Shape–Preserved CoFeNi–MOF/NF Exhibiting Superior Performance for Overall Water Splitting across Alkaline and Neutral Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CoFeNi–LDH/NF
2.3. Preparation of CoFeNi–MOF/NF
2.4. Electrochemical Measurement
2.5. Characterizations
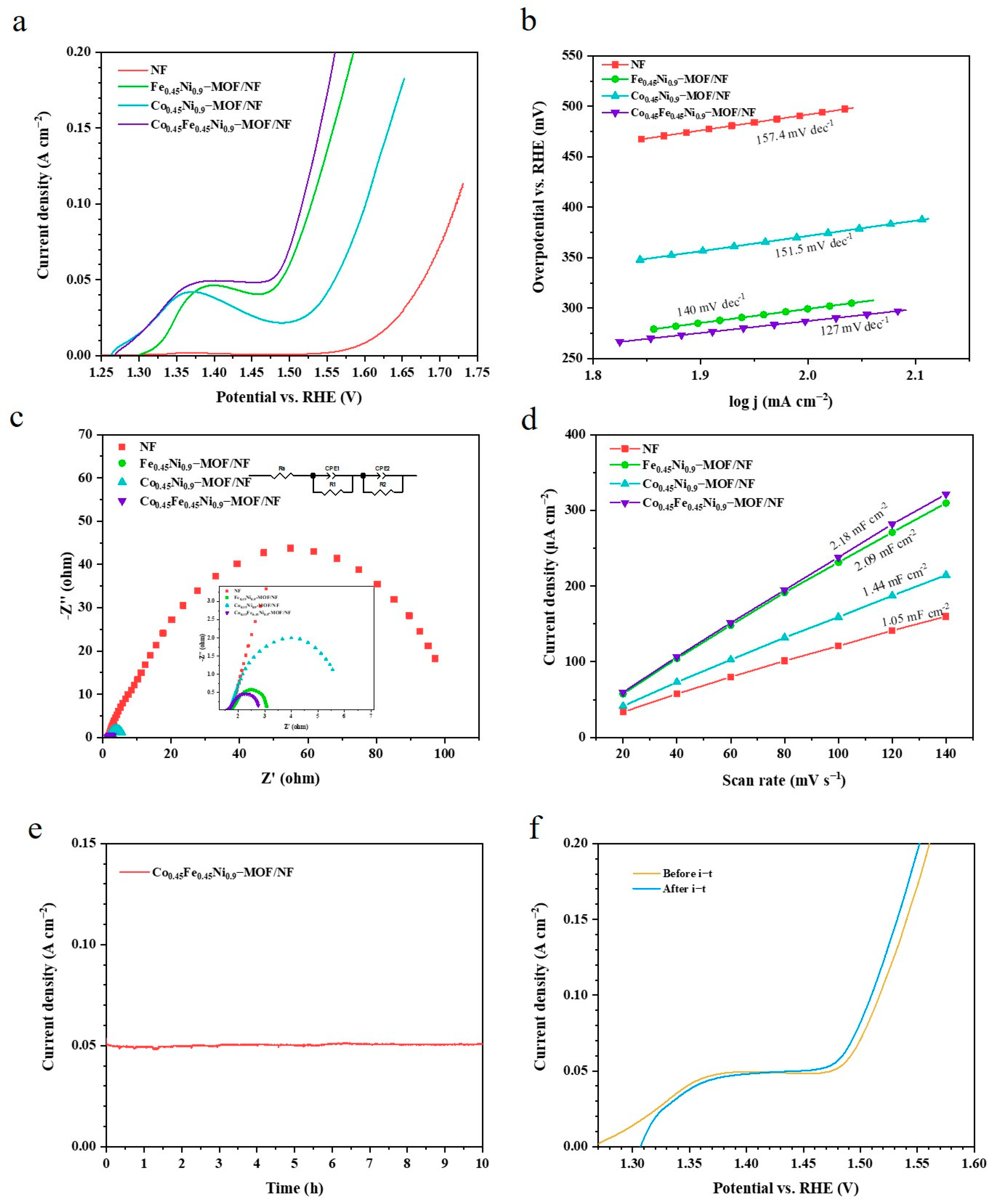
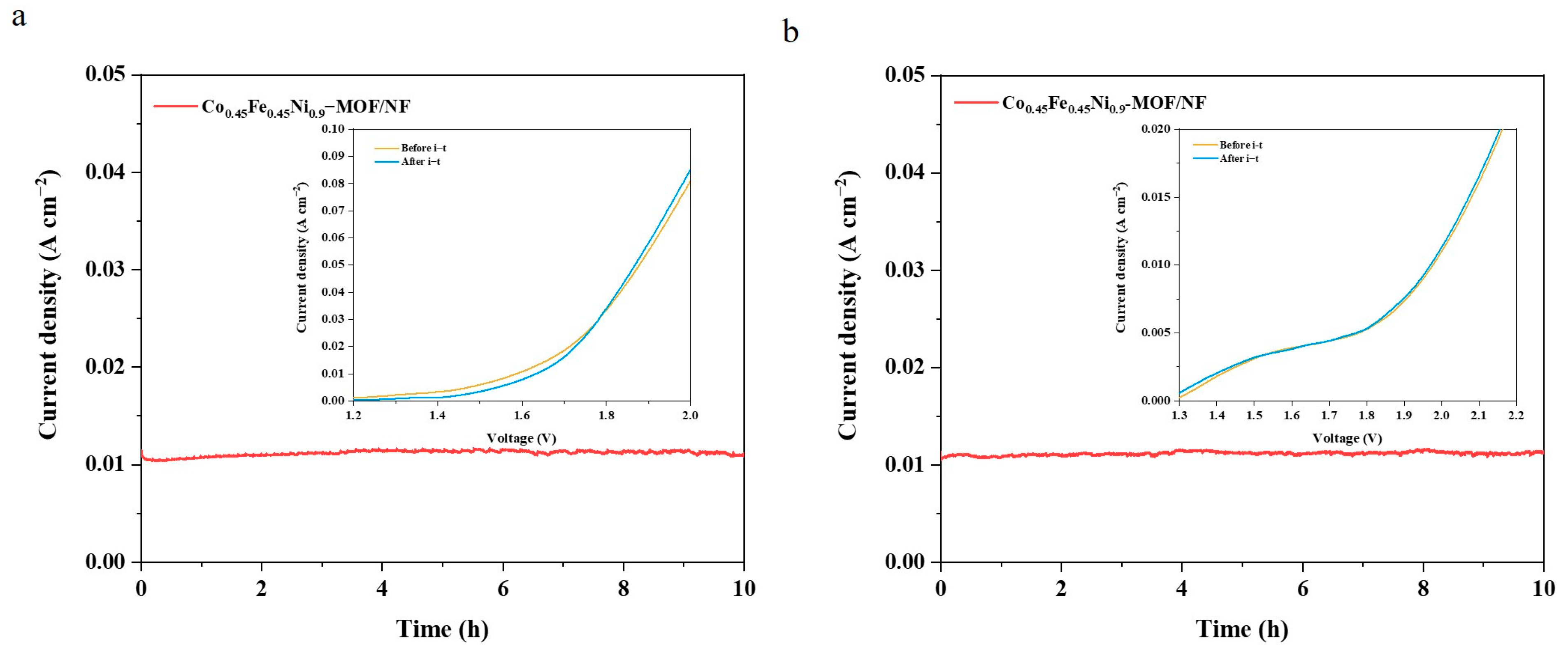
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajagopal, K.; Kathiresan, M.; Rajaram, A.; Natarajan, A.; Natesan, K. Development of robust noble–metal free lanthanum, neodymium doped Li1.05Ni0.5Mn1.5O4 as a bifunctional electrocatalyst for electrochemical water splitting. RSC Adv. 2023, 13, 23829–23840. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.N.S.; Tang, Y.; Dong, L.; Yu, X.Y.; Hong, Z. N2 plasma–activated NiO nanosheet arrays with enhanced water splitting performance. Nanotechnology 2020, 31, 455709. [Google Scholar] [CrossRef]
- Santhosh Kumar, R.; Ramakrishnan, S.; Prabhakaran, S.; Kim, A.R.; Kumar, D.R.; Kim, D.H.; Yoo, D.J. Structural, electronic, and electrocatalytic evaluation of spinel transition metal sulfide supported reduced graphene oxide. J. Mater. Chem. A 2022, 10, 1999–2011. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.D.; Liu, S.; Teng, C.P.; Han, M.Y. Recent Progress in Energy–Driven Water Splitting. Adv. Sci. 2017, 4, 37438–37475. [Google Scholar] [CrossRef]
- Gao, T.; Yu, S.; Chen, Y.; Li, X.; Tang, X.; Wu, S.; He, B.; Lan, H.; Li, S.; Yue, Q.; et al. Regulating the thickness of the carbon coating layer in iron/carbon heterostructures to enhance the catalytic performance for oxygen evolution reaction. J. Colloid. Interface Sci. 2023, 642, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Seuferling, T.E.; Larson, T.R.; Barforoush, J.M.; Leonard, K.C. Carbonate–Derived Multi–Metal Catalysts for Electrochemical Water–Splitting at High Current Densities. ACS Sustain. Chem. Eng. 2021, 9, 16678–16686. [Google Scholar] [CrossRef]
- Niyitanga, T.; Jeong, H.K. Hydrogen and oxygen evolution reactions of molybdenum disulfide synthesized by hydrothermal and plasma method. J. Electroanal. Chem. 2019, 849, 113383. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Gao, T.; Tang, X.; Li, X.; Lan, H.; Yu, S.; Wu, S.; Yue, Q.; Xiao, D. Surface reconstructing hierarchical structures as robust sulfion oxidation catalysts to produce hydrogen with ultralow energy consumption. Inorg. Chem. Front. 2023, 10, 1447–1456. [Google Scholar] [CrossRef]
- Das, D.; Santra, S.; Nanda, K.K. In Situ Fabrication of a Nickel/Molybdenum Carbide–Anchored N–Doped Graphene/CNT Hybrid: An Efficient (Pre)catalyst for OER and HER. ACS Appl. Mater. Interfaces 2018, 10, 35025–35038. [Google Scholar] [CrossRef]
- Zubaid, S.; Khan, J.; Sherazi, T.A. The influence of nanostructure and electrolyte concentration on the performance of nickel sulfide (Ni3S2) catalyst for electrochemical overall water splitting. J. Colloid. Interface Sci. 2024, 660, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Munawar, T.; Bashir, A.; Batoo, K.M.; Mukhtar, F.; Nadeem, M.S.; Hussain, S.; Manzoor, S.; Ashiq, M.N.; Khan, S.A.; Koc, M.; et al. Efficient rGO–supported CeO2–Y2O3–Nd2O3 nanocomposite electrocatalyst for water splitting (HER/OER) in alkaline medium. J. Korean Ceram. Soc. 2024, 15. [Google Scholar] [CrossRef]
