Advances in Endangered Plant Research: Ammopiptanthus’s Responses to Biotic and Abiotic Stressors
Abstract
:1. Introduction
2. Defense Response in Ammopiptanthus
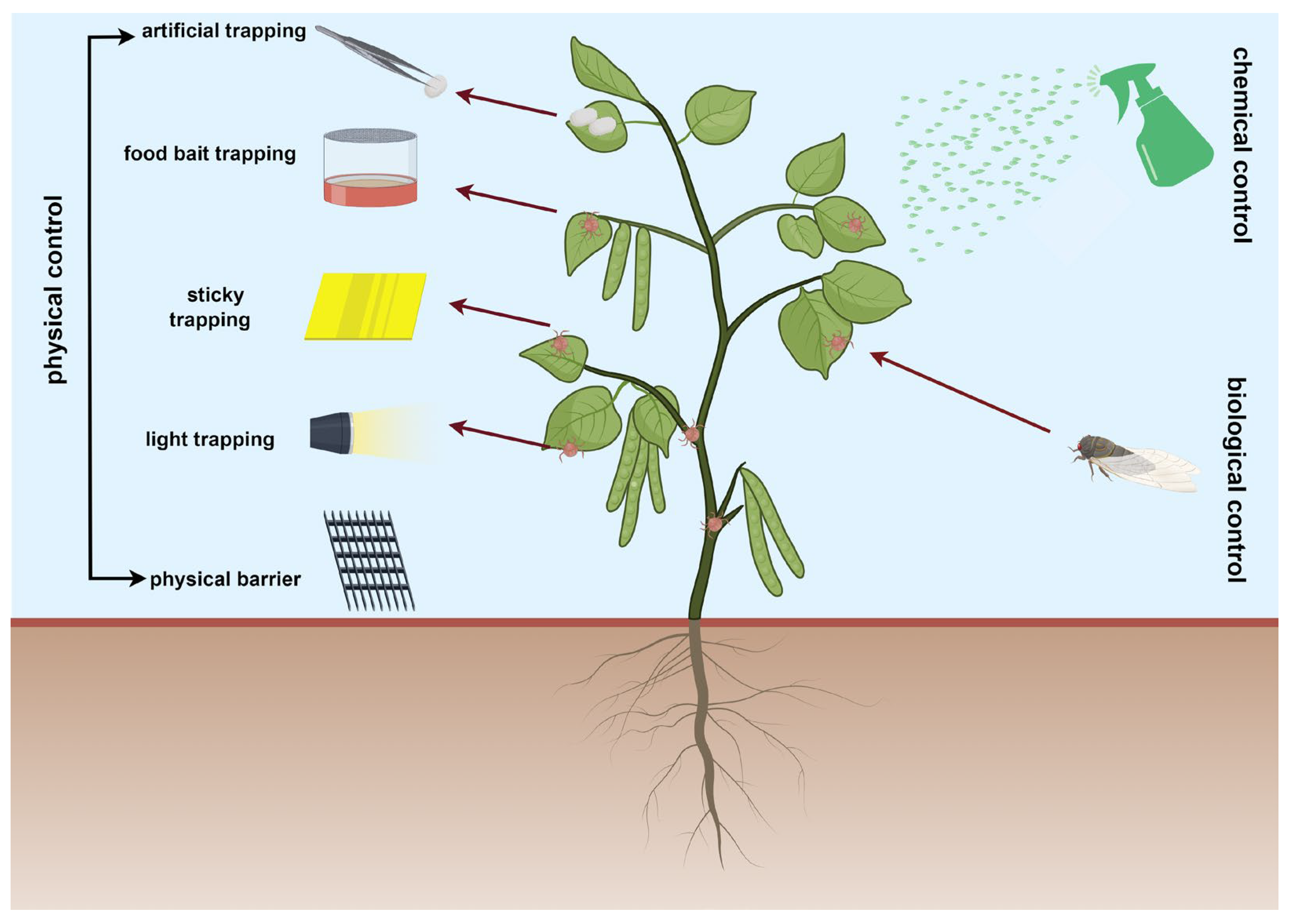
2.1. Insect Populations Affecting on Ammopiptanthus
2.2. Early Biotic Stress Signaling in Ammopiptanthus
2.3. Metabolic Changes in Ammopiptanthus
3. Abiotic Resistance Mechanisms in Ammopiptanthus
3.1. Physiological Adjustments
3.2. Modulation of Protective Enzyme Systems
3.3. Metabolic Level Alterations
4. Synergistic Effects of Multiple Environmental Pressures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, F.; Wang, X.; Li, X.; Xu, M.; Li, H.; Merhaba, A.; Sun, H.; Wei, S.; Feng, J.; Zhou, Y. Long-read sequencing and de novo genome assembly of Ammopiptanthus nanus, a desert shrub. GigaScience 2018, 7, giy074. [Google Scholar] [PubMed]
- Du, Z.; He, Y.; Wang, H.; Wang, C.; Duan, Y. Potential geographical distribution and habitat shift of the genus Ammopiptanthus in China under current and future climate change based on the MaxEnt model. J. Arid. Environ. 2021, 184, 104328. [Google Scholar] [CrossRef]
- Li, R.; Yan, C.; Zhao, Y.; Wang, P.; Qiu, G.Y. Discriminating growth stages of an endangered mediterranean relict plant (Ammopiptanthus mongolicus) in the arid Northwest China using hyperspectral measurements. Sci. Total Environ. 2019, 657, 270–278. [Google Scholar] [PubMed]
- Chen, Y.; Cao, C.; Guo, Z.; Zhang, Q.; Li, S.; Zhang, X.; Gong, J.; Shen, Y. Herbivore exposure alters ion fluxes and improves salt tolerance in a desert shrub. Plant Cell Environ. 2020, 43, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yu, H.; Liu, Y.; Jiao, P.; Zhou, S.; Zhang, S.; Li, W.; Fu, F. Heterologous expression of antifreeze protein gene AnAFP from Ammopiptanthus nanus enhances cold tolerance in Escherichia coli and tobacco. Gene 2014, 539, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhou, X.; Wang, Y.; Zhou, S.; Fu, F.; Li, W. A betaine aldehyde dehydrogenase gene from Ammopiptanthus nanus enhances tolerance of Arabidopsis to high salt and drought stresses. Plant Growth Regul. 2017, 83, 265–276. [Google Scholar] [CrossRef]
- Lu, C.; Yin, L.; Li, K. Proteome expression patterns in the stress tolerant evergreen Ammopiptanthus nanus under conditions of extreme cold. Plant Growth Regul. 2010, 62, 65–70. [Google Scholar] [CrossRef]
- Chen, S.; Geng, X.; Lou, J.; Huang, D.; Mao, H.; Lin, X. Overexpression of a plasmalemma Na+/H+ antiporter from the halophyte Nitraria sibirica enhances the salt tolerance of transgenic poplar. Plant Sci. 2024, 343, 112061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Chen, L.; Ma, H.; Ruan, Y.; Xu, T.; Xu, C.; He, Y.; Qi, M. Molecular cloning and expression of an encoding galactinol synthase gene (AnGolS1) in seedling of Ammopiptanthus nanus. Sci. Rep. 2016, 6, 36113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Bai, L.; Zhang, L.; Chen, G.; Fan, J.; Xu, S.; Guo, Z. Identification and characterization of AnICE1 and AnCBFs involved in cold tolerance from Ammopiptanthus nanus. Plant Physiol. Biochem. 