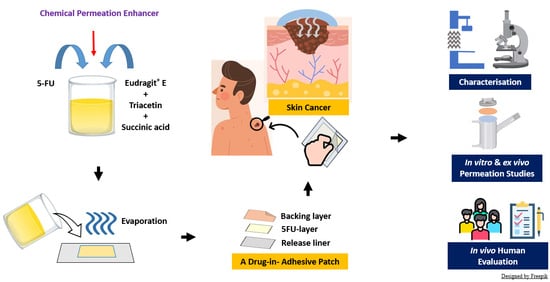
Enhanced Skin Permeation of 5-Fluorouracil through Drug-in-Adhesive Topical Patches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Slide Crystallisation Studies
2.3. Preparation of 5-FU-Loaded Drug-in-Adhesive Patches
2.4. HPLC Method for Quantification of 5-FU
2.5. Characterisations of the Patches
2.5.1. Content Uniformity
2.5.2. Thickness, Weight, Folding Endurance, and Surface pH
2.5.3. Percentage Moisture Content
2.5.4. Percentage Moisture Absorption
2.5.5. Scanning Electron Microscopy Analysis
2.5.6. Probe Tack Test
2.5.7. The 90° Peel Adhesion Test
2.6. Ex Vivo Permeation and Deposition Studies
2.6.1. Porcine Skin Preparation
2.6.2. Skin Integrity Evaluation
2.6.3. Ex Vivo Permeation Studies
2.6.4. Quantification of 5-FU Deposition in Skin Samples
2.7. In Vitro Permeation Studies Using Strat-M® Membranes
2.8. Stability Studies
2.9. In Vivo Evaluation of the Adhesive Properties and Safety of the Patches
2.10. Statistical Analysis
3. Results and Discussion
3.1. Determination of Clinically Relevant 5-FU Dose in the Patches
3.2. Slide Crystallisation Studies
3.3. Preparation of 5-FU-Loaded Drug-in-Adhesive Patches
3.4. Effect of Permeation Enhancers on Adhesive Properties of 5-FU-Loaded Drug-in-Adhesive Patches
3.5. Ex Vivo Permeation and Deposition Studies
3.6. In Vitro Permeation Studies for 72 H
3.7. Stability Studies
3.8. In Vivo Evaluation of the Adhesive Properties and Safety of the Patches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceilley, R.I. Mechanisms of action of topical 5-fluorouracil: Review and implications for the treatment of dermatological disorders. J. Dermatol. Treat. 2012, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bagatin, E. 5-fluorouracil for actinic keratoses. Expert Rev. Dermatol. 2010, 5, 131–139. [Google Scholar] [CrossRef]
- Kim, S.; Fouladian, P.; Afinjuomo, F.; Song, Y.; Youssef, S.H.; Vaidya, S.; Garg, S. Effect of plasticizers on drug-in-adhesive patches containing 5-fluorouracil. Int. J. Pharm. 2022, 611, 121316. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Zawislak, A.A.; Woolfson, A.D. Design and physicochemical characterisation of a bioadhesive patch for dose-controlled topical delivery of imiquimod. Int. J. Pharm. 2006, 307, 318–325. [Google Scholar] [CrossRef]
- Kim, S.; Day, C.M.; Song, Y.; Holmes, A.; Garg, S. Innovative Topical Patches for Non-Melanoma Skin Cancer: Current Challenges and Key Formulation Considerations. Pharmaceutics 2023, 15, 2577. [Google Scholar] [CrossRef]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; Gerber, M.; du Plessis, L.; du Preez, J.; du Plessis, J. Topical Delivery of 5-Fluorouracil from Pheroid Formulations and the In vitro Efficacy Against Human Melanoma. AAPS PharmSciTech 2015, 16, 1390–1399. [Google Scholar] [CrossRef]
- Mutalik, S.; Shetty, P.K.; Kumar, A.; Kalra, R.; Parekh, H.S. Enhancement in deposition and permeation of fluorouracil through human epidermis assisted by peptide dendrimers. Drug Deliv. 2014, 21, 44–54. [Google Scholar] [CrossRef]
- Crisóstomo, L.C.C.F.; Carvalho, G.S.G.; Leal, L.K.A.M.; de Araújo, T.G.; Nogueira, K.A.B.; da Silva, D.A.; de Oliveira Silva Ribeiro, F.; Petrilli, R.; Eloy, J.O. Sorbitan Monolaurate–Containing Liposomes Enhance Skin Cancer Cell Cytotoxicity and in Association with Microneedling Increase the Skin Penetration of 5-Fluorouracil. AAPS PharmSciTech 2022, 23, 212. [Google Scholar] [CrossRef]
