Using Polymers as Crystal Inhibitors to Prevent the Crystallization of the Rotigotine Patch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Screening of Crystal Growth Inhibitors
2.2.2. Effect of Polymer Ratio on ROT Crystallization
2.2.3. Characterization of ROT Patch
X-ray Diffraction (XRD)
Scanning Electron Microscope (SEM)
Energy Dispersive X-ray Spectroscopy (EDS)
2.2.4. Crystal Inhibition Mechanism
Molecular Docking
Determination of Crystal Nucleation Time and Growth Rate
2.2.5. In Vitro Skin Permeation Test
3. Results and Discussion
3.1. Screening of Potential Inhibitors for Crystal Growth
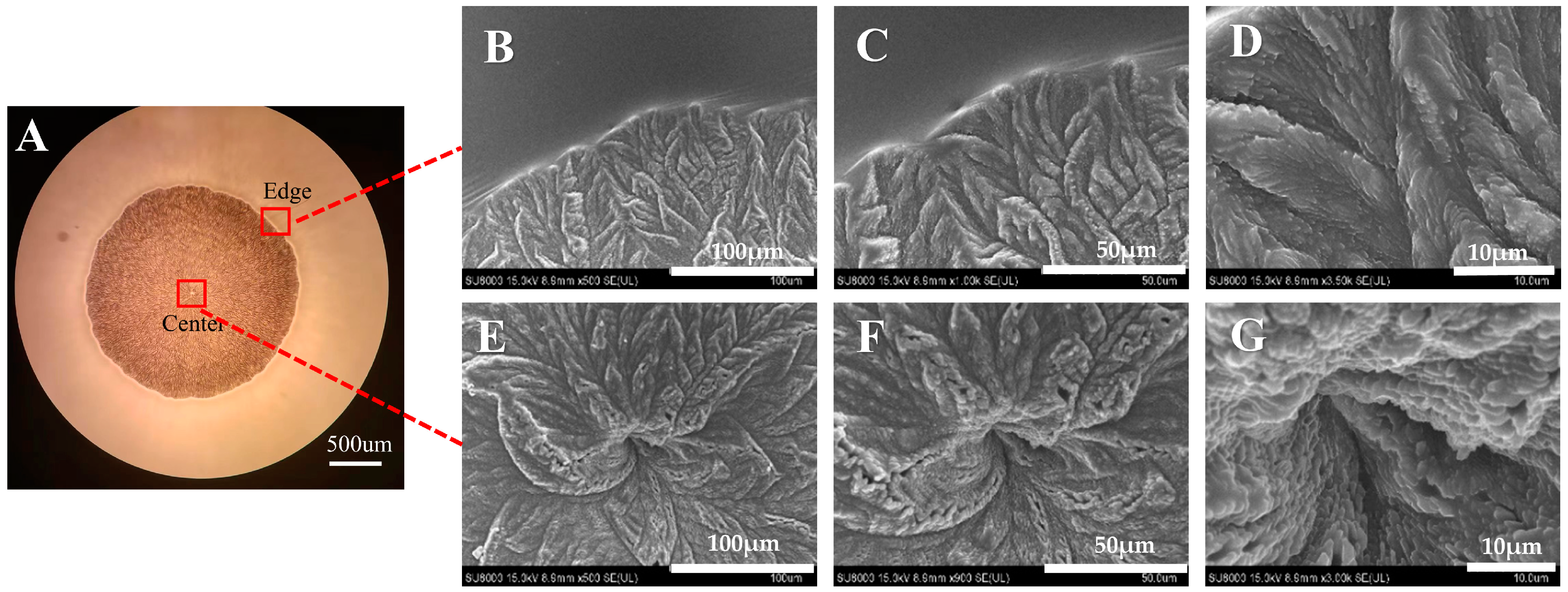
3.2. Crystal Morphology Observation of ROT
3.3. Effect of Polymer Ratios on ROT Crystallization
3.4. Characterization of ROT Crystallization in Different Polymers
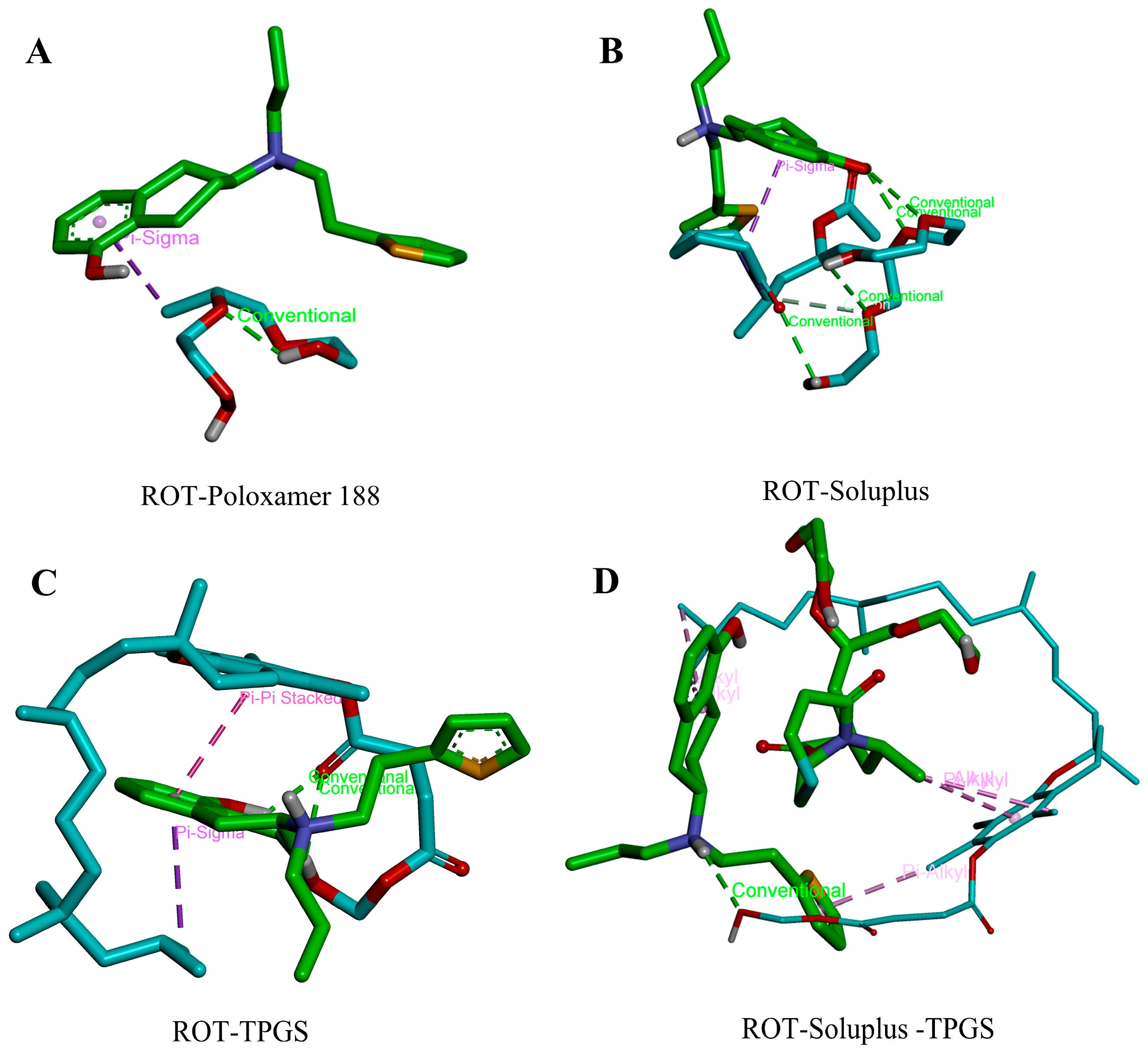
3.5. Molecular Docking of ROT with Different Polymers
3.6. Evaluation of Crystal Nucleation Time and Growth Rate
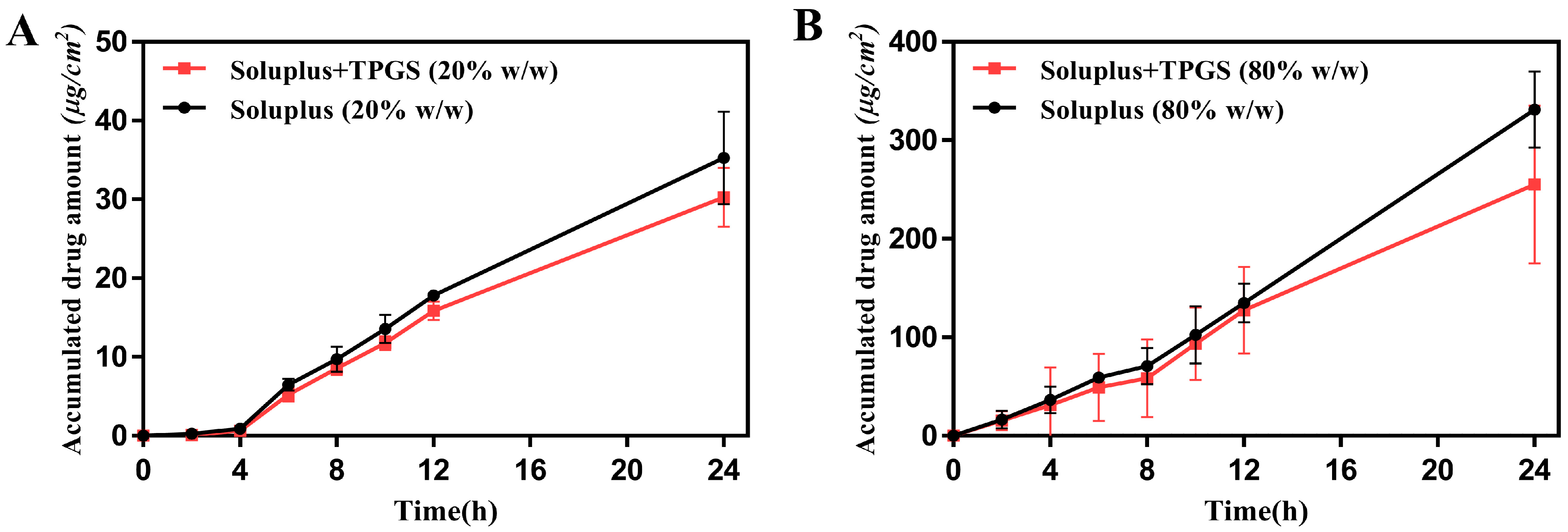
3.7. In Vitro Skin Permeation Study of ROT Patch
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raeder, V.; Boura, I.; Leta, V.; Jenner, P.; Reichmann, H.; Trenkwalder, C.; Klingelhoefer, L.; Chaudhuri, K.R. Rotigotine Transdermal Patch for Motor and Non-Motor Parkinson’s Disease: A Review of 12 Years’ Clinical Experience. CNS Drugs 2021, 35, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Ferraiolo, M.; Hermans, E. The Complex Molecular Pharmacology of the Dopamine D2 Receptor: Implications for Pramipexole, Ropinirole, and Rotigotine. Pharmacol. Ther. 2023, 245, 108392. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Barbosa, R.; Rascol, O. Off-Time Treatment Options for Parkinson’s Disease. Neurol. Ther. 2023, 12, 391–424. [Google Scholar] [CrossRef]
- Boroojerdi, B.; Wolff, H.-M.; Braun, M.; Scheller, D.K.A. Rotigotine Transdermal Patch for the Treatment of Parkinson’s Disease and Restless Legs Syndrome. Drugs Today Barc. Spain 2010, 46, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Rogers, R.D. Ionic Liquids: New Forms of Active Pharmaceutical Ingredients with Unique, Tunable Properties. Chem. Rev. 2023, 123, 11894–11953. [Google Scholar] [CrossRef] [PubMed]