2021, 168, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Mahboob, M.G.; Arena, C.; Kader, M.A.; Sultana, S.; Hasan, A.K.; Wang, J.; Sarker, T.; Zhang, R.; Barmon, M. The modulation of water, nitrogen, and phosphorous supply for growth optimization of the evergreen shrubs Ammopiptanthus mongolicus for revegetation purpose. Front. Plant Sci. 2021, 12, 766523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Q.; Liu, F.; Zheng, L.; Bing, J.; Zhou, Y.; Gao, F. Gene profiling of the ascorbate oxidase family genes under osmotic and cold stress reveals the role of AnAO5 in cold adaptation in Ammopiptanthus nanus. Plants 2023, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Zhang, H.; Shen, Y. The role of 1-penten-3-one in plant defense responses. Plant Physiol. J. 2019, 55, 225–231. [Google Scholar] [CrossRef]
- Eulgem, T. Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci. 2005, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yu, Y.; Yuan, Y.; Huang, H.; Yan, C. Genetic diversity and geographic differentiation in endangered Ammopiptanthus (Leguminosae) populations in desert regions of northwest China as revealed by ISSR analysis. Ann. Bot. 2005, 95, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, R.C.; Tarr, C.L.; Pratt, T.K. Genetic structure and mating system in the palila, an endangered Hawaiian honeycreeper, as assessed by DNA fingerprinting. Mol. Ecol. 2008, 3, 383–392. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Feng, B. The types of pests and their damage areas on the endangered plant Ammopiptanthus nanus. In Proceedings of the Congress and Symposium on the 60th Anniversary of the Entomological Society of China, Chongqing, China, 9–13 November 2004. [Google Scholar]
- Li, X.; Yang, Y.; Zhang, D.; Mu, T.; Yu, F.; Zhou, Y. Damage rate and spatial distribution type of Etiella zinckenella on Ammopiptanthus in different habitats. North Hortic. 2016, 24, 108–111. [Google Scholar]
- Oliveira, J.L.D.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Maischak, H.; Grigoriev, P.A.; Vogel, H.; Boland, W.; Mithofer, A. Oral secretions from herbivorous lepidopteran larvae exhibit ion channel-forming activities. FEBS Lett. 2007, 581, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Arimura, G.; Mithofer, A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 2012, 29, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithofer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P. Receptor kinases in plant responses to herbivory. Curr. Opin. Biotechnol. 2021, 70, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.R.; Mithofer, A.; Will, T.; Felle, H.H.; Furch, A.C. Herbivore-triggered electrophysiological reactions: Candidates for systemic signals in higher plants and the challenge of their identification. Plant Physiol. 2016, 170, 2407–2419. [Google Scholar] [CrossRef] [PubMed]
- Furstenberg-Hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Maffei, M.E. Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem. Biophys. Res. Commun. 2010, 400, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.; Zhang, W.; Su, Y.; Xiao, L.; Deng, H.; Xie, D. Injury activates Ca2+/Calmodulin-Dependent Phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Mol. Cell 2018, 70, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; Tumlinson, J.H., III.; Teal, P.E.A. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA 2009, 106, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, J.; Shen, Y. Regurgitant from Orgyia ericae Germar induces calcium influx and accumulation of hydrogen peroxide in Ammopiptanthus mongolicus (Maxim. ex Kom.) Cheng f. cells. Acta Ecol. Sin. 2012, 32, 6520–6526. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, Y.-T.; Liu, Y.-X.; Hao, G.-F.; Yang, X.-Q. Molecular interaction network of plant-herbivorous insects. Adv. Agrochem. 2024, 3, 74–82. [Google Scholar] [CrossRef]
