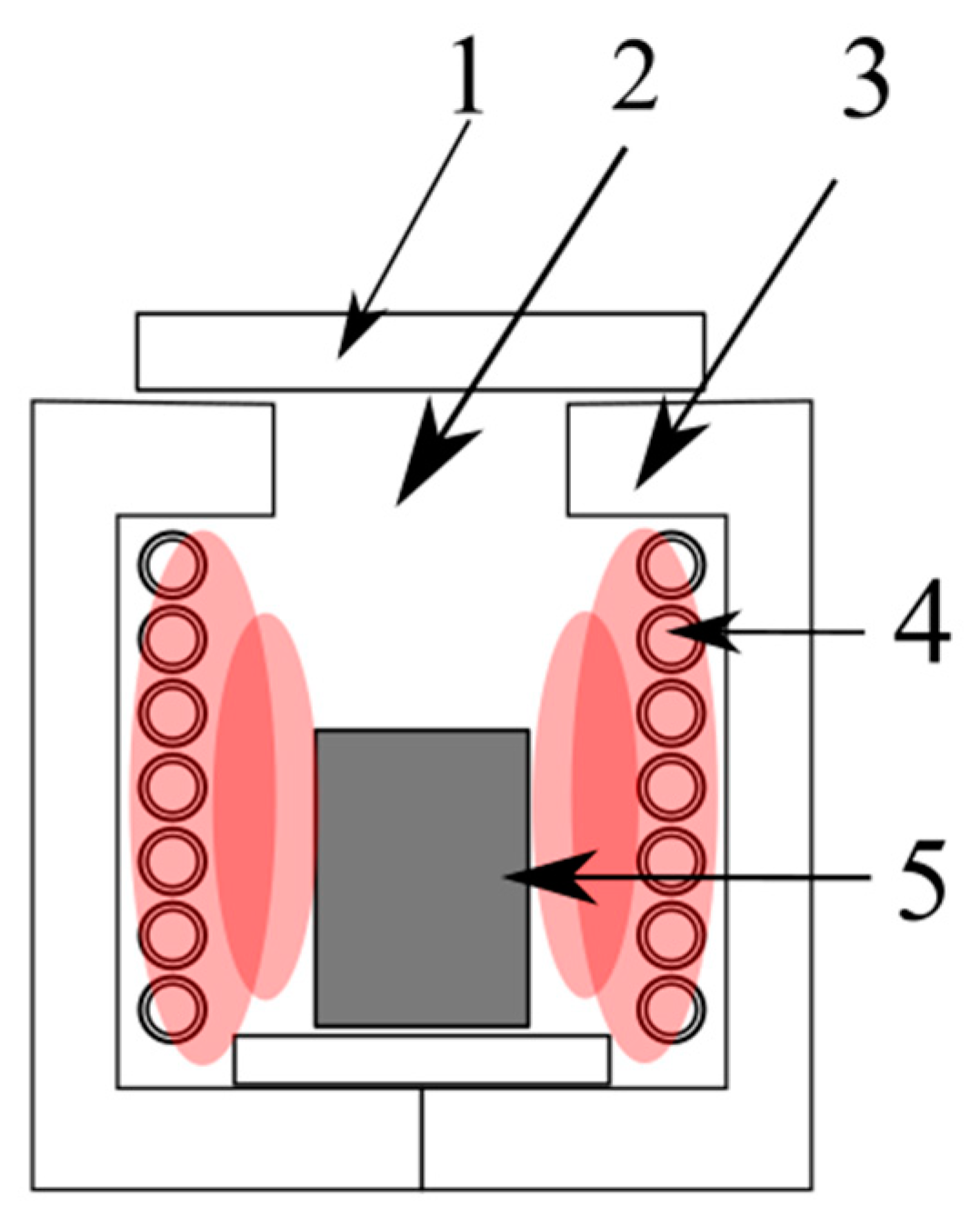
4.2. Hydrometallurgical Methods
4.2.1. Chemical Leaching
- –
- Those passing into solution during the leaching process, including Cu, Cd, Fe, Mg, and Ni;
- –
- Those forming insoluble salts, including PbSO4, CaSO4∙2H2O, BaSO4.