- McAfee, D.A.; Hadgraft, J.; Lane, M.E. Rotigotine: The First New Chemical Entity for Transdermal Drug Delivery. Eur. J. Pharm. Biopharm. 2014, 88, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An Extraordinary Example of Conformational Polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.V.; Kandasubramanian, B.; Sidharth, S. A Review on Recent Trends in Bio-Based Pressure Sensitive Adhesives. J. Adhes. 2023, 99, 2145–2166. [Google Scholar] [CrossRef]
- Hancock, B.C.; Carlson, G.T.; Ladipo, D.D.; Langdon, B.A.; Mullarney, M.P. Comparison of the Mechanical Properties of the Crystalline and Amorphous Forms of a Drug Substance. Int. J. Pharm. 2002, 241, 73–85. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Céolin, R. Rotigotine: Unexpected Polymorphism with Predictable Overall Monotropic Behavior. J. Pharm. Sci. 2015, 104, 4117–4122. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Drug Crystallization—Implications for Topical and Transdermal Delivery. Expert Opin. Drug Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Al-Shboul, T.; Sagala, F.; Nassar, N.N. Role of Surfactants, Polymers, Nanoparticles, and Its Combination in Inhibition of Wax Deposition and Precipitation: A Review. Adv. Colloid Interface Sci. 2023, 315, 102904. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Taylor, L.S. Small Scale Screening to Determine the Ability of Different Polymers to Inhibit Drug Crystallization upon Rapid Solvent Evaporation. Mol. Pharm. 2010, 7, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Fakhreeva, A.V.; Nosov, V.V.; Voloshin, A.I.; Dokichev, V.A. Polysaccharides as Effective and Environmentally Friendly Inhibitors of Scale Deposition from Aqueous Solutions in Technological Processes. Polymers 2023, 15, 1478. [Google Scholar] [CrossRef] [PubMed]