- Kallure, G.S.; Kumari, A.; Shinde, B.A.; Giri, A.P. Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 2022, 193, 113008. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Shen, Y.; Huang, Q. Influx of Ca2+-dependent H+ to suspended cells of Ammopiptanthus mongolicus triggered by mechanical stimulation. Sci. Silvae Sin. 2012, 48, 36–41. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, H.; Xu, F.; Yan, F.; Xu, W. H+-ATPases in plant growth and stress responses. Annu. Rev. Plant Biol. 2022, 73, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.; Yang, Y.; Guo, Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, X.; Ma, L.; Wu, Y.; Liu, X.; Fu, H.; Liu, G.; Lei, X.; Guo, Y. Dynamic changes of phosphatidylinositol and phosphatidylinositol 4-phosphate levels modulate H+-ATPase and Na+/H+ antiporter activities to maintain ion homeostasis in Arabidopsis under salt stress. Mol. Plant 2021, 14, 2000–2014. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, H.; Zhang, T.; Cao, C.; Zong, S.; Luo, Y.; Shen, Y. Metabolites of Ammopiptanthus mongolicus induced by Orgyia ericae attack and mechanical wounding. Plant Physiol. Biochem. 2013, 69, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Cao, C.; Mei, X.; Wang, N.; Yan, S.; Zong, S.; Luo, Y.; Yang, H.; Shen, Y. Similar metabolic changes induced by HIPVs exposure as herbivore in Ammopiptanthus mongolicus. PLoS ONE 2014, 9, e95474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Wu, M.; Li, X.; Liu, H.; Niu, N.; Li, S.; Chen, L. Volatile organic compounds (VOCs) from plants: From release to detection. TrAC Trend Anal. Chem. 2023, 158, 116872. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Hao, X.; Wang, S.; Fu, Y.; Liu, Y.; Shen, H.; Jiang, L.; McLamore, E.S.; Shen, Y. The WRKY46–MYC2 module plays a critical role in E-2-hexenal-induced anti-herbivore responses by promoting flavonoid accumulation. Plant Commun. 2023, 5, 100734. [Google Scholar] [CrossRef]
- Xu, G.; Pan, B.; Mingling, X. A study on alkaloids of Ammopiptanthus mongolicus. Arid. Zone Res. 1994, 11, 50–52. [Google Scholar]
- Gao, W.; Zhu, G.; Liu, Q. On the nematocidal activity of Ammopiptanthus mongolicus and Peganum harmala L against Bursaphelenchus xylophilus. J. Tianjin Norm. Univ. 2009, 29, 55–58. [Google Scholar] [CrossRef]
- Lei, X.; He, D.; He, Y.; Ma, Y. Inhibitory effects of the methanol extracts from the caudices of Ammopiptanthus mongolicus on development of the larvae of the diamondback moth, Plutella xylostella. Plant Prot. 2008, 34, 100–103. [Google Scholar] [CrossRef]
- Poelman, E.H.; Broekgaarden, C.; Van Loon, J.J.A.; Dicke, M. Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol. Ecol. 2008, 17, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Chalmers, J.A.; Raj, S.; Thaler, J.S. Induced plant responses to multiple damagers: Differential effects on an herbivore and its parasitoid. Oecologia 2005, 143, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Shen, Y.; Yang, H. Metabolic effects of early-season herbivores on the response to conspecific attack in later-season in Ammopiptanthus mongolicus (Maxim.) Cheng f. Plant Physiol. J. 2014, 50, 61–67. [Google Scholar] [CrossRef]
