Effects of Environment Change Scenarios on the Potential Geographical Distribution of Cunninghamia lanceolata (Lamb.) Hook. in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Species Distribution Data and Environmental Factors
2.2. Preprocessing of Environmental Factor Data
2.3. Model Building and Accuracy Evaluation
2.4. Dynamic Changes in the Distribution of Suitable Habitats under Different Climatic Scenarios in the Future and Core Distributional Shifts under Different Climatic Scenarios
3. Results
3.1. Accuracy Test of MaxEnt Model and the Leading Environmental Factors Affecting the Distribution of C. lanceolata
3.2. Potential Geographical Distribution of Suitable Habitats in Different Periods
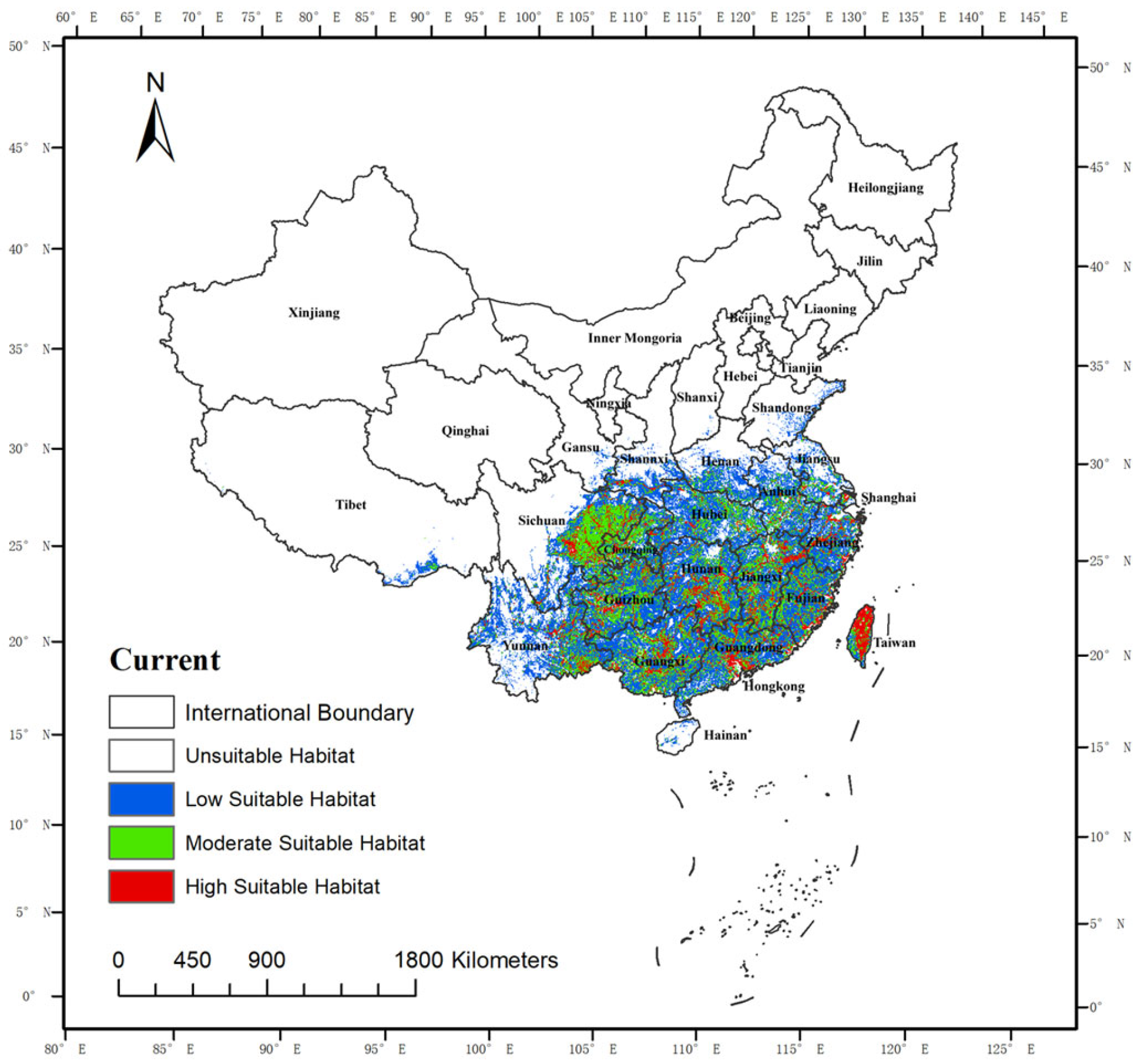
3.2.1. Current Geographical Distribution of Suitable Habitats
3.2.2. Potential Geographical Distribution of Suitable Habitats in the Future
3.3. Dynamic Changes of Distribution Habitats under Different Climatic Scenarios in the Future
3.4. The Core Distributional Shifts under Different Climatic Scenarios
4. Discussion
4.1. Changes in Suitable Habitats of C. lanceolata in Different Periods
4.2. Ecological Characteristics of the Distribution of C. lanceolata Habitats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.F. Characteristics of Cunninghamia lanceolata and afforestation techniques of improved varieties. Mod. Agric. Sci. Technol. 2022, 5, 97–98+101. (In Chinese) [Google Scholar]
- Shen, G.W.; Shen, G.H.; Fu, D. Cultivation techniques and application of urban roadside trees Cunninghamia lanceolata. Xiandai Nongcun Keji 2013, 17, 52. (In Chinese) [Google Scholar]
- Hou, B.X. The origin and development history of Chinese fir. Agric. Archaeol. 1996, 1, 161–171. (In Chinese) [Google Scholar]
- Xu, H.; Sun, Y.J.; Wang, X.J.; Wang, J.; Fu, Y. Linear mixed-effects models to describe individual tree crown width for China-fir in Fujian Province, southeast China. PLoS ONE 2015, 10, e0122257. [Google Scholar] [CrossRef]
- Duan, H.J.; Cao, S.; Zheng, H.Q.; Hu, D.H.; Lin, J.; Lin, H.Z.; Hu, R.Y.; Sun, Y.H.; Li, Y. Variation in the growth traits and wood properties of Chinese fir from six provinces of southern China. Forests 2016, 7, 192. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.Z.; Huang, M.S.; Wu, P.F.; Ma, X.Q. Genetic diversity and relationships of ancient Chinese fir (Cunninghamia lanceolata) genotypes revealed by sequence-related amplified polymorphism markers. Genet. Resour. Crop Evol. 2017, 64, 1087–1099. [Google Scholar] [CrossRef]