- Jamesh, M.-I.; Harb, M. Tuning the electronic structure of the earth–abundant electrocatalysts for oxygen evolution reaction (OER) to achieve efficient alkaline water splitting—A review. J. Energy Chem. 2021, 56, 299–342. [Google Scholar] [CrossRef]
- Sardar, K.; Petrucco, E.; Hiley, C.I.; Sharman, J.D.B.; Wells, P.P.; Russell, A.E.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Water–Splitting Electrocatalysis in Acid Conditions Using Ruthenate–Iridate Pyrochlores. Angew. Chem. Int. Ed. 2014, 53, 10960–10964. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Xiong, H.; Zhang, T.; Fan, X.; Wang, J.; Xu, F. Recent progress in noble–metal–free electrocatalysts for alkaline oxygen evolution reaction. Front. Chem. 2022, 10, 1071274. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, T.; Lee, K.; Li, J. Recent Advances in Transition Metal Phosphide Electrocatalysts for Water Splitting under Neutral pH Conditions. ChemElectroChem 2020, 7, 3578–3589. [Google Scholar] [CrossRef]
- Anantharaj, S.; Aravindan, V. Developments and Perspectives in 3d Transition–Metal–Based Electrocatalysts for Neutral and Near–Neutral Water Electrolysis. Adv. Energy Mater. 2019, 10, 1902666. [Google Scholar] [CrossRef]
- Jothi, M.; Gnanasekar, P.; Kulandaivel, J. NiCo–Metal Organic Frameworks for Highly Stable Electrocatalytic Water Splitting under Alkaline and Neutral pH Ranges. Energy Fuels 2022, 36, 13713–13721. [Google Scholar] [CrossRef]
- Subramaniam, M.R.; Ramakrishnan, S.; Sidra, S.; Karthikeyan, S.C.; Vijayapradeep, S.; Huang, J.; Mamlouk, M.; Kim, D.H.; Yoo, D.J. Carbon core–shell Pt nanoparticle embedded porphyrin Co–MOF derived N–doped porous carbon for the alkaline AEM water electrolyzer application. J. Mater. Chem. A 2024, 12, 5967–5979. [Google Scholar] [CrossRef]
- Dey, G.; Shadab; Aijaz, A. Metal–Organic Framework Derived Nanostructured Bifunctional Electrocatalysts for Water Splitting. ChemElectroChem 2021, 8, 3782–3803. [Google Scholar] [CrossRef]
- Ma, Y.; Miao, Y.; Mu, G.; Lin, D.; Xu, C.; Zeng, W.; Xie, F. Highly Enhanced OER Performance by Er–Doped Fe–MOF Nanoarray at Large Current Densities. Nanomaterials 2021, 11, 1847. [Google Scholar] [CrossRef]
- Park, K.; Kwon, J.; Jo, S.; Choi, S.; Enkhtuvshin, E.; Kim, C.; Lee, D.; Kim, J.; Sun, S.; Han, H.; et al. Simultaneous electrical and defect engineering of nickel iron metal–organic–framework via co–doping of metalloid and non–metal elements for a highly efficient oxygen evolution reaction. Chem. Eng. J. 2022, 439, 135720. [Google Scholar] [CrossRef]
- Gu, J.; He, J.; Zheng, H.; Sun, C. Morphology control in the synthesis of [Mo3S13]2–/Co–MOF–74 composite catalysts and their application in the oxygen evolution reaction. New J. Chem. 2023, 47, 8507–8514. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.X.; Jiang, H.L. Metal–organic framework–based CoP/reduced graphene oxide: High–performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef]
- Wang, K.; Kang, J.; Jin, L.; Yang, L.; Liu, Y.; Li, Y.; Chen, G.; Xu, H. Defect engineering of MOF toward enhanced electrocatalytic water oxidation. Ionics 2023, 29, 5397–5403. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Khan, A.; Hakeem, A.S. Binary CoNi and Ternary FeCoNi Alloy Thin Films as High–Performance and Stable Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 9556–9567. [Google Scholar] [CrossRef]