- Wari, D.; Aboshi, T.; Shinya, T.; Galis, I. Integrated view of plant metabolic defense with particular focus on chewing herbivores. J. Integr. Plant Biol. 2022, 64, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonates are signals in the biosynthesis of secondary metabolites—Pathways, transcription factors and applied aspects—A brief review. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Liao, Y.; Jian, G.; Jia, Y.; Zeng, L.; Gu, D.; Li, H.; Yang, Y. Light induces an increasing release of benzyl nitrile against diurnal herbivore Ectropis grisescens Warren attack in tea (Camellia sinensis) plants. Plant Cell Environ. 2023, 46, 3464–3480. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Moeder, W.; Yoshioka, K.; Shan, L. A tale of many families: Calcium channels in plant immunity. Plant Cell 2022, 34, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xi, J.; Du, L.; Suttle, J.C.; Poovaiah, B.W. Coupling calcium/calmodulin-mediated signaling and herbivore-induced plant response through calmodulin-binding transcription factor AtSR1/CAMTA3. Plant Mol. Biol. 2012, 79, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kloth, K.J.; Wiegers, G.L.; Busscher-Lange, J.; van Haarst, J.C.; Kruijer, W.; Bouwmeester, H.J.; Dicke, M.; Jongsma, M.A. AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J. Exp. Bot. 2016, 67, 3383–3396. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Yang, H.; Lu, Q.; Zhang, X. Endemic shrubs in temperate arid and semiarid regions of northern China and their potentials for rangeland restoration. AoB Plants 2015, 7, plv063. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Guo, M.; Yue, G.; Li, J.; Yang, S.; Zhao, P.; Su, Y. An unusual strategy of stomatal control in the desert shrub Ammopiptanthus mongolicus. Plant Physiol. Biochem. 2018, 125, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, J.; Xin, Z.; Huang, Y.; Han, C.; Li, Y.; Lu, Q. Ecological stoichiometric characteristics in organs of Ammopiptanthus mongolicus in different habitats. Plants 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z. Probe into drought-resisting mechanism of Ammopitanthus mongolicus (Maxim) Cheng F. J. Desert Res. 2000, 20, 71–74. [Google Scholar] [CrossRef]
- Wang, H.; Jia, G.; Ding, Q. Research progress of abiotic stress tolerant mechanisms and application prospect of Ammopiptanthus mongolicus Maxim. Chin. Agric. Sci. Bull. 2005, 21, 121–125. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; Feng, J.; Jia, X. Advances of dhought-resistance and frigid-resistance mechanism recearch on Ammopiptanthus mongolicus. J. Desert Res. 2001, 21, 312–316. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Lu, T.; Wang, J.; Shi, W. Identification of the efficacy of ex situ conservation of Ammopiptanthus nanus based on its ETS-SSR markers. Plants 2023, 12, 2670. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Han, H.; Zhao, X.; Chen, S.; Li, G.; Shi, S.; Feng, J. Physiological and transcriptome analyses reveal the response of Ammopiptanthus mongolicus to extreme seasonal temperatures in a cold plateau desert ecosystem. Sci. Rep. 2022, 12, 10630. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wang, L.; Zhou, L.; Su, X.; Guo, H.; Cheng, H. Overexpression of AmCBF1 enhances drought and cold stress tolerance, and improves photosynthesis in transgenic cotton. PeerJ 2022, 10, e13422. