Root Respiration–Trait Relationships Are Influenced by Leaf Habit in Tropical Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Information
2.2. Root Sample Collection
2.3. Root Respiration Rate
2.4. Root Morphological and Chemical Traits
2.5. Statistical Analysis
3. Results
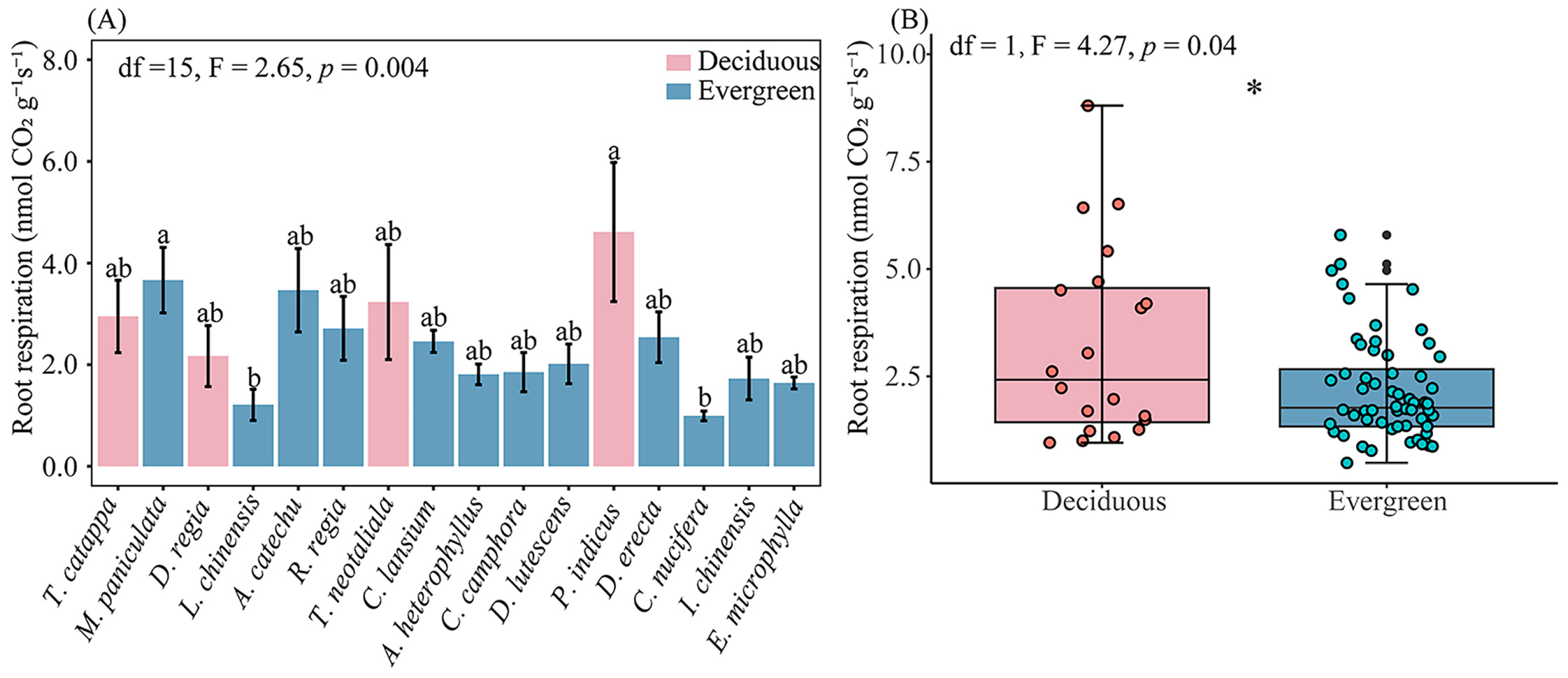
3.1. Root Respiration Rate Variation among Plant Species and Leaf Habits
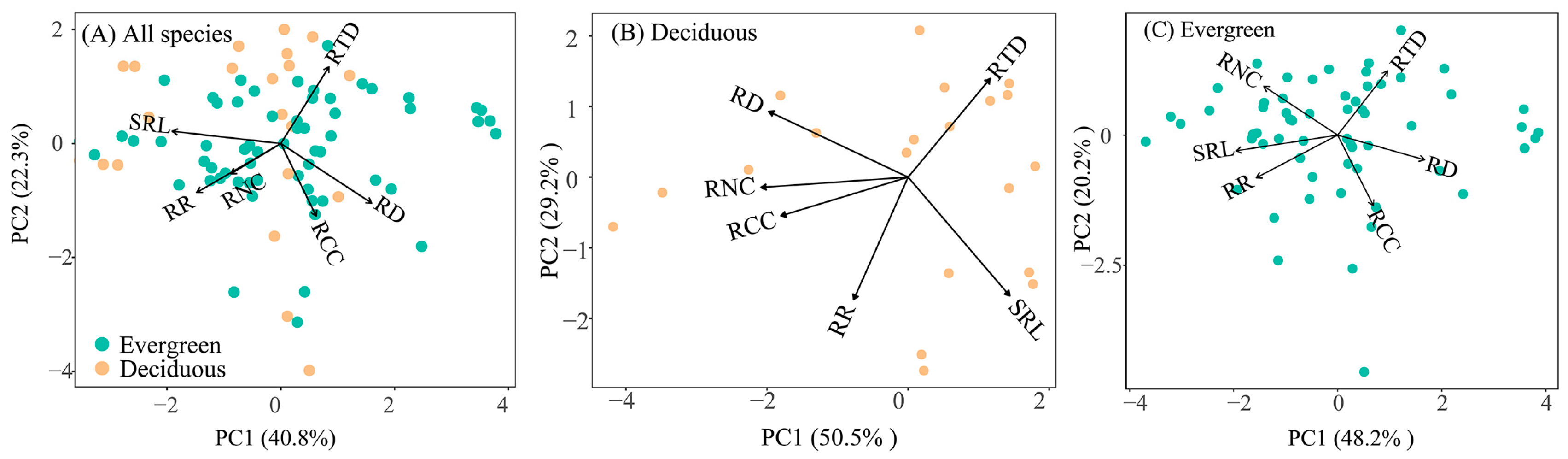
3.2. Correlations among Root Respiration Rate and Other Root Traits
3.3. Effects of Root Traits on Root Respiration Rate
4. Discussion
4.1. Effects of Plant Leaf Habit on Root Respiration and Root Respiration–Trait Relationships in Tropical Plants
4.2. Tropical Plant Root Traits Followed a Multidimensional Pattern of Variation Rather Than a One-Dimensional RES
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faucon, M.P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 20, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Sun, L.; Gan, D.; Fu, L.; Zhu, B. Root functional traits are key determinants of the rhizosphere effect on soil organic matter decomposition across 14 temperate hardwood species. Soil Biol. Biochem. 2020, 151, 108019. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2018, 221, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Shi, Z.; Liu, S.; Chen, M.; Xu, G.; Cao, X.; Zhang, M.; Chen, J.; Li, F. Leaf traits divergence and correlations of woody plants among the three plant functional types on the eastern Qinghai-Tibetan Plateau, China. Front. Plant Sci. 2023, 14, 1128227. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-K.; Pan, X.; Liu, G.-F.; Li, W.-B.; Dai, W.-H.; Tang, S.-L.; Zhang, Y.-L.; Xiao, T.; Chen, L.-Y.; Xiong, W.; et al. Novel evidence for within-species leaf economics spectrum at multiple spatial scales. Front. Plant Sci. 2015, 6, 901. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Lavely, E.; Ma, Z. Fine-root and mycorrhizal traits help explain ecosystem processes and responses to global change. New Phytol. 2014, 204, 455–458. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Flaig, I.C.; Rillig, M.C. Root trait responses to drought are more heterogeneous than leaf trait responses. Funct. Ecol. 2020, 34, 2224–2235. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Marañón, T.; Pérez-Ramos, I.M.; Navarro-Fernández, C.M.; Olmo, M.; Villar, R. Root traits across environ-mental gradients in Mediterranean woody communities: Are they aligned along the root economics spectrum? Plant Soil 2018, 424, 35–48. [Google Scholar] [CrossRef]
- Liu, C.; Xiang, W.; Zou, L.; Lei, P.; Zeng, Y.; Ouyang, S.; Deng, X.; Fang, X.; Liu, Z.; Peng, C. Variation in the functional traits of fine roots is linked to phy-logenetics in the common tree species of Chinese subtropical forests. Plant Soil 2019, 436, 347–364. [Google Scholar] [CrossRef]