- Tang, L.; Cai, M.; Zhang, M.; Chen, X.; Cai, Z. LDH–assisted growth of FeCo bimetal–MOF nanorods for electrocatalytic oxygen evolution. RSC Adv. 2022, 12, 25112–25117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wu, Z.P.; Luan, D.; Zang, S.Q.; Lou, X.W.D. Synergetic Cobalt–Copper–Based Bimetal–Organic Framework Nanoboxes toward Efficient Electrochemical Oxygen Evolution. Angew. Chem. Int. Ed. Engl. 2021, 60, 26397–26402. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-B.; Wu, J.-Q.; Zhao, J.-W.; Xie, L.-J.; Li, G.-R. Highly dispersed ultrafine Ni particles embedded into MOF–74 arrays by partial carbonization for highly efficient hydrogen evolution. Mater. Adv. 2020, 1, 1212–1219. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, R.; Xie, H.; Li, R.; Liu, X.; Pan, M.; Lei, Y. Effect of the valence state of initial iron source on oxygen evolution activity of Fe–doped Ni–MOF. Chem. Pap. 2020, 74, 2775–2784. [Google Scholar] [CrossRef]
- Ravipati, M.; Durai, L.; Badhulika, S. Single–Pot Solvothermal Synthesis of Single–Crystalline Nickel–Metal Organic Framework Nanosheets for Direct Iron Fuel Cell Applications. ACS Appl. Energy Mater. 2023, 6, 6901–6909. [Google Scholar] [CrossRef]
- Sun, T.; Lin, S.; Xu, Z.; Li, L. In situ growth of an Fe–doped NiCo–MOF electrocatalyst from layered double hydroxide effectively enhances electrocatalytic oxygen evolution performance. CrystEngComm 2021, 23, 7650–7657. [Google Scholar] [CrossRef]
- Vanaraj, R.; Vinodh, R.; Periyasamy, T.; Madhappan, S.; Babu, C.M.; Asrafali, S.P.; Haldhar, R.; Jayprakash Raorane, C.; Hwang, H.; Kim, H.-J.; et al. Capacitance Enhancement of Metal–Organic Framework (MOF) Materials by Their Morphology and Structural Formation. Energy Fuels 2022, 36, 4978–4991. [Google Scholar] [CrossRef]
- Sarawade, P.; Tan, H.; Polshettiwar, V. Shape– and Morphology–Controlled Sustainable Synthesis of Cu, Co, and In Metal Organic Frameworks with High CO2 Capture Capacity. ACS Sustain. Chem. Eng. 2012, 1, 66–74. [Google Scholar] [CrossRef]
- Deng, T.; Lu, Y.; Zhang, W.; Sui, M.; Shi, X.; Wang, D.; Zheng, W. Inverted Design for High–Performance Supercapacitor Via Co(OH)2–Derived Highly Oriented MOF Electrodes. Adv. Energy Mater. 2017, 8, 1702294. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; She, X.; Pan, L.; Xi, C.; Wang, D.; Yi, J.; Yang, J. Ni–modified FeOOH integrated electrode by self–source corrosion of nickel foam for high–efficiency electrochemical water oxidation. J. Colloid. Interface Sci. 2023, 652, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xie, L.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. A Zn–doped Ni3S2 nanosheet array as a high–performance electrochemical water oxidation catalyst in alkaline solution. Chem. Commun. 2017, 53, 12446–12449. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhan, C.; Gao, J. The direct growth of Mn0.6Ni0.4CO3 nanosheet assemblies on Ni foam for high–performance supercapacitor electrodes. New J. Chem. 2022, 46, 2635–2640. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+1–xAl3+x Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Peng, C.K.; Bu, L.; Chiang, C.L.; Tian, K.; Zhao, Y.; Zhao, J.; Lin, Y.G.; Lee, J.M.; et al. Co–Induced Electronic Optimization of Hierarchical NiFe LDH for Oxygen Evolution. Small 2020, 16, e2002426. [Google Scholar] [CrossRef]