- Yao, M.M.; Yang, Y.Q.; Song, J.L.; Yu, Y.; Jin, Y.C. Melamine formaldehyde modified furfurylation to improve Chinese fir’s dimensional stability and mechanical properties. BioResources 2017, 12, 3057–3066. [Google Scholar] [CrossRef]
- Yan, X.X.; Chang, Y.J. Investigation of waterborne thermochromic topcoat film with color-changing microcapsules on Chinese fir surface. Prog. Org. Coat. 2019, 136, 105262. [Google Scholar] [CrossRef]
- Sun, Q.X.; Peng, Z.H.; Zhang, Q.S. Volatiles of wood of Chinese fir in nature and its effect on human heaith. J. Anhui Agric. Univ. 2004, 2, 158–163. (In Chinese) [Google Scholar] [CrossRef]
- Kurpis, J.; Serrato-Cruz, M.A.; Feria Arroyo, T.P. Modeling the effects of climate change on the distribution of Tagetes lucida Cav. (Asteraceae). Glob. Ecol. Conserv. 2019, 20, e00747. [Google Scholar] [CrossRef]
- Gilani, H.; Arif Goheer, M.; Ahmad, H.; Hussain, K. Under predicted climate change: Distribution and ecological niche modelling of six native tree species in Gilgit-Baltistan, Pakistan. Ecol. Indic. 2020, 111, 106049. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Ulukan, H. Climate change and global warming effect(s) on wheat Landraces: A General Approach. In Wheat Landraces; Zencirci, N., Baloch, F.S., Habyarimana, E., Chung, G., Eds.; Springer International Publisher: Berlin, Germany, 2021; pp. 169–191. [Google Scholar] [CrossRef]
- Steel, E.J.; Fontaine, J.B.; Ruthrof, K.X.; Burgess, T.I.; Hardy, G.E.S.J. Changes in structure of over- and midstory tree species in a Mediterranean-type forest after an extreme drought-associated heatwave. Austral Ecol. 2019, 44, 1438–1450. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.X.; Brouwers, N.C.; Dell, B.; Hardy, G.S.J. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. Eur. J. For. Res. 2013, 132, 497–510. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Liu, H.C.; Jacquemyn, H.; He, X.Y.; Chen, W.; Huang, Y.Q.; Yu, S.; Lu, Y.P.; Zhang, Y. The Impact of human pressure and climate change on the habitat availability and protection of Cypripedium (Orchidaceae) in Northeast China. Plants 2021, 10, 84. [Google Scholar] [CrossRef]
- Garah, K.; Bentouati, A. Using the MaxEnt model for assessing the impact of climate change on the Aurasian Aleppo pine distribution in Algeria. Afr. J. Ecol. 2019, 57, 500–511. [Google Scholar] [CrossRef]