- Latsch, S.; Selzer, T.; Fink, L.; Kreuter, J. Determination of the Physical State of Norethindrone Acetate Containing Transdermal Drug Delivery Systems by Isothermal Microcalorimetry, X-ray Diffraction, and Optical Microscopy. Eur. J. Pharm. Biopharm. 2004, 57, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.F.; Ang, K.P.; Sethi, G.; Looi, C.Y. Recent Advancement of Medical Patch for Transdermal Drug Delivery. Medicina 2023, 59, 778. [Google Scholar] [CrossRef]
- Tahir, M.A.; Ali, M.E.; Lamprecht, A. Nanoparticle Formulations as Recrystallization Inhibitors in Transdermal Patches. Int. J. Pharm. 2020, 575, 118886. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L. Prodrug Strategy for Enhancing Drug Delivery via Skin. Curr. Drug Discov. Technol. 2006, 3, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Kotiyan, P.N.; Vavia, P.R. Eudragits: Role as Crystallization Inhibitors in Drug-in-Adhesive Transdermal Systems of Estradiol. Eur. J. Pharm. Biopharm. 2001, 52, 173–180. [Google Scholar] [CrossRef]
- Ma, X.; Taw, J.; Chiang, C.-M. Control of Drug Crystallization in Transdermal Matrix System. Int. J. Pharm. 1996, 142, 115–119. [Google Scholar] [CrossRef]
- Mackellar, A.J.; Buckton, G.; Newton, J.M.; Orr, C.A. The Controlled Crystallisation of a Model Powder: 2. Investigation into the Mechanism of Action of Poloxamers in Changing Crystal Properties. Int. J. Pharm. 1994, 112, 79–85. [Google Scholar] [CrossRef]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications—A Review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.; Zhang, H.; Zhao, C.; Sun, L.; Zhao, Y. Emerging Functional Biomaterials as Medical Patches. ACS Nano 2021, 15, 5977–6007. [Google Scholar] [CrossRef] [PubMed]
- Overhoff, K.A.; McConville, J.T.; Yang, W.; Johnston, K.P.; Peters, J.I.; Williams, R.O. Effect of Stabilizer on the Maximum Degree and Extent of Supersaturation and Oral Absorption of Tacrolimus Made by Ultra-Rapid Freezing. Pharm. Res. 2008, 25, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Riess, G. Micellization of Block Copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced Drug Delivery Devices via Self-Assembly of Amphiphilic Block Copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Kuldipkumar, A.; Tan, Y.T.F.; Goldstein, M.; Nagasaki, Y.; Zhang, G.G.Z.; Kwon, G.S. Amphiphilic Block Copolymer as a Crystal Habit Modifier. Cryst. Growth Des. 2005, 5, 1781–1785. [Google Scholar] [CrossRef]