4.2.2. Bioleaching
5. Discussion and Conclusions
- –
- Metallurgical slags from zinc and lead production contain significant amounts of metals and semi-metals, dominantly Si, Al, Ca, Ma, Zn, and Pb. Among the slags of Zn-Pb metallurgy, refining slags are distinctive due to their much higher contents of Zn, Pb, and Cu and significantly lower concentrations of Si, Al, Ca, and Mg.
- –
- The mineral composition of Zn-Pb metallurgical slags is dominated by multiphase crystalline conglomerates formed by high-temperature processes. These take the form of intergrowths of fine, intercalated clusters of individual phases, among which one is always dominant. Typical mineral constituents in these slags include Zn and Fe oxides, Fe hydroxides, Zn, Pb, Fe and Cu sulphides, Pb sulphates, and hydrated Zn, Ca, Cu sulphates, Zn silicates, olivine group silicates, melilites (Ca,Na)2(Al,Mg)[(Si,Al)2O7], Pb and Zn carbonates, spinels, and multicomponent metal alloys of Pb, Zn, Cu, Fe, As, and Sb. In addition, tochilinite [Fe0.9]6S6[Mg0.71Fe0.29(OH)2]5 is present in refining slags [85,86], whereas this component has not been identified in shaft slags from Zn-Pb metallurgy.
- –
- The choice of slag processing technology is determined primarily by the mineral composition of the slag, which in turn is determined by various factors, including the diversity of the feedstock, the variability in the technological parameters, and the rate of slag cooling (which determines the ratio of the amorphous to the crystalline phase). Slag processing methods commonly applied on an industrial scale include pyrometallurgical techniques, such as fuming, slag remelting in Isasmelt or Kaldo furnaces, and thermal electrolysis, and hydrometallurgical leaching, which can be performed using acid solutions (such as sulphuric or hydrochloric acid), ammonia solutions, or alkaline hydroxide solutions. The choice of the leaching medium depends on the chemical form in which the target metal is present in the waste. Various other methods have also been developed to recover metals from slag; however, most of these have only been tested on a laboratory scale. For example, the bioleaching method, despite its high efficiency, has not been applied on an industrial scale due to its long process duration, low yields, and problems with separating metals from the solution.
- –
- The pyrometallurgical and hydrometallurgical methods described in detail in this article are used on an industrial scale due to their high efficiency (amounting to >80% average metal recovery) and effective waste neutralisation by obtaining high-purity metallic products while minimising the formation of toxic secondary waste. Other Zn-Pb slag processing methods described in the literature, i.e., the IBDR-ZIPP pyrometallurgical process or hydrometallurgical processes (e.g., Española del Zinc, Placid), represent technological concepts verified on a pilot scale.
- –
- The presented technologies in this article represent a rational approach to using secondary materials from Zn-Pb metallurgy and are consistent with current circular economy trends. These methods also consider complementary activities aimed at the following:
- Improving the efficiency of using Zn-Pb slags as a secondary raw material;
- Comprehensive processing of Zn-Pb metallurgical waste;
- Reducing the amount of waste generated at individual stages of the technological process.
- –
- The processing of metallurgical slag is an extremely important topic as, in addition to the economic benefits of the process, it significantly neutralises the potential of slag to act as a source of environmental pollution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brodny, J.; Dziemba, Ł. Analiza możliwości przerobu produktów ubocznych wyrobów hutniczych. Syst. Wspomagania Inżynierii Prod. 2015, 2, 192–200. [Google Scholar]
- Matinde, E.; Steenkamp, J.D. Metallurgical Overview and Production of Slags. In Metallurgical Slags: Environmental Geochemistry and Resource Potential, 1st ed.; Piatak, N., Ettler, V., Eds.; Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Piatak, N.M.; Ettler, V.; Hoppe, D. Geochemistry and Mineralogy of Slags. In Metallurgical Slags: Environmental Geochemistry and Resource Potential, 1st ed.; Piatak, N., Ettler, V., Eds.; Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Sitko, J. Technologie utylizacji żużli metalurgicznych—Studium literaturowe. Syst. Wspomagania Inżynierii Prod. 2015, 2, 200–210. [Google Scholar]
- Wowkonowicz, P.; Bojanowicz-Bablok, A.; Gworek, B. Wykorzystanie odpadów z przemysłu wydobywczego i hutnictwa w drogownictwie. Rocz. Ochr. Sr. 2018, 20, 1335–1349. [Google Scholar]
- Pozzi, M.; Nowińska, K. Dystrybucja Wybranych Pierwiastków Towarzyszących Koncentratom Zn—Pb w Technologii Imperial Smelting Process; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2006. [Google Scholar]
- Sohn, H.Y.; Olivas-Martinez, M. Zinc and Lead Production. In Treatise on Process Metallurgy, 1st ed.; Seshadri Seetharaman; Elsevier: Amsterdam, The Netherlands, 2014; p. 671. [Google Scholar]
- Hoang, J.; Reuter, M.; Matusewicz, R.; Hughes, S.; Piret, N. Top submerged lance direct zinc smelting. Miner. Eng. 2009, 22, 742–751. [Google Scholar] [CrossRef]
- Sahu, K.; Agrawal, A. Lead, Zinc Extraction Processes. In Proceedings of the Extraction of Nonferrous Metals and Their Recycling—A Training Programme, Jamshedpur, India, 27–29 February 2008. [Google Scholar]
- ZSG. Lead and Zinc Statistics. Available online: https://www.ilzsg.org/static/statistics.aspx?from=1 (accessed on 15 November 2023).