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.B.; Cao, P.X.; Gao, S.Q.; Wang, B.; Wei, L.B.; Zhao, J.; Chen, G.; Wang, B.H. Purification and structure analysis of antifreeze proteins from Ammopiptanthus mongolicus. Prep. Biochem. Biotechnol. 2008, 38, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, L.; Wang, G. Multistep purification of an antifreeze protein from Ammopiptanthus mongolicus by chromatographic and electrophoretic methods. J. Chromatogr. Sci. 2003, 41, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, L. Purification of boiling-soluble antifreeze protein from the Legume Ammopiptanthus mongolicus. Prep. Biochem. Biotechnol. 2003, 33, 67–80. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The key roles of ROS and RNS as a signaling molecule in plant–microbe interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Niu, M.X.; Feng, C.H.; He, F.; Zhang, H.; Bao, Y.; Liu, S.J.; Liu, X.; Su, Y.; Liu, C.; Wang, H.L.; et al. The miR6445-NAC029 module regulates drought tolerance by regulating the expression of glutathione S-transferase U23 and reactive oxygen species scavenging in Populus. New Phytol. 2024, 242, 2043–2058. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, M.; Shi, J.; Zheng, G.; Wang, Y.; Wang, J.; Chen, Y.; Lu, C.; Yin, W. Reference gene selection for qPCR in Ammopiptanthus mongolicus under abiotic stresses and expression analysis of seven ROS-scavenging enzyme genes. Plant Cell Rep. 2012, 31, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zheng, L.; Cao, S.; Liu, Q.; Wei, S.; Zhou, Y.; Gao, F. AnDREB5.1, a A5 group DREB gene from desert shrub Ammopiptanthus nanus, confers osmotic and cold stress tolerances in transgenic tobacco. Physiol. Plant. 2024, 176, e14272. [Google Scholar] [CrossRef] [PubMed]
- Dorjee, T.; Cui, Y.; Zhang, Y.; Liu, Q.; Li, X.; Sumbur, B.; Yan, H.; Bing, J.; Geng, Y.; Zhou, Y.; et al. Characterization of NAC gene family in Ammopiptanthus mongolicus and functional analysis of AmNAC24, an osmotic and cold-stress-induced NAC gene. Biomolecules 2024, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, H.; Miao, Y.; Li, G. Impacts of low temperature on photosynthesis physiology of Ammopiptanthus nanus (Leguminosae) and Ammopiptanthus mongolicus seedlings. J. Xinjiang Univ. 2009, 5, 342–346. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Jin, M.; Su, Y. Functional analysis of a type 2C protein phosphatase gene from Ammopiptanthus mongolicus. Gene 2018, 653, 29–42. [Google Scholar] [CrossRef]
- Klotke, J.; Kopka, J.; Gatzke, N.; Heyer, A.G. Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation—Evidence for a role of raffinose in cold acclimation. Plant Cell Environ. 2004, 27, 1395–1404. [Google Scholar] [CrossRef]
- Kito, K.; Yamane, K.; Yamamori, T.; Matsuhira, H.; Tanaka, Y.; Takabe, T. Isolation, functional characterization and stress responses of raffinose synthase genes in sugar beet. J. Plant Biochem. Biotechnol. 2017, 27, 36–45. [Google Scholar] [CrossRef]
- Yu, H.Q.; Yong, T.M.; Li, H.J.; Liu, Y.P.; Zhou, S.F.; Fu, F.L.; Li, W.C. Overexpression of a phospholipase Dα gene from Ammopiptanthus nanus enhances salt tolerance of phospholipase Dα1-deficient Arabidopsis mutant. Planta 2015, 242, 1495–1509. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Yu, T.; Chen, S.; Chen, Y.; Lu, C. Heterologous expression of three Ammopiptanthus mongolicus dehydrin genes confers abiotic stress tolerance in Arabidopsis thaliana. Plants 2020, 9, 193. [Google Scholar] [CrossRef]