- Wang, G.Z.; Geng, Q.F.; Xiao, M.Y.; Zhang, M.Y.; Zhang, Y.Y.; Wang, Z.S. Predicting Pseudolarix amabilis potential habitat based on four Niche models. Sheng Tai Xue Bao 2020, 40, 6096–6104. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.W.; Xiang, W.H.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Chen, Y.G.; Yue, X.G.; Chen, Y.H.; Cheng, W.X.; Du, J.G.; Zhong, Q.L.; Cheng, D.L. Identification of potential distribution area of Cunninghamia lanceolata in China under climate change based on the MaxEnt model. Ying Yong Sheng Tai Xue Bao 2022, 33, 1207–1214. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, Q.; Feng, C.H.; Shi, Z.P. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Sheng Tai Xue Bao 2017, 37, 8556–8566. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.P.; Zhang, Q.; Zhang, D.F.; Xie, C.X.; Liu, X. Prediction for the potential distribution area of Codonopsis pilosula at global scale based on Maxent model. Ying Yong Sheng Tai Xue Bao 2017, 28, 992–1000. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.G.; Li, R.M.; Wang, X.L.; Cheng, J.H.; Yang, Q.L.; Kong, H. AHP-GIS and MaxEnt for delineation of potential distribution of Arabica coffee plantation under future climate in Yunnan, China. Ecol. Indic. 2021, 132, 108339. [Google Scholar] [CrossRef]
- Etherington, T.R. Python based GIS tools for landscape genetics: Visualising genetic relatedness and measuring landscape connectivity. Methods Ecol. Evol. 2011, 2, 52–55. [Google Scholar] [CrossRef]
- Hong, G.X.; Lv, S.Y.; Peng, J.Y.; Jiang, Z.Y. Genetic geography and conservation of Cunninghamia lanceolata and Cunnighamia konishii. Nat. Conserv. Q. 2000, 31, 33–36. (In Chinese) [Google Scholar]
- Hu, Z.J.; Zhang, Y.L.; Yu, H.B. Simulation of Stipa purpurea distribution pattern on Tibetan Plateau based on MaxEnt model and GIS. Ying Yong Sheng Tai Xue Bao 2015, 26, 505–511. (In Chinese) [Google Scholar] [CrossRef]
- Ding, W.N.; Ree, R.; Spicer, R.; Xing, Y. Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 2020, 369, 578–581. [Google Scholar] [CrossRef] [PubMed]
- An, J. Study on Molecular Phylogeography of Cunninghamia lanceolata (Lamb.) Hook. Master’s Thesis, Central South University of Forestry and Technology, Hunan, China, 2012. (In Chinese). [Google Scholar]
- Wang, K.F.; Xu, S. Quaternary Palynology; Guizhou People’s Publishing House: Guizhou, China, 1988; p. 333. (In Chinese) [Google Scholar]
- Song, Z.C. Late Cenozoic palyno-flora from Zhaotong, Yunnan. J. Nanjing Inst. Geol. Paleontol. 1988, 24, 1–108. (In Chinese) [Google Scholar]