- Habashi, F. Retorts in the Production of Metals. A Historical Survey. Metall 2012, 66, 149–155. [Google Scholar]
- Santos, S.M.C.; Ismael, M.R.C.; Correia, M.J.N.; Reis, M.T.A.; Deep, A.; Carvalho, J.M.R. Hydrometallurgical Treatment of a Zinc Concentrate by Atmospheric Direct Leach Process. In Proceedings of the European Congress of Chemical Engineering (ECCE-6), Copenhagen, Denmark, 16–20 September 2007. [Google Scholar]
- Filippou, D. Innovative Hydrometallurgical Processes for the Primary Processing of Zinc. Miner. Process. Extr. Rev. 2004, 25, 205–252. [Google Scholar] [CrossRef]
- Babu, M.N.; Sahu, K.K.; Pandey, B.D. Zinc Recovery from Sphalerite Concentrate by Oxidative Leaching with Ammonium, Sodium and Potassium Persulphates. Hydrometallurgy 2002, 64, 119–129. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The Leaching of Sulphide Minerals in Chloride Media. Hydrometallurgy 1991, 29, 1–45. [Google Scholar] [CrossRef]
- Chodkowski, S. Metalurgia Metali Nieżelaznych, 1st ed.; Wydawnictwo Śląsk: Katowice, Poland, 1971. [Google Scholar]
- Vignes, A. Extractive Metallurgy 3, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zhao, B. Lead and zinc sintering. In Sintering Applications; InTech: Rijeka, Croatia, 2013; pp. 165–199. [Google Scholar]
- Nowińska, K. Formy Występowania Metali w Żużlach z Hutnictwa Cynku i Ołowiu w Aspekcie Środowiskowym i Możliwości Ich Odzysku; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2022. [Google Scholar]
- Adamczyk, Z.; Melaniuk-Wolny, E.; Nowińska, K. The Mineralogical and Chemical Study of Feedstock Mixtures and By-Products from Pyrometallurgical Process of Zinc and Lead Production; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2010. [Google Scholar]
- De Andrade Lima, L.R.P.; Bernardez, L.A. Characterization of the lead smelter slag in Santo Amaro, Bahia, Brazil. J. Hazard. Mater. 2011, 189, 692–699. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Kříbek, B.; Šebek, O.; Mihaljevic, M. Mineralogy and environmental stability of slags from the Tsumeb smelter, Namibia. Appl. Geochem. 2009, 24, 1–15. [Google Scholar] [CrossRef]
- Kicińska, A. Physical and chemical characteristics of slag produced during Pb refining and the environmental risk associated with the storage of slag. Environ. Geochem. Health 2021, 43, 2723–2741. [Google Scholar] [CrossRef]
- Nowińska, K.; Adamczyk, Z. Slags of the imperial smelting process for Zn and Pb production. In Reference Module in Materials Science and Materials Engineering, 1st ed.; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–5. [Google Scholar]
- Harvey, W.; Downs-Rose, G. The Bay Mine, Wanlockhead, Scotland; British Mining, 2; Northern Mine Research Society: New York, NY, USA, 1976; pp. 1–9. [Google Scholar]
- Ettler, V.; Legendre, O.; Bodénan, F.; Touray, J. Primary phases and natural weathering of old lead–zinc pyrometallurgical slag from Příbram, Czech Republic. Can. Mineral. 2001, 39, 873–888. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Touray, J.C.; Jelínek, E. Zinc partitioning between glass and silicate phases in historical and modern lead–zinc metallurgical slags from the Příbram district, Czech Republic. Earth Planet. Sci. 2000, 331, 245–250. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Nowińska, K. Phase composition of metallurgical zinc and lead slags. Civ. Environ. Eng. Rep. 2013, 1, 13–21. [Google Scholar]