- Guo, H.; Lu, X.; He, J.; Zhang, H.; Zhang, H.; Dong, Y.; Zhou, D.; Xia, Q. Co–MOF nanosheet supported on ZSM–5 with an improved catalytic activity for air epoxidation of olefins. Mater. Chem. Phys. 2023, 294, 127001. [Google Scholar] [CrossRef]
- Dai, M.; Wang, J.; Li, L.; Wang, Q.; Liu, M.; Zhang, Y. High–performance Oxygen Evolution Catalyst Enabled by Interfacial Effect between CeO2 and FeNi Metal–organic Framework. Acta Chim. Sin. 2020, 78, 355–362. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, Q.; Cao, X.; Chi, B.; Zhang, J.; Zhang, C.; Liu, T.; Wang, X.; Su, Y. Electronic structure and electrocatalytic activity of cerium–doped tantalum oxide. J. Electroanal. Chem. 2012, 681, 139–143. [Google Scholar] [CrossRef]
- Wiktor, C.; Turner, S.; Zacher, D.; Fischer, R.A.; Tendeloo, G.V. Imaging of intact MOF–5 nanocrystals by advanced TEM at liquid nitrogen temperature. Microporous Mesoporous Mater. 2012, 162, 131–135. [Google Scholar] [CrossRef]
- Gong, X.; Gnanasekaran, K.; Chen, Z.; Robison, L.; Wasson, M.C.; Bentz, K.C.; Cohen, S.M.; Farha, O.K.; Gianneschi, N.C. Insights into the Structure and Dynamics of Metal–Organic Frameworks via Transmission Electron Microscopy. J. Am. Chem. Soc. 2020, 142, 17224–17235. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, G.; Wen, H.; Guan, X.; Sun, X.; Feng, H.; Tian, W.; Zheng, D.; Cheng, X.; Yao, Y. Constructing a highly oriented layered MOF nanoarray from a layered double hydroxide for efficient and long–lasting alkaline water oxidation electrocatalysis. J. Mater. Chem. A 2019, 7, 8771–8776. [Google Scholar] [CrossRef]
- Li, Z.; Xing, X.; Chu, J.; Wang, K.-L.; Yu, C.; Wei, Z.; Wen, Y.; Sun, H.; Wang, Z.-K. MOF confined in macroporous–mesoporous–TiO2 for light–boosting electrocatalytical oxygen production. Mater. Today Energy 2019, 13, 125–133. [Google Scholar] [CrossRef]
- Ni, S.; Qu, H.; Xu, Z.; Zhu, X.; Chen, L.; Xing, H.; Wu, X.; Liu, H.; Yang, L. Regulating the Spin State of Metal and Metal Carbide Heterojunctions for Efficient Oxygen Evolution. ACS Appl. Mater. Interfaces 2023, 15, 36423–36433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, X.; Zhu, K.; Yuan, W.; Jiang, T.; Xue, H.; Tian, J. Fe–doping induced electronic structure reconstruction in Ni–based metal–organic framework for improved energy–saving hydrogen production via urea degradation. J. Power Sources 2022, 520, 230882. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, L.; Quan, F.; Guo, R. Recent developments of Co3O4–based materials as catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2022, 12, 436–461. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, G.; Wang, L.; Liu, X.; Qu, Y.; Zhou, Y.; Zhou, F.; Li, Z.; Li, Y.; Yao, T.; et al. Single Ru Atoms Stabilized by Hybrid Amorphous/Crystalline FeCoNi Layered Double Hydroxide for Ultraefficient Oxygen Evolution. Adv. Energy Mater. 2020, 11, 2002816. [Google Scholar] [CrossRef]
- Ghadge, S.D.; Velikokhatnyi, O.I.; Datta, M.K.; Damodaran, K.; Shanthi, P.M.; Kumta, P.N. Highly Efficient Fluorine Doped Ni2P Electrocatalysts for Alkaline Mediated Oxygen Evolution Reaction. J. Electrochem. Soc. 2021, 168, 064512. [Google Scholar] [CrossRef]
- Karmakar, A.; Kundu, S. A concise perspective on the effect of interpreting the double layer capacitance data over the intrinsic evaluation parameters in oxygen evolution reaction. Mater. Today Energy 2023, 33, 101259. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kang, P.W.; Klingenhof, M.; Lee, H.; Dionigi, F.; Strasser, P. Active Surface Area and Intrinsic Catalytic Oxygen Evolution Reactivity of NiFe LDH at Reactive Electrode Potentials Using Capacitances. ACS Catal. 2023, 13, 1186–1196. [Google Scholar] [CrossRef]