- Gao, Y.; Olsen, K.W. Drug–Polymer Interactions at Water–Crystal Interfaces and Implications for Crystallization Inhibition: Molecular Dynamics Simulations of Amphiphilic Block Copolymer Interactions with Tolazamide Crystals. J. Pharm. Sci. 2015, 104, 2132–2141. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.H. Transdermal Drug Delivery. In Drug Delivery; Schäfer-Korting, M., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 197, pp. 399–410. [Google Scholar]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent Advances in Transdermal Drug Delivery Systems: A Review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef]
- Oh, D.-W.; Chon, J.; Na, M.-J.; Kang, J.-H.; Park, E.-S.; Rhee, Y.-S.; Kim, J.-Y.; Shin, D.H.; Kim, D.-W.; Park, C.-W. Preparation and Physicochemical Characterization of Rotigotine Drug-in-Adhesive Patch Containing Crystal Growth Inhibitor. J. Drug Deliv. Sci. Technol. 2019, 53, 101193. [Google Scholar] [CrossRef]
- Isooka, N.; Miyazaki, I.; Kikuoka, R.; Wada, K.; Nakayama, E.; Shin, K.; Yamamoto, D.; Kitamura, Y.; Asanuma, M. Dopaminergic Neuroprotective Effects of Rotigotine via 5-HT1A Receptors: Possibly Involvement of Metallothionein Expression in Astrocytes. Neurochem. Int. 2020, 132, 104608. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liang, R.; Liu, W.; Sha, C.; Li, Y.; Sun, K. Effect of Palmitic Acid on the Characteristics and Release Profiles of Rotigotine-Loaded Microspheres. Pharm. Dev. Technol. 2016, 21, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qu, X.; Song, L.; Shang, R.; Wan, X.; Fang, L. Investigation on the Effect of Deep Eutectic Formation on Drug-Polymer Miscibility and Skin Permeability of Rotigotine Drug-in-Adhesive Patch. Int. J. Pharm. 2020, 574, 118852. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Asgharnejad, M.; Bauer, L.; Ramirez, F.; Jeon, B. Evaluation of Rotigotine Transdermal Patch for the Treatment of Depressive Symptoms in Patients with Parkinson’s Disease. Expert Opin. Pharmacother. 