- Bril, H.; Zainou, K.; Puziewicz, J.; Court In-Nomade, A.; Vanaecker, M.; Bollinger, J.-C. Secondary phases from the alteration of a pile of zinc-smelting slag as indicators of environmental conditions: An example from Świętochłowice, Upper Silesia, Poland. Can. Mineral. 2008, 46, 1235–1248. [Google Scholar] [CrossRef]
- Kierczak, J.; Pietranik, A. Mineralogy and composition of historical Cu slags from the Rudawy Janowickie mountains, southwestern Poland. Can. Mineral. 2011, 49, 1281–1296. [Google Scholar] [CrossRef]
- Kierczak, J.; Bril, H.; Neel, C.; Puziewicz, J. Pyrometallurgical Slags in Upper and Lower Silesia (Poland): From Environmental Risks to Use of Slag-based Products—A Review. Arch. Environ. Prot. 2010, 36, 111–126. [Google Scholar]
- Nowińska, K.; Adamczyk, Z.; Melaniuk-Wolny, E. Pyrometallurgical slags as a potential source of selected metals recovery. Metalurgija 2014, 53, 577–580. [Google Scholar]
- Nowińska, K. Mineralogical and chemical characteristics of slags from the pyrometallurgical extraction of zinc and lead. Minerals 2020, 10, 371. [Google Scholar] [CrossRef]
- Mendecki, M.; Warchulski, R.; Szczuka, M.; Środek, D.; Pierwoła, J. Geophysical and petrological studies of the former lead smelting waste dump in Sławków, Poland. J. Appl. Geophys. 2020, 179, 104080. [Google Scholar] [CrossRef]
- Piatak, N.M.; Seal, R.R. Mineralogy and the release of trace elements from slag from the Hegeler Zinc smelter, Illinois (USA). Appl. Geochem. 2010, 25, 302–320. [Google Scholar] [CrossRef]
- Pan, D.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. Resour. Conserv. Recycl. 2019, 146, 140–155. [Google Scholar] [CrossRef]
- Parsons, M.B.; Bird, D.K.; Einaudi, M.T.; Alpers, C.N. Geochemical and mineralogical controls on trace element release from the Penn Mine base-metal slag dump, California. Appl. Geochem. 2001, 16, 1567–1573. [Google Scholar] [CrossRef]
- Warchulski, R.; Doniecki, T.; Gawęda, A.; Szopa, K. Composition and weathering of Zn-Pb slags from Bytom—Piekary Śląskie area: A case of heavy metal concentration and mobility. Mineral.-Spec. Pap. 2012, 40, 132–133. [Google Scholar]
- Warchulski, R.; Doniecki, T.; Gawęda, A.; Szopa, K. Secondary phases from the Zn-Pb smelting slags from Katowice—Piekary Śląskie area, Upper Silesia, Poland: A SEM—XRD overview. Mineral.-Spec. Pap. 2014, 42, 110. [Google Scholar]
- Warchulski, R.; Gawęda, A.; Janeczek, J.; Kędziołka-Gaweł, M. Mineralogy and origin of coarse-grained segregations in the pyrometallurgical Zn-Pb slags from Katowice-Wełnowiec (Poland). Mineral. Petrol. 2016, 110, 681–692. [Google Scholar] [CrossRef]
- Warchulski, R.; Gawęda, A.; Kądziołka-Gaweł, M.; Szopa, K. Composition and element mobilization in pyrometallurgical slags from the Orzeł Biały smelting plant in the Bytom-Piekary Śląskie area, Poland. Mineral. Mag. 2015, 79, 459–483. [Google Scholar] [CrossRef]