- Benck, J.D.; Chen, Z.; Kuritzky, L.Y.; Forman, A.J.; Jaramillo, T.F. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into the Origin of their Catalytic Activity. ACS Catal. 2012, 2, 1916–1923. [Google Scholar] [CrossRef]
- Muthukumar, P.; Arunkumar, G.; Pannipara, M.; Al–Sehemi, A.G.; Moon, D.; Anthony, S.P. Enhancing the electrocatalytic OER activity of Co–MOFs through labile solvents coordination. New J. Chem. 2023, 47, 20831–20837. [Google Scholar] [CrossRef]
- Joshi, A.; Gaur, A.; Sood, P.; Singh, M. One–Pot Crystallization of 2D and 3D Cobalt–Based Metal–Organic Frameworks and Their High–Performance Electrocatalytic Oxygen Evolution. Inorg. Chem. 2021, 60, 12685–12690. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Honorato, A.M.B.; Tremiliosi Filho, G.; Varela, H. Trifunctional catalytic activities of trimetallic FeCoNi alloy nanoparticles embedded in a carbon shell for efficient overall water splitting. J. Mater. Chem. A 2020, 8, 9021–9031. [Google Scholar] [CrossRef]
- Pasquini, C.; D’Amario, L.; Zaharieva, I.; Dau, H. Operando Raman spectroscopy tracks oxidation–state changes in an amorphous Co oxide material for electrocatalysis of the oxygen evolution reaction. J. Chem. Phys. 2020, 152, 194202. [Google Scholar] [CrossRef]
- Ao, K.; Dong, J.; Fan, C.; Wang, D.; Cai, Y.; Li, D.; Huang, F.; Wei, Q. Formation of Yolk–Shelled Nickel–Cobalt Selenide Dodecahedral Nanocages from Metal–Organic Frameworks for Efficient Hydrogen and Oxygen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 10952–10959. [Google Scholar] [CrossRef]
- Ray, C.; Lee, S.C.; Jin, B.; Kundu, A.; Park, J.H.; Jun, S.C. Stacked Porous Iron–Doped Nickel Cobalt Phosphide Nanoparticle: An Efficient and Stable Water Splitting Electrocatalyst. ACS Sustain. Chem. Eng. 2018, 6, 6146–6156. [Google Scholar] [CrossRef]
- Sondermann, L.; Jiang, W.; Shviro, M.; Spieß, A.; Woschko, D.; Rademacher, L.; Janiak, C. Nickel-Based Metal-Organic Frameworks as Electrocatalysts for the Oxygen Evolution Reaction (OER). Molecules 2022, 27, 1241. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.B.Z.; Zander, J.; Weiss, M.; Simon, C.; Gerschel, P.; Sanden, S.A.; Smialkowski, M.; Tetzlaff, D.; Kull, T.; Marschall, R.; et al. FeNi2S4 –A Potent Bifunctional Efficient Electrocatalyst for the Overall Electrochemical Water Splitting in Alkaline Electrolyte. Small 2024, 20, 2311627. [Google Scholar] [CrossRef] [PubMed]
- Thangasamy, P.; Shanmuganathan, S.; Subramanian, V. A NiCo–MOF nanosheet array based electrocatalyst for the ox-ygen evolution reaction. Nanoscale Adv. 2020, 2, 2073–2079. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Indra, A.; Feng, Y.; Han, H.; Song, T. Promoting electrocatalytic overall water splitting with nanohybrid of transition metal nitride-oxynitride. Appl. Catal. B Environ. 2018, 241, 521–527. [Google Scholar] [CrossRef]
- Beglau, T.H.Y.; Rademacher, L.; Oestreich, R.; Janiak, C. Synthesis of Ketjenblack Decorated Pillared Ni(Fe) Metal-Organic Frameworks as Precursor Electrocatalysts for Enhancing the Oxygen Evolution Reaction. Molecules 2023, 28, 4464. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, J. Oxygen evolution catalyst containing CoNi nanoalloy and carbon nanotube maximized the electrochemical surface area and intrinsic activity. Solid State Sci. 2023, 135. [Google Scholar] [CrossRef]