- Liu, H.L.; Wang, D.Y. A study on the remains of ancient forest in Mianning, Sichuan. Sci. Silvae Sin. 1984, 20, 175–184. (In Chinese) [Google Scholar]
- Huang, Z.G.; Li, P.R.; Zhang, Z.Y.; Li, K.H.; Qiao, P.N. Formation, Development and Evolution of Pearl River Delta; Science and Technology of China Press: Guangzhou, China, 1982; p. 274. (In Chinese) [Google Scholar]
- Gu, H.B. Sporopollen Analysis and Paleoenvironment Discussion of Pengtoushan Site in Lixian County, Hunan Province. Cult. Relics 1990, 8, 30–32. (In Chinese) [Google Scholar]
- Peng, Z.H. On the origin of Chinese fir from historical documents. J. Anhui Agric. Univ. 1984, 2, 23–32. (In Chinese) [Google Scholar] [CrossRef]
- Huang, B.L.; Lan, T.G. Preliminary discussion on the history of cultivation and utilization of Chinese fir. J. Nanjing For. Univ. (Nat. Sci. Ed.) 1988, 2, 54–59. (In Chinese) [Google Scholar] [CrossRef]
- Hou, B.X.; Chen, F.S.; Cheng, Z.H. Origin of distribution, utilization and cultivation history of Chinese fir in Hunan. Hunan For. Sci. Technol. 1995, 3, 1–6. (In Chinese) [Google Scholar]
- Li, Y.X. Genetic Diversity and Genetic Divergence of Cunninghamia lanceolata (Lamb.) Hook. Geographical Provenances. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2015. (In Chinese). [Google Scholar]
- Wu, Z.L. Preliminary study on the distribution of Chinese fir. Acta Geogr. Sin. 1955, 3, 273–285. (In Chinese) [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5. [Google Scholar] [CrossRef]
- Shugart, H.H.; Antonovsky, M.Y.; Jarvis, P.G.; Sandford, A.P. CO2 climatic change and forest ecosystems. In The Greenhouse Effect, Climatic Change and Ecosystems; Bolin, B., Doeoes, B.R., Jaeger, J., Warrick, R.A., Eds.; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Chen, G.X.; Yu, K.W.; Liao, L.P.; Xu, G.S. Effect of human activities on forest ecosystems: N cycle and soil fertility. Nutr. Cycl. Agroecosyst. 2000, 57, 47–54. [Google Scholar] [CrossRef]
- Liang, D.L.; Wang, B.; Jiang, L.L.; Chen, K. The population and its spatial characteristic analysis from 2000 to 2010 in Taiwan. J. Shanxi Norm. Univ. (Nat. Sci. Ed.) 2016, 30, 90–97. (In Chinese) [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, S.B.; Lyu, Y.Q.; Zhao, Y.; Lyu, S.H. Characteristics and future projections of summer extreme precipitation in Sichuan Province, China. J. Mt. Sci. 2020, 17, 1696–1711. [Google Scholar] [CrossRef]
- Xu, C.C.; Wu, W.X.; Ge, Q.S. Impact assessment of climate change on rice yields using the ORYZA model in the Sichuan Basin, China. Int. J. Climatol. 2018, 38, 2922–2939. [Google Scholar] [CrossRef]