- Yin, N.H. Weathering of Metallurgical Slags: A Comprehensive Study on the Importance of Chemical and Biological Contributions. Ph.D. Dissertation, Université Paris-Est, Paris, France, 2014. [Google Scholar]
- Sueoka, Y.; Sakakibara, M. Primary Phases and Natural Weathering of Smelting Slag at an Abandoned Mine Site in Southwest Japan. Minerals 2013, 3, 412–426. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R., II. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Bernasowski, M.; Klimczyk, A.; Stachura, R. Overview of Zinc Production in Imperial Smelting Process. In Proceedings of the Conference Iron and Steelmaking, Horní Bečva, Czech Republic, 4–6 October 2017. [Google Scholar]
- Potysz, A.; Van Hullebusch, E.D. Secondary metal recovery from Slags. In Metallurgical Slags, 1st ed.; Piatak, N., Ettler, V., Eds.; Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Jarosiński, A. Innowacyjne i Proekologiczne Metody Przeróbki Materiałów Cynkonośnych; Wydawnictwo IGSMiE PAN: Kraków, Poland, 2012. [Google Scholar]
- Ulewicz, M. Procesy Odzysku i Recyklingu Metali Nieżelaznych i Stali; Wydawnictwo Politechniki Częstochowskiej: Częstochowa, Poland, 2015. [Google Scholar]
- Reddy, R.G.; Prabhu, V.L.; Mantha, D. Zinc fuming from lead blast furnace slag. High Temp. Mater. Process. 2002, 21, 377–386. [Google Scholar] [CrossRef]
- Jak, E.; Hayes, P. Phase equilibria and thermodynamics of zinc fuming slags. Can. Metall. Q. 2002, 41, 163–174. [Google Scholar] [CrossRef]
- Verscheure, K.; Van Camp, M.; Blanpain, B.; Wollants, P.; Hayes, P.; Jak, E. Continuous fuming of zinc bearing residues: Part I. Model Development. Metall. Mater. Trans. B 2007, 38B, 13–20. [Google Scholar] [CrossRef]
- Verscheure, K.; Van Camp, M.; Blanpain, B.; Wollants, P.; Hayes, P.; Jak, E. Continuous fuming of zinc bearing residues: Part II. The submerged plasma zinc fuming process. Metall. Mater. Trans. 2007, 38, 21–33. [Google Scholar] [CrossRef]
- Cybulski, A.; Prajsnar, R.; Kulawik, S.; Michalski, R.; Basiura, B. Rozwiązania techniczno-technologiczne w pirometalurgii cynku i ołowiu na Świecie. Rudy Met. 2016, 63, 19–27. [Google Scholar] [CrossRef]
- Bakker, M.L.; Alvear, G.R.F.; Kreuh, M. IsasmeltTM—Making a splash for nickiel. Miner. Eng. 2011, 24, 610–619. [Google Scholar] [CrossRef]
- Arthur, P.; Edwards, J.; Technology, X. IsasmeltTM—A Quiet Revolution. In Proceedings of the European Metallurgical Conference, Hannover, Germany, 16–19 September 2023. [Google Scholar]
- Errington, W.J.; Fewings, J.H.; Keran, V.P.; Denholm, W.T. The Isasmelt lead smelting process. Trans. Inst. Min. Metall. Sect. C 1987, 96, 1–6. [Google Scholar]
- Hogg, B.; Prince, M.; Letchford, M.; Burrows, A.; Tokzhigitov, T.; Azekenov, T. Successful Development and Optimisation of Lead ISASMELT™ Furnace Slag Tapping System at Kazzinc Ltd. In Furnace Tapping, 1st ed.; Steenkamp, J.D., Gregurek, D., Reynolds, Q.G., Alvear Flores, G., Joubert, H., Mackey, P.J., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2022; pp. 245–249. [Google Scholar]
- Shanmuganathan, P.; Lakshmipathiraj, P.; Srikanth, S.; Nachiappan, A.L.; Sumathy, A. Toxicity characterization and long-term stability studies on copper slag from the ISASMELT process. Resour. Conserv. Recycl. 2008, 52, 601–611. [Google Scholar] [CrossRef]