- Lee, J.-I.; O., S.G.; Kim, Y.J.; Park, S.J.; Sin, G.S.; Kim, J.H.; Ryu, J.H. Electrocatalytic properties of Te incorporated Ni(OH)2 microcrystals grown on Ni foam. J. Korean Cryst. Growth Cryst. Technol. 2021, 31, 96–101. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, S.H.; Youn, D.H. Direct One–Step Growth of Bimetallic Ni2Mo3N on Ni Foam as an Efficient Oxygen Evolution Electrocatalyst. Materials 2021, 14, 4768. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef]
- Gutiérrez-Tarriño, S.; Olloqui-Sariego, J.L.; Calvente, J.J.; Espallargas, G.M.; Rey, F.; Corma, A.; Oña-Burgos, P. Cobalt Metal–Organic Framework Based on Layered Double Nanosheets for Enhanced Electrocatalytic Water Oxidation in Neutral Media. J. Am. Chem. Soc. 2020, 142, 19198–19208. [Google Scholar] [CrossRef]
- Jeong, D.; Jin, K.; Jerng, S.E.; Seo, H.; Kim, D.; Nahm, S.H.; Kim, S.H.; Nam, K.T. Mn5O8 Nanoparticles as Efficient Water Oxidation Catalysts at Neutral pH. ACS Catal. 2015, 5, 4624–4628. [Google Scholar] [CrossRef]
- Xu, K.; Cheng, H.; Liu, L.; Lv, H.; Wu, X.; Wu, C.; Xie, Y. Promoting Active Species Generation by Electrochemical Acti-vation in Alkaline Media for Efficient Electrocatalytic Oxygen Evolution in Neutral Media. Nano Lett. 2016, 17, 578–583. [Google Scholar] [CrossRef]
- Jin, K.; Chu, A.; Park, J.; Jeong, D.; Jerng, S.E.; Sim, U.; Jeong, H.-Y.; Lee, C.W.; Park, Y.-S.; Yang, K.D.; et al. Partially Oxidized Sub-10 nm MnO Nanocrystals with High Activity for Water Oxidation Catalysis. Sci. Rep. 2015, 5, 10279. [Google Scholar] [CrossRef]
- Zaharieva, I.; Chernev, P.; Risch, M.; Klingan, K.; Kohlhoff, M.; Fischer, A.; Dau, H. Electrosynthesis, functional, and structural characterization of a water–oxidizing manganese oxide. Energy Environ. Sci. 2012, 5, 7081–7089. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Xu, H.; Wu, Z.; Wang, H.; Liang, Y. Iron-Doped Cobalt Monophosphide Nanosheet/Carbon Nanotube Hybrids as Active and Stable Electrocatalysts for Water Splitting. Adv. Funct. Mater. 2017, 27, 1606635. [Google Scholar] [CrossRef]
- Liang, X.; Wang, S.; Feng, J.; Xu, Z.; Guo, Z.; Luo, H.; Zhang, F.; Wen, C.; Feng, L.; Wan, C.; et al. Structural transformation of metal–organic frameworks and identification of electrocatalytically active species during the oxygen evolution reaction under neutral conditions. Inorg. Chem. Front. 2023, 10, 2961–2977. [Google Scholar] [CrossRef]
- Jin, K.; Park, J.; Lee, J.; Yang, K.D.; Pradhan, G.K.; Sim, U.; Jeong, D.; Jang, H.L.; Park, S.; Kim, D.; et al. Hydrated manganese(II) phosphate (Mn3(PO4)2.3H2O) as a water oxidation catalyst. J. Am. Chem. Soc. 2014, 136, 7435–7443. [Google Scholar] [CrossRef]
- Wang, N.; Cao, Z.; Zheng, X.; Zhang, B.; Kozlov, S.M.; Chen, P.; Zou, C.; Kong, X.; Wen, Y.; Liu, M.; et al. Hydration-Effect-Promoting Ni–Fe Oxyhydroxide Catalysts for Neutral Water Oxidation. Adv. Mater. 2020, 32, 1906806. [Google Scholar] [CrossRef]
- Khiarak, B.N.; Hasanzadeh, M.; Simchi, A. Electrocatalytic hydrogen evolution reaction on graphene supported transition metal-organic frameworks. Inorg. Chem. Commun. 2021, 127, 108525. [Google Scholar] [CrossRef]