- Weigelt, A.; Mommer, L.; Andraczek, K.; Iversen, C.M.; Bergmann, J.; Bruelheide, H.; Fan, Y.; Freschet, G.T.; Guerrero-Ramírez, N.R.; Kattge, J.; et al. An integrated framework of plant form and function: The belowground perspective. New Phytol. 2021, 232, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Weigelt, A.; van der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bai, W.; Zhang, Y.; Zhang, W. Multi-dimensional patterns of variation in root traits among coexisting herbaceous species in temperate steppes. J. Ecol. 2018, 106, 2320–2331. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenes that reduce the metabolic costs of soil exploration: Opportunities for 21st century agriculture. Plant Cell Environ. 2015, 38, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhu, B. Linking root respiration to chemistry and morphology across species. Glob. Chang. Biol. 2021, 27, 190–201. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Erktan, A.; Roumet, C.; Bouchet, D.; Stokes, A.; Pailler, F.; Munoz, F. Two dimensions define the variation of fine root traits across plant communities under the joint influence of ecological succession and annual mowing. J. Ecol. 2018, 106, 2031–2042. [Google Scholar] [CrossRef]
- An, N.; Lu, N.; Fu, B.; Chen, W.; Keyimu, M.; Wang, M. Evidence of differences in covariation among root traits across plant growth forms, mycorrhizal types, and biomes. Front. Plant Sci. 2022, 12, 785589. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Tjoelker, M.G.; Pregitzer, K.S.; Wright, I.J.; Oleksyn, J.; Machado, J. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 2008, 11, 793–801. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2020, 229, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Iii, F.S.C.; Tryon, P.R. Habitat and leaf habit as determinants of growth, nutrient absorption, and nutrient use by Alaskan taiga forest species. Can. J. For. Res. 1983, 13, 818–826. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, J.; Ding, J.; Zou, T.; Zhang, Z.; Liu, Q.; Yin, H. Differences in root exudate inputs and rhizosphere effects on soil N transformation between deciduous and evergreen trees. Plant Soil 2021, 458, 277–289. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Niklas, K.J.; Sun, J.; Wang, Z.; Zhong, Q.; Hu, D.; Cheng, D. A whole-plant economics spectrum including bark functional traits for 59 subtropical woody plant species. J. Ecol. 2020, 110, 248–261. [Google Scholar] [CrossRef]
- Chen, X.; Le, X.; Niklas, K.J.; Hu, D.; Zhong, Q.; Cheng, D. Divergent leaf nutrient-use strategies of coexistent evergreen and deciduous trees in a subtropical forest. J. Plant Ecol. 2023, 16, rtac093. [Google Scholar] [CrossRef]