- Fu, X.; Xiao, Z.; Gao, F.; Zhou, Y. Proteomics analysis of Ammopiptanthus mongolicus leaves under drought stress. Biotechnol. Bull. 2017, 33, 69–80. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Hao, X.; Wang, Z.; Chen, Y.; Qu, Y.; Yao, H.; Shen, Y. AnWRKY29 from the desert xerophytic evergreen Ammopiptanthus nanus improves drought tolerance through osmoregulation in transgenic plants. Plant Sci. 2023, 336, 111851. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hao, X.; Liu, Y.; Chen, Y.; Qu, Y.; Wang, Z.; Shen, Y. AnWRKY29 and AnHSP90 synergistically modulate trehalose levels in a desert shrub leaves during osmotic stress. Physiol. Plant. 2024, 176, e14237. [Google Scholar] [CrossRef] [PubMed]
- Sumbur, B.; Zhou, M.; Dorjee, T.; Bing, J.; Ha, S.; Xu, X.; Zhou, Y.; Gao, F. Chemical and transcriptomic analyses of leaf cuticular wax metabolism in Ammopiptanthus mongolicus under osmotic stress. Biomolecules 2024, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Bobadilla, M.; Vitiello, A.; Erb, M.; Poelman, E.H. Plant defense strategies against attack by multiple herbivores. Trends Plant Sci. 2022, 27, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Ryu, C.M. Insect stings to change gear for healthy plant: Improving maize drought tolerance by whitefly infestation. Plant Signal. Behav. 2016, 11, e1179420. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kannaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenstrom, E.; Niinemets, U. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, X.; Luo, Y.; Gong, Z.; Hu, X.; Wu, M.; Liu, Y.; Yan, F.; Zhang, X.; Zhang, W.; et al. R2R3 MYB-dependent auxin signalling regulates trichome formation, and increased trichome density confers spider mite tolerance on tomato. Plant Biotechnol. J. 2021, 19, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Sun, L.; Dong, M.; Fan, S.; Shi, K.; Qu, Y.; Zhu, L.; Shi, J.; Wang, W.; Liu, Y.; et al. Novel players in organogenesis and flavonoid biosynthesis in cucumber glandular trichomes. Plant Physiol. 2023, 192, 2723–2736. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Li, L.; Zhang, F.; Wang, M. Cloning and expression analysis of a trypsin inhibitor gene AmTI from Ammopiptanthus mongolicus. J. Inn. Mong. Agr. Univ. 2012, 33, 103–108. [Google Scholar] [CrossRef]
- Green, T.R.; Ryan, C.A. Wound-induced proteinase inhibitor in plant leaves: A possible defense mechanism against insects. Science 1972, 175, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cheng, J.; Yin, M.; Wu, J. NaMLP, a new identified Kunitz trypsin inhibitor regulated synergistically by JA and ethylene, confers Spodoptera litura resistance in Nicotiana attenuata. Plant Cell Rep. 2023, 42, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Song, N.; Chen, S.; Wu, J. NaKTI2, a Kunitz trypsin inhibitor transcriptionally regulated by NaWRKY3 and NaWRKY6, is required for herbivore resistance in Nicotiana attenuata. Plant Cell Rep. 2020, 40, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Collinge, D.B.; Kragh, K.M.; Mikkelsen, J.D.; Nielsen, K.K.; Rasmussen, U.; Vad1, K. Plant chitinases. Plant J. 1993, 3, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Alves, P.C.M.S.; Ahmad, I.; Gaffoor, I.; Acevedo, F.E.; Peiffer, M.; Jin, S.; Han, Y.; Shakeel, S.; Felton, G.W.; et al. Turnabout is fair play: Herbivory-induced plant chitinases excreted in fall armyworm frass suppress herbivore defenses in maize. Plant Physiol. 