- Yamane, M.A.; Williams, A.C.; Barry, B.W. Effects of terpenes and oleic acid as skin penetration enhancers towards 5-fluorouracil as assessed with time; permeation, partitioning and differential scanning calorimetry. Int. J. Pharm. 1995, 116, 237–251. [Google Scholar] [CrossRef]
- Gadag, S.; Narayan, R.; Nayak, Y.; Garg, S.; Nayak, U.Y. Design, development and evaluation of Resveratrol transdermal patches for breast cancer therapy. Int. J. Pharm. 2023, 632, 122558. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Thakkar, H.P. QbD-driven development of dissolving microneedle patch loaded with ultradeformable liposomes encapsulated Noopept: Exploring a patient friendly, once-daily option to manage dementia. Eur. J. Pharm. Sci. 2021, 164, 105909. [Google Scholar] [CrossRef]
- Tuntiyasawasdikul, S.; Sripanidkulchai, B. Development and clinical trials on anti-inflammatory effect of transdermal patch containing a combination of Kaempferia parviflora and Curcuma longa extracts. J. Drug Deliv. Sci. Technol. 2022, 68, 103093. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, G.; Du, F.; Yi, J.; Quan, L.; Liu, H.; Zhou, X.; Gong, W.; Han, J.; Wang, Y.; et al. Formulation and Evaluation of a Drug-in-Adhesive Patch for Transdermal Delivery of Colchicine. Pharmaceutics 2022, 14, 2245. [Google Scholar] [CrossRef]
- Puri, A.; Bhattaccharjee, S.A.; Zhang, W.; Clark, M.; Singh, O.; Doncel, G.F.; Banga, A.K. Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV. Pharmaceutics 2019, 11, 173. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, R.B.; Singh, J. Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. Int. J. Pharm. 2005, 298, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Skorik, Y.A. Patches as Polymeric Systems for Improved Delivery of Topical Corticosteroids: Advances and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 12980. [Google Scholar] [CrossRef] [PubMed]
- Ganti, S.S.; Bhattaccharjee, S.A.; Murnane, K.S.; Blough, B.E.; Banga, A.K. Formulation and evaluation of 4-benzylpiperidine drug-in-adhesive matrix type transdermal patch. Int. J. Pharm. 2018, 550, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.H.; Afinjujuomo, F.; Song, Y.; Garg, S. Development of a novel chromatographic method for concurrent determination of 5-fluorouracil and cisplatin: Validation, greenness evaluation, and application on drug-eluting film. Microchem. J. 2021, 2021, 106510. [Google Scholar] [CrossRef]
- Al-Mayahy, M.H.; Sabri, A.H.; Rutland, C.S.; Holmes, A.; McKenna, J.; Marlow, M.; Scurr, D.J. Insight into imiquimod skin permeation and increased delivery using microneedle pre-treatment. Eur. J. Pharm. Biopharm. 2019, 139, 33–43. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Schwartz, R.A. Topical pharmacotherapy for skin cancer: Part I. Pharmacology. J. Am. Acad. Dermatol. 2014, 70, 965.e1–965.e12; quiz 977–968. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. Australian Product Information—Efudix Cream. 2020. Available online: https://www.tga.gov.au/resources/artg/13721 (accessed on 4 February 2024).