- Galmán, A.; Abdala-Roberts, L.; Zhang, S.; Berny-Mier y Teran, J.C.; Rasmann, S.; Moreira, X. A global analysis of ele-vational gradients in leaf herbivory and its underlying drivers: Effects of plant growth form, leaf habit and climatic correlates. J. Ecol. 2018, 106, 413–421. [Google Scholar] [CrossRef]
- Schweiger, R.; Castells, E.; Da Sois, L.; Martínez-Vilalta, J.; Müller, C. highly species-specific foliar metabolomes of diverse woody species and relationships with the leaf economics spectrum. Cells 2021, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, K.W.; van Langevelde, F.; Ward, D.; Bongers, F.; da Silva, D.A.; Prins, H.H.T.; de Bie, S.; Sterck, F.J. Deciduous and evergreen trees differ in juvenile biomass allometries because of differences in allocation to root storage. Ann. Bot. 2013, 112, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, S.P.; Garland, T., Jr.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877–884. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Mo, W.; Lee, M.-S.; Uchida, M.; Inatomi, M.; Saigusa, N.; Mariko, S.; Koizumi, H. Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agric. For. Meteorol. 2005, 134, 81–94. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Craine, J.M.; Wedin, D.; Reich, P.B.; Tilman, D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 2005, 167, 493–508. [Google Scholar] [CrossRef]
- Makita, N.; Kosugi, Y.; Dannoura, M.; Takanashi, S.; Niiyama, K.; Kassim, A.R.; Nik, A.R. Patterns of root respiration rates and morphological traits in 13 tree species in a tropical forest. Tree Physiol. 2012, 32, 303–312. [Google Scholar] [CrossRef]
- Liang, S.; Guo, H.; McCormack, M.L.; Qian, Z.; Huang, K.; Yang, Y.; Xi, M.; Qi, X.; Ou, X.; Liu, Y.; et al. Positioning absorptive root respiration in the root economics space across woody and herbaceous species. J. Ecol. 2023, 111, 2710–2720. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally dis-tributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Zhang, W.; Han, B.; Zhou, H.; Lu, X.; Deng, Y.; Liu, K.; Shao, X. Arbuscular Mycorrhizal Fungi Alter the Interaction Effects Between Bacillus and Rhizobium on Root Morphological Traits of Medicago ruthenica L. J. Soil Sci. Plant Nutr. 2023, 23, 2868–2877. [Google Scholar] [CrossRef]
- Whitehead, D.C.; Goulden, K.M.; Hartley, R.D. The distribution of nutrient elements in cell wall and other fractions of the herbage of some grasses and legumes. J. Sci. Food Agric. 1985, 36, 311–318. [Google Scholar] [CrossRef]
- Jia, S.; McLaughlin, N.B.; Gu, J.; Li, X.; Wang, Z. Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 2013, 33, 579–589. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidi-mensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Jarvi, M.P.; Burton, A.J. Root respiration and biomass responses to experimental soil warming vary with root diameter and soil depth. Plant Soil 2020, 451, 435–446. [Google Scholar] [CrossRef]