- Zhao, B.; Errington, B.; Yang, G.; Wang, J.; Dong, Y.; Jak, E.; Hayes, P.C. Characterisation of ISASMELT™ slag and lead blast furnace sinters. In Proceedings of the International Symposium on Lead and Zinc Processing, Kyoto, Japan, 17–19 October 2005. [Google Scholar]
- Derin, B.; Şahin, F.C.; Yűcel, O. The electrical characteristics of copper slags in a 270 kVA DC arc furnace. In Proceedings of the 3rd BMC Conference, Ohrid, North Macedonia, 2–3 July 2003. [Google Scholar]
- Lia, Y.; Weid, C.; Liud, C.; Jiangd, J.; Wangd, F. Sulfidation roasting of low grade lead–zinc oxide ore with elemental sulfur. Miner. Eng. 2010, 23, 563–566. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Abdel Basin, S.M.; Rabah, M.A. Hydrometallurgical recovery of metal values from brass melting slag. Hydrometallurgy 1999, 53, 31–44. [Google Scholar] [CrossRef]
- RaO, S.; Liu, Z.; Wang, D.; Cao, H.; Zhu, W.; Zhang, K.; Tao, J. Hydrometallurgical process for recovery of Zn, Pb, Ga and Ge from Zn refinery residues. Trans. Nonferrous Met. Soc. China 2021, 31, 555–564. [Google Scholar] [CrossRef]
- Kul, M.; Topkaya, Y. Recovery of germanium and other valuable metals from zinc plant residue. Hydrometallurgy 2008, 92, 87–94. [Google Scholar] [CrossRef]
- Kurama, H.; Göktepe, F. Recovery of zinc from waste material using hydro metallurgical processes. Environ. Prog. 2004, 22, 161–166. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, L.; Li, H.; Koppala, S.; Yin, S.; Li, S.; Yang, K.; Zhu, F. Efficient recycling of Pb from zinc leaching residues by using the hydrometallurgical method. Mater. Res. Express 2019, 6, 075505. [Google Scholar] [CrossRef]
- Limpa, J.L.; Figueiredo, J.M.; Amer, S.; Luis, A. The CENIM-LNETI process: A new process for the hydrometallurgical treatment of complex sulphides in ammonium chloride solutions. Hydrometallurgy 1997, 28, 149–161. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Ram, R. The aqueous chemistry of the copper-ammonia system and its implications for the sustainable recovery of copper. Clean. Eng. Technol. 2022, 9, 100515. [Google Scholar] [CrossRef]
- Forte, F.; Horckmans, L.; Broos, K.; Kim, E.; Kukurugya, F.; Binnemans, K. Closed-loop solvometallurgical process for recovery of lead from iron-rich secondary lead smelter residues. RSC Adv. 2017, 7, 49999–50005. [Google Scholar] [CrossRef]
- Kim, E.; Horckmans, L.; Spooren, J.; Vrancken, K.C.; Quaghebeur, M.; Broos, K. Hydrometallurgy, Selective leaching of Pb, Cu, Ni and Zn from secondary lead smelting residues. Hydrometallurgy 2017, 169, 372–381. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, Q.; Zhao, Z.; Liu, Z.; Zeng, L. Removal of arsenic from Waelz zinc oxide using a mixed NaOH–Na2S leach. Hydrometallurgy 2011, 108, 165–170. [Google Scholar] [CrossRef]
- Piatak, N.M. Environmental Characteristics and Utilization Potential of Metallurgical Slag. In Environmental Geochemistry, 2nd ed.; De Vivo, B., Belkin, H.E., Lima, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Radzymińska-Lenarcik, E.; Sulewski, M.; Urbaniak, W. Wydobywanie metali z odpadów hydrometalurgicznej przeróbki rud cynkowych. Przemysł Chem. 2017, 96, 1000–1004. [Google Scholar] [CrossRef]