2016, 17, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, T.; Xue, J.; Xu, H.; Wu, S. Hydrogen Bonding Principle-Based Molecular Design of a Polymer Excipient and Impacts on Hydrophobic Drug Properties: Molecular Simulation and Experiment. Biomacromolecules 2023, 24, 1675–1688. [Google Scholar] [CrossRef]
- Dai, W.-G.; Dong, L.C.; Li, S.; Deng, Z. Combination of Pluronic/Vitamin E TPGS as a Potential Inhibitor of Drug Precipitation. Int. J. Pharm. 2008, 355, 31–37. [Google Scholar] [CrossRef]
- Akash, S.Z.; Lucky, F.Y.; Hossain, M.; Bepari, A.K.; Rahman, G.M.S.; Reza, H.M.; Sharker, S.M. Remote Temperature-Responsive Parafilm Dermal Patch for On-Demand Topical Drug Delivery. Micromachines 2021, 12, 975. [Google Scholar] [CrossRef]








| Polymer:ROT (w/w) | Samples | Nucleation Time |
|---|---|---|
| 1:1 | ROT | 22 h |
| Poloxamer188 | 36 h | |
| Soluplus | — | |
| Soluplus-TPGS | — | |
| 1:2 | ROT | 22 h |
| Poloxamer188 | 32 h | |
| Soluplus | — | |
| Soluplus-TPGS | — | |
| 1:4 | ROT | 23 h |
| Poloxamer188 | 26 h | |
| Soluplus | 11 d | |
| Soluplus-TPGS | 19 d | |
| 1:8 | ROT | 22 h |
| Poloxamer188 | 22 h | |
| Soluplus | 4 d | |
| Soluplus-TPGS | 8 d |
| Receptor | Ligand | Binding Free Energy (kcal/mol) |
|---|---|---|
| ROT | Poloxamer 188 | −1.5 |
| Soluplus | −2.1 | |
| TPGS | −4.0 | |
| TPGS-Soluplus | −5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Li, X.; Liu, B.; Kong, J.; Wang, Q.; Gao, Z. Using Polymers as Crystal Inhibitors to Prevent the Crystallization of the Rotigotine Patch. Pharmaceutics 2024, 16, 630. https://doi.org/10.3390/pharmaceutics16050630
Liu Q, Li X, Liu B, Kong J, Wang Q, Gao Z. Using Polymers as Crystal Inhibitors to Prevent the Crystallization of the Rotigotine Patch. Pharmaceutics. 2024; 16(5):630. https://doi.org/10.3390/pharmaceutics16050630
Chicago/Turabian StyleLiu, Qiantong, Xing Li, Bo Liu, Jiahao Kong, Qing Wang, and Zhigang Gao. 2024. "Using Polymers as Crystal Inhibitors to Prevent the Crystallization of the Rotigotine Patch" Pharmaceutics 16, no. 5: 630. https://doi.org/10.3390/pharmaceutics16050630