- Duraisamy, S.; Ganguly, A.; Sharma, P.K.; Benson, J.; Davis, J.; Papakonstantinou, P. One–Step Hydrothermal Synthesis of Phase–Engineered MoS2/MoO3 Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2021, 4, 2642–2656. [Google Scholar] [CrossRef]
- Sannasi, V.; Thangasamy, P.; Raj, J.A.; Sathish, M. Transformation of sluggish higher valent molybdenum into electro-catalytically active amorphous carbon doped MoO2/MoO3–x nanostructures using phyllanthus reticulatus fruit extract as natural reducing agent in supercritical fluid processing. Int. J. Hydrog. Energy 2019, 44, 21692–21702. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Multifunctional Carbon–Based Metal–Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. Adv Mater 2017, 29, 1604942. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.S.; Bolar, S.; Murmu, N.C.; Ganesh, R.S.; Inokawa, H.; Banerjee, A.; Kuila, T. Synthesis of Tri-functional Core-shell CuO@carbon Quantum Dots@carbon Hollow Nanospheres Heterostructure for Non-enzymatic H2O2 Sensing and Overall Water Splitting Applications. Electroanalysis 2019, 31, 2120–2129. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, J.; Xie, Y.; Shao, Y.; Ling, Y.; Chen, Y.; Zhang, Y. CoS2 particles loaded on MOF-derived hollow carbon spheres with enhanced overall water splitting. Electrochimica Acta 2023, 458, 142511. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, W.; Ran, S.; Boles, S.T.; Lee, L.Y.S. Overall Water-Splitting Electrocatalysts Based on 2D CoNi-Metal-Organic Frameworks and Its Derivative. Adv. Mater. Interfaces 2018, 5, 1800849. [Google Scholar] [CrossRef]
- Arunkumar, P.; Gayathri, S.; Han, J.H. Impact of an Incompatible Atomic Nickel-Incorporated Metal–Organic Framework on Phase Evolution and Electrocatalytic Activity of Ni-Doped Cobalt Phosphide for the Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 2975–2992. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, J.; Zhao, Y.; Zheng, Y.; Qiao, S.Z. Engineering 2D Metal–Organic Framework/MoS2 Interface for Enhanced Alkaline Hydrogen Evolution. Small 2019, 15, 1805511. [Google Scholar] [CrossRef]
- Nady, H.; El-Rabiei, M.; Deyab, M.; Samy, M.; El-Hafez, G.M.A. Ni–Cr alloys for effectively enhancing hydrogen evolution processes in phosphate-buffered neutral electrolytes. Int. J. Hydrog. Energy 2022, 47, 39030–39046. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Chen, M.; Qi, J.; Zhang, W.; Cao, R. Electrosynthesis of NiPx nanospheres for electrocatalytic hydrogen evolution from a neutral aqueous solution. Chem. Commun. 2017, 53, 5507–5510. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Wu, R.; Yu, Y.; Zhang, B. Anchoring CoO Domains on CoSe2 Nanobelts as Bifunctional Electrocatalysts for Overall Water Splitting in Neutral Media. Adv. Sci. 2016, 3, 1500426. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, B.; Gao, Q.; Zheng, Y.R.; Zheng, X.S.; Zhu, J.F.; Gao, M.R.; Yu, S.H. A Janus Nickel Cobalt Phosphide Catalyst for High-Efficiency Neutral-pH Water Splitting. Angew. Chem. Int. Ed. 2018, 57, 15445–15449. [Google Scholar] [CrossRef]
- Feng, J.X.; Xu, H.; Ye, S.H.; Ouyang, G.; Tong, Y.X.; Li, G.R. Silica–Polypyrrole Hybrids as High-Performance Metal-Free Electrocatalysts for the Hydrogen Evolution Reaction in Neutral Media. Angew. Chem. Int. Ed. 2017, 56, 8120–8124. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, R.; Kang, L.; Yang, M.; He, X.; Li, W.; Wang, R.; Brett, D.J.L.; He, G.; Chai, G.; et al. Enhancing Hydrogen Evolution Electrocatalytic Performance in Neutral Media via Nitrogen and Iron Phosphide Interactions. Small Sci. 2021, 1, 2100032. [Google Scholar] [CrossRef]