2016, 171, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Li, Z.; Shi, W.; Gao, F.; Zhou, Y.; Zhang, G.; Feng, J. Genome-wide identification and expression analyses of the chitinases under cold and osmotic stress in Ammopiptanthus nanus. Genes 2019, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Louis, J.; Ayre, B.G.; Reese, J.C.; Pegadaraju, V.; Shah, J. TREHALOSE PHOSPHATE SYNTHASE11-dependent trehalose metabolism promotes Arabidopsis thaliana defense against the phloem-feeding insect Myzus persicae. Plant J. 2011, 67, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, H.; Shen, Y. Effects of MeJA fumigation to leaves on ion flux in apical region of Ammopiptanthus nanus and salt resistance. Plant Physiol. J. 2013, 50, 477–482. [Google Scholar] [CrossRef]
- Mishra, S.; Roychowdhury, R.; Ray, S.; Hada, A.; Kumar, A.; Sarker, U.; Aftab, T.; Das, R. Salicylic acid (SA)-mediated plant immunity against biotic stresses: An insight on molecular components and signaling mechanism. Plant Stress. 2024, 11, 100427. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [PubMed]
- Chen, J.; Clinton, M.; Qi, G.; Wang, D.; Liu, F.; Fu, Z.Q.; Spoel, S. Reprogramming and remodeling: Transcriptional and epigenetic regulation of salicylic acid-mediated plant defense. J. Exp. Bot. 2020, 71, 5256–5268. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, C.; Gobbato, E.; Choi, J.; Riemann, M.; Johnston, M.G.; Summers, W.; Carbonnel, S.; Mansfield, C.; Yang, S.-Y.; Nadal, M.; et al. Rice perception of symbiotic arbuscular mycorrhizal fungi requires the karrikin receptor complex. Science 2015, 350, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, T.; Wang, Y.; Yang, J.; Yang, X. Growth promotion and mechanism of arbuscular mycorrhizal fungi (AMF) on Ammopiptanthus mongolicus seedlings. Arid. Zone Res. 2023, 40, 78–89. [Google Scholar]
- Ma, J.; Wang, W.; Yang, J.; Qin, S.; Yang, Y.; Sun, C.; Pei, G.; Zeeshan, M.; Liao, H.; Liu, L.; et al. Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 2022, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front. Plant Sci. 2018, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.P.; Oresnik, I.J. The rhizobium-legume symbiosis: Co-opting successful stress management. Front. Plant Sci. 2022, 12, 796045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, X.; Huo, H.; Yuan, G.; Sun, Y.; Zhang, D.; Cao, Y.; Xu, L.; Wei, G. Phylogenetic diversity of Ammopiptanthus rhizobia and distribution of rhizobia associated with Ammopiptanthus mongolicus in diverse regions of northwest China. Microb. Ecol. 2016, 72, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, X.; Hou, L.; Ren, Y.; Wang, S.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 7896. [Google Scholar] [CrossRef]
- Hou, L.; Yu, J.; Zhao, L.; He, X. Dark septate endophytes improve the growth and the tolerance of medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed]


| Order | Family | Feeding Site | Representative Species |
|---|---|---|---|
| Coleooptera | Teneberionidae | Leaves; flowers; young fruits; seeds; roots | Platyope mongolica Faldermann; Anatolica mucronate Reitter; Platyope ordossica Semenow; Anatolica amoenula Reitter; Pterocoma reiterri Frivoldszky et al. |