- Rhodes, J.; Clay, C.; Phillips, M. The surface area of the hand and the palm for estimating percentage of total body surface area: Results of a meta-analysis. Br. J. Dermatol. 2013, 169, 76–84. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Drug crystallization—Implications for topical and transdermal delivery. Expert Opin. Drug Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef]
- Kim, S.; Youssef, S.H.; Song, Y.; Garg, S. Development and application of a chromatographic method for simultaneous quantification of 5-fluorouracil and imiquimod in drug-in-adhesive topical patches. Sustain. Chem. Pharm. 2022, 27, 100711. [Google Scholar] [CrossRef]
- Chauhan, M.K.; Sharma, P.K. Optimization and characterization of rivastigmine nanolipid carrier loaded transdermal patches for the treatment of dementia. Chem. Phys. Lipids 2019, 224, 104794. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.R.; Ahmad, M.; Abrar, A.; Sarfraz, R.M.; Mahmood, A. Formulation design and development of matrix diffusion controlled transdermal drug delivery of glimepiride. Drug Des. Dev. Ther. 2018, 12, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Lee, C.-J.; Lin, Y.-Y. The Effect of Plasticizers on Compatibility, Mechanical Properties, and Adhesion Strength of Drug-Free Eudragit E Films. Pharm. Res. 1991, 8, 1137–1143. [Google Scholar] [CrossRef]
- Kanikkannan, N.; Andega, S.; Burton, S.; Babu, R.J.; Singh, M. Formulation and in vitro evaluation of transdermal patches of melatonin. Drug Dev. Ind. Pharm. 2004, 30, 205–212. [Google Scholar] [CrossRef]
- Rajabalaya, R. Studies on the effect of plasticizer on in vitro release and ex vivo permeation from Eudragit E 100 based chlorpheniramine maleate matrix type transdermal delivery system. J. Excip. Food Chem. 2010, 1, 3–12. [Google Scholar]
- Cilurzo, F.; Gennari, C.G.; Minghetti, P. Adhesive properties: A critical issue in transdermal patch development. Expert Opin. Drug Deliv. 2012, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.I.; Katas, H.; Amin, M.C.I.M.; Ng, S.-F.; Zulfakar, M.H.; Jamil, A. In-vivo dermal pharmacokinetics, efficacy, and safety of skin targeting nanoparticles for corticosteroid treatment of atopic dermatitis. Int. J. Pharm. 2016, 507, 72–82. [Google Scholar] [CrossRef]
- Kim, S.; Abdella, S.; Abid, F.; Afinjuomo, F.; Youssef, S.H.; Holmes, A.; Song, Y.; Vaidya, S.; Garg, S. Development and Optimization of Imiquimod-Loaded Nanostructured Lipid Carriers Using a Hybrid Design of Experiments Approach. Int. J. Nanomed. 2023, 18, 1007–1029. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Z.; Yang, Y.; Li, Y.; Wang, C. Novel skin permeation enhancers based on amino acid ester ionic liquid: Design and permeation mechanism. Int. J. Pharm. 2020, 576, 119031. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic Study to Determine the Structural Similarities between Artificial Membrane Strat-M™ and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of Formulation Parameters on Permeation of Ibuprofen from Topical Formulations Using Strat-M® Membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane properties for permeability testing: Skin versus synthetic membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-M™. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a Capsaicin-Loaded Nanoemulsion for Improving Skin Penetration. J. Agric. Food Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Milanowski, B.; Wosicka-Frąckowiak, H.; Główka, E.; Sosnowska, M.; Woźny, S.; Stachowiak, F.; Suchenek, A.; Wilkowski, D. Optimization and Evaluation of the In vitro Permeation Parameters of Topical Products with Non-Steroidal Anti-Inflammatory Drugs through Strat-M® Membrane. Pharmaceutics 2021, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Boelsma, E.; Tanojo, H.; Boddé, H.E.; Ponec, M. Assessment of the potential irritancy of oleic acid on human skin: Evaluation in vitro and in vivo. Toxicol. Vitr. 1996, 10, 729–742. [Google Scholar] [CrossRef]