- Chen, T.; Lin, C.; Song, T.; Guo, R.; Cai, Y.; Chen, W.; Xiong, D.; Jiang, Q.; Chen, G. Does root respiration of subtropical Chinese fir seedlings acclimate to seasonal temperature variation or experimental soil warming? Agric. For. Meteorol. 2021, 308, 108612. [Google Scholar] [CrossRef]
- Salazar, A.; Rousk, K.; Jónsdóttir, I.S.; Bellenger, J.; Andrésson, S. Faster nitrogen cycling and more fungal and root biomass in cold ecosystems under experimental warming: A meta-analysis. Ecology 2020, 101, e02938. [Google Scholar] [CrossRef]
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.A.; Meier, I.C.; Kögel-Knabner, I.; Mueller, C.W. Root exudates induce soil macroaggregation facilitated by fungi in subsoil. Front. Environ. Sci. 2018, 6, 140. [Google Scholar] [CrossRef]
- Staszel-Szlachta, K.; Lasota, J.; Szlachta, A.; Błońska, E. The impact of root systems and their exudates in different tree species on soil properties and microorganisms in a temperate forest ecosystem. BMC Plant Biol. 2024, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Steidinger, B.S.; Crowther, T.W.; Liang, J.; van Nuland, M.E.; Werner, G.D.; Reich, P.B.; Nabuurs, G.J.; De-Miguel, S.; Zhou, M.; Picard, N.; et al. Climatic controls of decompo-sition drive the global biogeography of forest-tree symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Kong, D.; Zhang, Z.; Cai, Q.; Xiao, J.; Liu, Q.; Yin, H. Climate and soil nutrients differentially drive multidimensional fine root traits in ectomycorrhizal-dominated alpine coniferous forests. J. Ecol. 2020, 108, 2544–2556. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, M.; Shao, J.; Zhou, G.; Liu, R.; Zhou, L.; Liu, H.; He, Y.; Chen, Y.; Zhou, X. Fine root trait-function relationships affected by mycorrhizal type and climate. Geoderma 2021, 394, 115011. [Google Scholar] [CrossRef]




| Species | Family | Leaf Habit | Mean DBH 1/Ground Diameter 2 (cm) | Height (m) |
|---|---|---|---|---|
| Areca catechu L. | Arecaceae | Evergreen | 12.58 ± 0.46 | 8.14 ± 0.31 |
| Artocarpus heterophyllus Lam. | Moraceae | Evergreen | 61.08 ± 6.72 | 12.42 ± 1.28 |
| Cinnamomum camphora (L.) J. Presl | Lauraceae | Evergreen | 67.06 ± 2.56 | 22.78 ± 0.77 |
| Clausena lansium (Lour.) Skeels | Rutaceae | Evergreen | 9.56 ± 1.21 | 4.78 ± 0.17 |
| Cocos nucifera L. | Arecaceae | Evergreen | 34.78 ± 1.13 | 9.22 ± 0.33 |
| Dypsis lutescens (H. Wendl.) | Arecaceae | Evergreen | 7.80 ± 0.58 | 3.66 ± 0.16 |
| Duranta erecta L. | Verbenaceae | Evergreen | 2.16 ± 0.12 | 0.87 ± 0.01 |
| Ehretia microphylla Lam. | Boraginaceae | Evergreen | 2.22 ± 0.27 | 0.62 ± 0.03 |
| Ixora chinensis Lam. | Rubiaceae | Evergreen | 1.78 ± 0.14 | 0.51 ± 0.03 |