| Curculionidea | Leaves; flowers; young fruits; seeds; roots | Deracanthus jakovlevi Suvorov; Chloebius psittacinus Boheman et al. | |
| Rutelidae | Leaves; flowers; young fruits | Proagopertha lucidula Faldermann | |
| Melolonthidae | Leaves; flowers; young fruits | Chioneosoma reitteri Semenov | |
| Meloidae | Flowers; young fruits | Mylabris mongolica (Dokhlouroff) | |
| Cleridea | Flowers; young fruits | Trichodes sinae Chevrolat | |
| Chrysomelidae | Leaves; flowers; young fruits | Diorhabda rybakowi Weise | |
| Elateridae | Roots | Pleonomus canaliculatus Faldermann | |
| Homoptera | Psyllidae | Leaves; flowers; young fruits | Psylla mongolicus Loginova |
| Lepidoptera | Lymantriidae | Leaves; young fruits | Orgyia ericae Germar |
| Hemiptera | Lygaeidae | Flowers; young fruits | Lygaeus equestris Linnaeus |
| Pentatomidae | Flowers; young fruits | Rhaphigaster nebulosa Poda; Brachynema germarii Kolenati | |
| Miridae | Flowers | ||
| Orthoptera | Tettigoniidae | Leaves; flowers; young fruits; seeds | |
| Thysanoptera | Thripidae | Flowers |
| Category | Pathway | Description | Involved | Stresses Addressed |
|---|---|---|---|---|
| Physical Defense | Trichome Formation | Increase density to cope with stress; dense trichomes on leaves and stems act as a physical barrier against pests and pathogens, and adapt to arid environments. | Trichomes | Drought, extreme temperatures, UV radiation, pathogens and pests |
| Ion Regulation | Selective Absorption of Ca2+, H+, K+, and Na+ in Roots | Such activation of H+-ATPase enhances H+ efflux, providing a proton motive force for the Na+/H+ antiporter to pump excess Na+ out of the cell. | H+-ATPase, Na+/H+ antiporter, calcium channel, etc. | Pests, salt stress |
| Chemical Defense | Stress Proteins | Involved in physiological processes induced by various stress factors | Trypsin inhibitors | Dehydration, low temperature, salt stress |
| Dehydrins | Water stress, salt stress, extreme temperatures, pathogen infections | |||
| Plant Defense Enzyme | Synthesis of plant defense enzymes | Chitinase; trehalose phosphate synthase | Pathogens, pests, and extreme temperatures; osmotic stress | |
| Plant Hormones | Plant hormones play pivotal roles in the plant’s response to various stresses and in signaling pathways. | SA, JA, ABA et al. | Water stress, biotic stress, extreme temperatures, salt stress, pathogens, pests | |
| Symbiotic Relationships | Mycorrhizal and Endophytic Symbiosis | Form symbiotic relationships with fungi, enhancing nutrient absorption and adaptation. | AMF, DSE et al. | Drought, pathogens, heavy metal pollution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liang, S.; Liu, Y.; Chen, Y. Advances in Endangered Plant Research: Ammopiptanthus’s Responses to Biotic and Abiotic Stressors. Forests 2024, 15, 890. https://doi.org/10.3390/f15050890
Wang S, Liang S, Liu Y, Chen Y. Advances in Endangered Plant Research: Ammopiptanthus’s Responses to Biotic and Abiotic Stressors. Forests. 2024; 15(5):890. https://doi.org/10.3390/f15050890
Chicago/Turabian StyleWang, Shuyao, Shenghua Liang, Yahui Liu, and Yingying Chen. 2024. "Advances in Endangered Plant Research: Ammopiptanthus’s Responses to Biotic and Abiotic Stressors" Forests 15, no. 5: 890. https://doi.org/10.3390/f15050890