| Formulation | Ingredients | |||||||
|---|---|---|---|---|---|---|---|---|
| 5-FU (g) | EuE (g) | Triacetin (g) | Succinic Acid (g) | Oleic Acid (g) | Transcutol® (g) | Tween 20 (g) | MeOH (g) | |
| Patch-CONT | 0.4 | 8 | 3.2 | 0.4 | To 20 g | |||
| Patch-OA | 0.8 | |||||||
| Patch-TRAN | 0.8 | |||||||
| Patch-T20 | 0.8 | |||||||
| Formulation | * Thickness (µm) | ^ Weight (mg/cm2) | Folding Endurance (Times) | Surface pH | % Moisture Content | % Moisture Absorption | Drug Content (µg/cm2) |
|---|---|---|---|---|---|---|---|
| Patch-CONT | 266.7 ± 15.1 | 12.4 ± 0.4 | >100 | 6.06 ± 0.03 | 2.16 ± 0.65 | 3.42 ± 1.00 | 424.8 ± 24.2 |
| Patch-OA | 286.7 ± 19.7 | 13.9 ± 1.0 | >100 | 6.33 ± 0.07 | 2.08 ± 0.99 | 3.38 ± 1.34 | 465.2 ± 42.6 |
| Patch-TRAN | 290.0 ± 11.0 | 13.4 ± 0.6 | >100 | 6.21 ± 0.04 | 1.98 ± 0.55 | 2.67 ± 0.85 | 462.7 ± 47.2 |
| Patch-T20 | 285.0 ± 10.5 | 14.0 ± 0.8 | >100 | 6.17 ± 0.06 | 1.60 ± 0.35 | 3.61 ± 0.46 | 447.7 ± 35.7 |
| Question | Score | Key Findings | |
|---|---|---|---|
| Patch-OA | Patch-TRAN | ||
| Q1. Ease of removal from the release liner | 4.9 ± 0.3 | 4.9 ± 0.3 | Most participants found the removal process of the patches from the release liner extremely easy; one participant reported it could potentially improve. |
| Q2. Initial tack | 4.8 ± 0.4 | 4.8 ± 0.4 | Most participants agreed that both patches provided excellent initial adhesion. |
| Q3. Ongoing adhesion for 72 h | 4.8 ± 0.4 | 4.9 ± 0.3 | Most participants reported suitable ongoing adhesion of the patches over 72 h on both arms; however, three participants reported that one Patch-OA accidentally fell off one arm after 48 to 60 h; one participant experienced the adhesion failure of one of Patch-TRAN at 60 h. |
| Q4. Wear comfort | 5.0 | 5.0 | All participants agreed that both patches were extremely comfortable to wear for 72 h. |
| Q5. Ease of removal from skin at 72 h | 4.5 ± 0.8 | 4.5 ± 0.7 | Three participants commented that both patches required a bit of effort to remove from the skin after 72 h. |
| Q6. Side effects | See key findings | Nil | One participant reported mild redness and swelling from Patch-OA around the application area; another participant experienced slight blemish surrounding the application area; symptoms subsided in 24 h; no side effects were reported from Patch-TRAN |
| Other comments | - | - | One participant commented that the transparency of the patches blended well with the skin, providing a good aesthetical look. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Youssef, S.H.; Lee, K.M.K.; Song, Y.; Vaidya, S.; Garg, S. Enhanced Skin Permeation of 5-Fluorouracil through Drug-in-Adhesive Topical Patches. Pharmaceutics 2024, 16, 379. https://doi.org/10.3390/pharmaceutics16030379
Kim S, Youssef SH, Lee KMK, Song Y, Vaidya S, Garg S. Enhanced Skin Permeation of 5-Fluorouracil through Drug-in-Adhesive Topical Patches. Pharmaceutics. 2024; 16(3):379. https://doi.org/10.3390/pharmaceutics16030379
Chicago/Turabian StyleKim, Sangseo, Souha H. Youssef, Kyung Min Kirsten Lee, Yunmei Song, Sachin Vaidya, and Sanjay Garg. 2024. "Enhanced Skin Permeation of 5-Fluorouracil through Drug-in-Adhesive Topical Patches" Pharmaceutics 16, no. 3: 379. https://doi.org/10.3390/pharmaceutics16030379