- Mikoda, B.; Kucha, H.; Potysz, A.; Kmiecik, E. Metallurgical slags from Cu production and Pb recovery in Poland—Their environmental stability and resource potential. Appl. Geochem. 2018, 98, 459–472. [Google Scholar] [CrossRef]
- Mikoda, B.; Kucha, H.; Potysz, A.; Kmiecik, E. Bacterial leaching of critical metal values from Polish copper metallurgical slags using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef]
- Pandey, S.C.; Pande, V.; Sati, D.; Samant, M. (Eds.) Bioremediation of heavy metals by soil-dwelling microbes: An environment survival approach. In Advanced Microbial Techniques in Agriculture, Environment, and Health Management, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Tezyapar Kara, I.; Kremser, K.; Wagland, S.T.; Coulon, F. Bioleaching metal-bearing wastes and by-products for resource recovery: A review. Environ. Chem. Lett. 2023, 21, 3329–3350. [Google Scholar] [CrossRef]
- Klimiuk, E.; Łebkowska, M. Biotechnologia w Ochronie Środowiska; PWN: Warszawa, Poland, 2008. [Google Scholar]
- Błaszczyk, M.K. Mikroorganizmy w Ochronie Środowiska; PWN: Warszawa, Poland, 2009. [Google Scholar]
- Tuovinen, O.H.; Särkijärvi, S.; Peuraniemi, E.; Junnikkala, S.; Puhakka, J.A.; Kaksonen, A.H. Acid Leaching of Cu and Zn from a Smelter Slag with a Bacterial Consortium. Adv. Mater. Res. 2015, 1130, 660–663. [Google Scholar] [CrossRef]
- Blažek, V.; Závada, J.; Bouchal, T.; Lébr, J.; Fečko, P. Ługowanie miedzi i cyny z odpadów elektronicznych za pomocą bakterii Acidithiobacillus ferrooxidans. Inżynieria Miner. 2012, 13, 1–7. [Google Scholar]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J. Perspectives regarding the use of metallurgical slags as secondary metal resources—A review of bioleaching approaches. J. Environ. Manag. 2018, 219, 138–152. [Google Scholar] [CrossRef]
- Potysz, A.; Pędziwiatr, A.; Hedwig, S.; Lenz, M. Bioleaching and toxicity of metallurgical wastes. J. Environ. Chem. Eng. 2020, 8, 104450. [Google Scholar] [CrossRef]
- Lee, M.R.; Lingren, P. Aqueous alteration of chondrules from the Murchison CM carbonaceous chondrite: Replacement, pore filling, and the genesis of polyhedral serpentine. Meteorit. Planet. Sci. 2016, 51, 1003–1021. [Google Scholar] [CrossRef]
- Pekova, I.V.; Sereda, E.V.; Polekhovsky, Y.S.; Britvin, S.N.; Chukanov, N.V.; Yapaskurt, V.O.; Bryzgalov, I.A. Ferrotochilinite, 6FeS·5Fe(OH)2, a New Mineral from the Oktyabr’sky Deposit, Noril’sk District, Siberia, Russia. Geol. Ore Depos. 2013, 55, 567–574. [Google Scholar] [CrossRef]







| Component | Zn-Pb Slags | Pb Refining Slags | ||||
|---|---|---|---|---|---|---|
| Min | Max | Average * | Min | Max | Average ** | |
| Al2O3 | 0.90 | 21.9 | 8.3 | 0.57 | 7.8 | 2.8 |
| CaO | 0.18 | 32.2 | 17.3 | 0.14 | 5.65 | 2.8 |
| FeOTotal | 0.88 | 59.6 | 16.7 | 8.3 | 31.1 | 20.0 |
| K2O | 0.02 | 3.91 | 0.60 | 0.04 | 0.27 | 0.15 |
| CuO | - | - | - | 0.98 | 20.93 | 11.5 |
| MgO | 0.61 | 15.9 | 5.4 | 0.10 | 0.81 | 1.1 |
| PbO | 0.0002 | 6.4 | 0.93 | 0.72 | 44.6 | 17.8 |
| ZnO | 0.03 | 47.2 | 4.93 | 6.6 | 18.07 | 12.2 |