- Li, N.; Bai, R.; Wu, L.; Li, W.; Chen, M.; Chen, X.; Fan, C.H.; Yang, G.S. Impacts of future climate change on spring phenology stages of rubber tree in Hainan, China. Ying Yong Sheng Tai Xue Bao 2020, 31, 1241–1249. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Z.Q. Preliminary study on growth and environmental factors of Cunninghamia lanceolata in the south of Yangtze River in Anhui province. Lin Ye Ke Xue Yan Jiu 1998, 1, 34–36. (In Chinese) [Google Scholar]
- Tian, D.L.; Xiang, W.H.; Chen, X.Y.; Yan, W.D.; Fang, X.; Kang, W.X.; Dan, X.W.; Peng, C.H.; Peng, Y.Y. A long-term evaluation of biomass production in first and second rotations of Chinese fir plantations at the same site. Forestry 2011, 84, 411–418. [Google Scholar] [CrossRef]



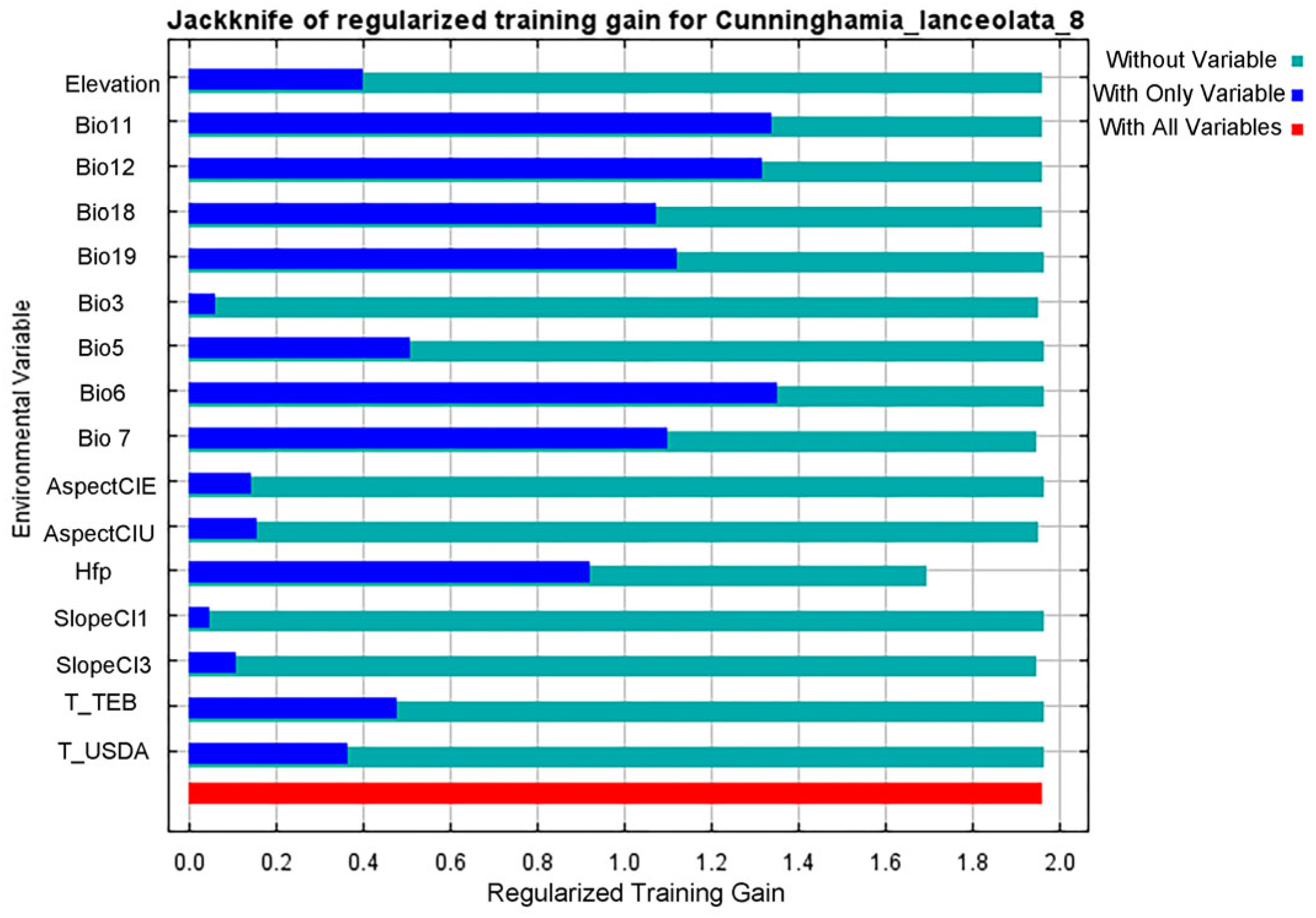




| Environmental Factors | Elevation (m) | Bio6 (°C) | Bio7 (°C) | Bio11 (°C) | Bio12 (mm) | Bio19 (mm) | Hfp |
|---|---|---|---|---|---|---|---|
| Suitable minimum value | 0 | 1 | 14 | 6 | 1100 | 120 | 40 |
| Suitable maximum value | 600 | 12 | 30 | 15 | 4700 | 510 | 105 |
| RCP2.6 | RCP4.5 | RCP6.0 | RCP8.5 | |||||
|---|---|---|---|---|---|---|---|---|
| 2050s | 2070s | 2050s | 2070s | 2050s | 2070s | 2050s | 2070s | |
| Expansion /×104 km2 | 22.76 | 23.16 | 23.27 | 17.94 | 27.33 | 24.88 | 22.83 | 16.44 |
| contraction /×104 km2 | 8.46 | 5.74 | 8.04 | 13.37 | 4.15 | 7.81 | 6.26 | 9.14 |
| No change /×104 km2 | 205.96 | 208.68 | 206.39 | 201.05 | 210.28 | 206.61 | 208.16 | 205.28 |
| No occupancy /×104 km2 | 722.82 | 722.42 | 722.31 | 727.64 | 718.25 | 720.70 | 722.75 | 729.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Cao, Y.; Manda, T.; Hwarari, D.; Chen, J.; Yang, L. Effects of Environment Change Scenarios on the Potential Geographical Distribution of Cunninghamia lanceolata (Lamb.) Hook. in China. Forests 2024, 15, 830. https://doi.org/10.3390/f15050830
Feng J, Cao Y, Manda T, Hwarari D, Chen J, Yang L. Effects of Environment Change Scenarios on the Potential Geographical Distribution of Cunninghamia lanceolata (Lamb.) Hook. in China. Forests. 2024; 15(5):830. https://doi.org/10.3390/f15050830
Chicago/Turabian StyleFeng, Jiajie, Yiwei Cao, Teja Manda, Delight Hwarari, Jinhui Chen, and Liming Yang. 2024. "Effects of Environment Change Scenarios on the Potential Geographical Distribution of Cunninghamia lanceolata (Lamb.) Hook. in China" Forests 15, no. 5: 830. https://doi.org/10.3390/f15050830