| Litchi chinensis Sonn. | Sapindaceae | Evergreen | 39.36 ± 4.56 | 9.36 ± 0.91 |
| Murraya paniculata (L.) Jack | Rutaceae | Evergreen | 2.08 ± 0.31 | 0.83 ± 0.02 |
| Roystonea regia (Kunth) O.F. Cook | Arecaceae | Evergreen | 35.28 ± 2.44 | 15.72 ± 1.00 |
| Delonix regia (Bojer ex Hook) Raffin | Fabaceae | Deciduous | 48.24 ± 5.57 | 14.24 ± 0.28 |
| Pterocarpus indicus Willd. | Fabaceae | Deciduous | 48.98 ± 2.05 | 20.72 ± 0.40 |
| Terminalia catappa L. | Combretaceae | Deciduous | 47.48 ± 4.84 | 24.38 ± 0.69 |
| Terminalia neotaliala Capuron | Combretaceae | Deciduous | 24.16 ± 1.45 | 12.08 ± 0.51 |
| Traits | Deciduous | Evergreen | t | df | p |
|---|---|---|---|---|---|
| SRL (m g−1) | 5.71 ± 0.69 | 5.26 ± 0.32 | 0.52 | 29.29 | 0.600 |
| RD (mm) | 1.05 ± 0.07 | 1.12 ± 0.03 | −1.21 | 27.45 | 0.240 |
| RTD (g cm−3) | 0.25 ± 0.01 | 0.24 ± 0.01 | 1.60 | 49.87 | 0.120 |
| RNC (mg g−1) | 76.10 ± 12.05 | 43.45 ± 2.44 | 2.87 | 26.74 | 0.008 |
| RCC (m g−1) | 429.21 ± 10.1 | 459.70 ± 5.96 | −2.50 | 28.42 | 0.020 |
| C/N | 8.04 ± 0.98 | 19.76 ± 1.13 | −3.60 | 31.00 | 0.001 |
| Regression Weights | Standardized Regression Weights | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | Std. Error | Beta | t | p | Vif | R2 | F(p) | ||
| All species | (Intercept) | −0.16 | 0.04 | 0.00 | −4.04 | <0.001 | |||
| SRL (m g−1) | 0.50 | 0.10 | 0.47 | 5.10 | <0.001 | 1.03 | 0.36 | 21.83 (p < 0.001) | |
| RNC (mg g−1) | 0.31 | 0.10 | 0.30 | 3.29 | 0.002 | 1.03 | |||
| Multiple regression analysis model | RR = −0.16 + 0.50SRL + 0.31RNC | ||||||||
| Deciduous | (Intercept) | −0.09 | 0.03 | 0.00 | −2.8 | 0.006 | |||
| SRL (m g−1) | 0.46 | 0.11 | 0.44 | 4.11 | <0.001 | 1.25 | |||
| RTD (mg g−1) | 3.83 | 4.23 | 0.09 | 0.90 | 0.370 | 1.02 | 0.30 | 10.88 (p < 0.001) | |
| RD (mm) | −0.03 | 0.02 | −0.16 | −1.57 | 0.120 | 1.23 | |||
| Multiple regression analysis model | RR= −0.09 + 0.46 SRL + 3.83 RTD − 0.03RD | ||||||||
| Evergreen | (Intercept) | −0.77 | 0.35 | 0.00 | −2.24 | 0.028 | |||
| SRL (m g−1) | 0.60 | 0.10 | 0.57 | 6.86 | < 0.001 | 1.06 | 0.31 | 17.44 (p < 0.001) | |
| RCC (mg g−1) | 0.15 | 0.06 | 0.20 | 1.62 | 0.11 | 1.06 | |||
| Multiple regression analysis model | RR = −0.77 + 0.60SRL + 0.15RCC | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, D.; Sun, Y.; Yang, M. Root Respiration–Trait Relationships Are Influenced by Leaf Habit in Tropical Plants. Forests 2024, 15, 806. https://doi.org/10.3390/f15050806
Deng D, Sun Y, Yang M. Root Respiration–Trait Relationships Are Influenced by Leaf Habit in Tropical Plants. Forests. 2024; 15(5):806. https://doi.org/10.3390/f15050806
Chicago/Turabian StyleDeng, Danting, Yanfei Sun, and Meiqiu Yang. 2024. "Root Respiration–Trait Relationships Are Influenced by Leaf Habit in Tropical Plants" Forests 15, no. 5: 806. https://doi.org/10.3390/f15050806