- Wang, K.; Si, Y.; Lv, Z.; Yu, T.; Liu, X.; Wang, G.; Xie, G.; Jiang, L. Efficient and stable Ni–Co–Fe–P nanosheet arrays on Ni foam for alkaline and neutral hydrogen evolution. Int. J. Hydrog. Energy 2020, 45, 2504–2512. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Y.; Zhang, X.; Guo, J. Synergistic effect between sulfur and CoFe alloys embedded in N-doped carbon nanosheets for efficient hydrogen evolution under neutral condition. Chem. Eng. J. 2021, 426, 131922. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Zhang, L.; Zhang, W.; Wang, X.; Cui, Z.; Song, H.; Liang, Z.; Du, L. Ultrafast Carbothermal Shock Constructing Ni3Fe1–xCrx Intermetallic Integrated Electrodes for Efficient and Durable Overall Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 19524–19533. [Google Scholar] [CrossRef]
- Woo, S.; Lee, J.; Lee, D.S.; Kim, J.K.; Lim, B. Electrospun Carbon Nanofibers with Embedded Co-Ceria Nanoparticles for Efficient Hydrogen Evolution and Overall Water Splitting. Materials 2020, 13, 856. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Z.; Yu, H.; Wen, H.; Yang, R.; Peng, S.; Sun, M.; Yu, L. Alkali treatment of layered double hydroxide nanosheets as highly efficient bifunctional electrocatalysts for overall water splitting. J. Colloid Interface Sci. 2023, 636, 11–20. [Google Scholar] [CrossRef]
- Moi, C.T.; Sahu, A.; Qureshi, M. Tapping the Potential of High–Valent Mo and W Metal Centers for Dynamic Electronic Structures in Multimetallic FeVO(OH)/Ni(OH)2 for Ultrastable and Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Zhang, Y.; Zhao, Y.; Zhang, Z.; Wang, S.; Cao, J.; Yang, Y.; Sun, S.; Pan, M.; Zhu, L. Room Temperature Preparation of Two–Dimensional Black Phosphorus@Metal Organic Framework Heterojunctions and Their Efficient Overall Water–Splitting Electrocatalytic Reactions. ACS Appl. Mater. Interfaces 2022, 14, 31502–31509. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, S.; Wang, T.; Yang, Y.; Wang, L.; Zhang, X.; Liu, Z.; Niu, L. Construction of CoFe bimetallic phosphide micro-flowers electrocatalyst for highly efficient overall water splitting. Catal. Commun. 2023, 175, 106607. [Google Scholar] [CrossRef]
- Chai, N.; Kong, Y.; Liu, T.; Ying, S.; Jiang, Q.; Yi, F.-Y. (FeMnCe)–co–doped MOF–74 with significantly improved per-formance for overall water splitting. Dalton Trans. 2023, 52, 11601–11610. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, P.; Wang, Z.; Gao, L. Shape–Preserved CoFeNi–MOF/NF Exhibiting Superior Performance for Overall Water Splitting across Alkaline and Neutral Conditions. Materials 2024, 17, 2195. https://doi.org/10.3390/ma17102195
Liu Y, Li P, Wang Z, Gao L. Shape–Preserved CoFeNi–MOF/NF Exhibiting Superior Performance for Overall Water Splitting across Alkaline and Neutral Conditions. Materials. 2024; 17(10):2195. https://doi.org/10.3390/ma17102195
Chicago/Turabian StyleLiu, Yu, Panpan Li, Zegao Wang, and Liangjuan Gao. 2024. "Shape–Preserved CoFeNi–MOF/NF Exhibiting Superior Performance for Overall Water Splitting across Alkaline and Neutral Conditions" Materials 17, no. 10: 2195. https://doi.org/10.3390/ma17102195