| MnO | 0.01 | 3.0 | 0.5 | 0.02 | 0.55 | 0.21 |
| SO3 | 0.05 | 14.9 | 2.99 | 5.83 | 23.1 | 13.6 |
| SiO2 | 2.04 | 57,1 | 28.6 | 2.45 | 35.5 | 11.1 |
| TiO2 | 0.07 | 1.14 | 0.4 | 0.04 | 0.34 | 0.16 |
| Element | Min | Max | Average * | Min | Max | Average ** |
| As | 1 | 10,710 | 1181 | 147 | 15,558 | 8843 |
| Ba | 76 | 17,914 | 1126 | 336 | 778 | 508 |
| Cd | 0.38 | 575 | 31.2 | 85 | 19,757 | 5595 |
| Co | 8.5 | 242 | 35.7 | 0.01 | 452 | 232 |
| Cr | 4 | 700 | 155 | 132 | 708 | 435 |
| Ni | 13 | 240 | 59.7 | 76 | 447 | 311 |
| Cu | 16 | 7400 | 802 | - | - | - |
| P | 43.57 | 26,400 | 5138 | - | - | - |
| Sb | 0.16 | 245 | 42.6 | 81 | 9869 | 472 |
| Sn | 0.1 | 500 | 23.4 | 0.01 | 617 | 323 |
| V | 6 | 9980 | 2294 | - | - | - |
| Group | Phase | Chemical Formula |
|---|---|---|
| Oxides | Zincite | ZnO |
| Wüstite | FeO | |
| Hematite | Fe2O3 | |
| Hydroxides | Goethite | FeO(OH) |
| Sulphides | Sphalerite | ZnS |
| Galena | PbS | |
| Pyrite | FeS2 | |
| Pyrrhotite | FeS | |
| Digenite | (Cu,Fe)9S5 | |
| Cubanite | CuFe2S3 | |
| Covellite | CuS | |
| Chalcocite | Cu2S | |
| Sulphate | Anglesite | PbSO4 |
| Hydrated sulphates | Goslarite | ZnSO4∙7H2O |
| Gypsum | CaSO4·2H2O | |
| Rapidcreekite | Ca2(SO4)(CO3)·4H2O | |
| Ktenasite | ZnCu4(SO4)2(OH)6·6H2O | |
| Posnjakite | Cu4[(OH)6|SO4]·H2O | |
| Silicates | Willemite | Zn2SiO4 |
| Fayalite | Fe2SiO4 | |
| Kirschsteinite | CaFe2+SiO4 | |
| Forsterite | Mg2SiO4 | |
| Aluminosilicate | Melilites | (Ca,Na)2(Al,Mg)[(Si,Al)2O7] |
| Carbonates | Cerussite | PbCO3 |
| Smithsonite | ZnCO3 | |
| Hydrozincite | Zn5[(OH)3/CO3]2 | |
| Hydrocerussite | Pb3(CO3)2(OH) | |
| Spinels | Magnetite | Fe3O4 |
| Hercynite | FeAl2O4 | |
| Franklinite | ZnFe2O4 | |
| Gahnite | ZnAl2O4 | |
| Ulvöspinel | Fe2TiO4 |
| Parameter | Unit | Value |
|---|---|---|
| The amount of slag processed | Mg/h | 10 |
| The amount of air for spraying and feeding the oil | Nm3/h | 10 |
| The amount of primary air for oil combustion | Nm3/h | 1.53 |
| The amount of secondary air for oxidation of vapours and afterburning of gases | Nm3/h | 1.54 |
| Slag temperature | K | 1520–1570 |
| Type of Material | Content (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Zn | Pb | Cu | S | FeO | SiO2 | CaO | Ag (g/Mg) | |
| Output slag | 6.5 | 0.5 | 0.4 | 2.7 | 37 | 20 | 14 | 30 |
| Dust | 60 | 12 | 0.1 | 0.5 | 0.3 | 0.1 | 0.1 | 100 |
| Waste slag | 3.0 | 0.1 | 0.5 | 2.6 | 40 | 19 | 13 | 25 |
| Element | Zn | Pb | Fe | Cu | S | SiO2 | CaO | MgO | MnO | Al2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 21.6 | 1.3 | 29.5 | 0.1 | 0.5 | 5.6 | 9.3 | 2.7 | 2.2 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowińska, K.; Adamczyk, Z. Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review. Materials 2023, 16, 7295. https://doi.org/10.3390/ma16237295
Nowińska K, Adamczyk Z. Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review. Materials. 2023; 16(23):7295. https://doi.org/10.3390/ma16237295
Chicago/Turabian StyleNowińska, Katarzyna, and Zdzisław Adamczyk. 2023. "Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review" Materials 16, no. 23: 7295. https://doi.org/10.3390/ma16237295





