Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep)
Abstract
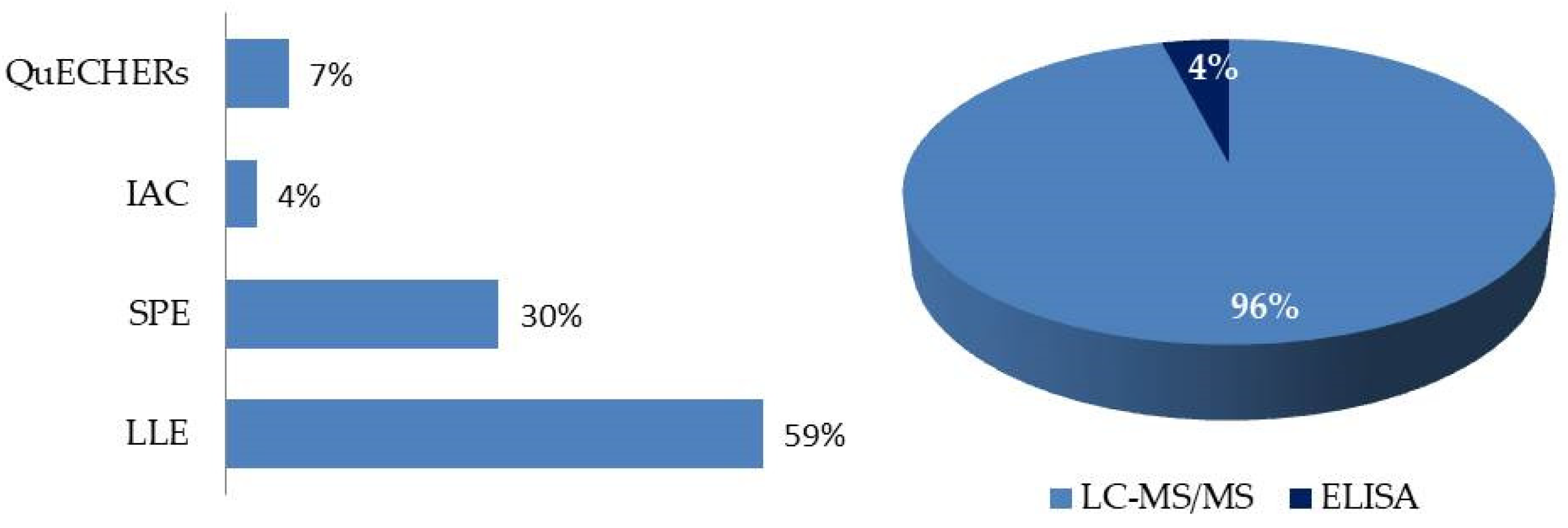
:1. Introduction
| Fungi | Mycotoxin | Ref. |
|---|---|---|
| Fusarium | DON, T-2, HT-2, DAS, NEO, NIV, 3-ADON, 15-ADON, FUS-X, FB1, FB2, FB3, ZEA, MON, ENNs, BEA | [7] |
| Aspergillus | AFB1, AFB2, AFG1, AFG2, OTA, FB2, FB4, STER, PAT | [9] |
| Penicillium | OTA, PAT, ROQC, CIT, cyclopiazonic acid | [10] |
2. Monitoring Mycotoxin Exposure in Food-Producing Animals
3. External Exposure
3.1. Analytical Methods
3.2. Feed Analysis
3.2.1. Ochratoxin A
3.2.2. Zearalenone
3.2.3. Deoxynivalenol and Its Derivatives
3.2.4. Aflatoxins
3.2.5. Fumonisins
3.2.6. T-2 and HT-2
3.2.7. Other Mycotoxins
3.2.8. Multiexposure
4. Internal Exposure
| Mycotoxins and Metabolites | Matrix | Animal | Year | Reference |
|---|---|---|---|---|
| OTA, OTα | Plasma Kidney, liver, muscle | Pig | 2023 | [109] |
| AFB1, AFB2, AFG1, AFG2, AFM1, OTA, OTB, ZEA, DON, 3- and 15-ADON, DOM-1, T-2, HT-2; STER, NEO, DAS, FUS-X, NIV (and their glucuronide or sulfate conjugates) | Plasma | Poultry Pig Sheep Cattle | 2023 | [112] |
| AFB1, AFB2, AFG1, AFG2, AFM1 and AFM2 | Plasma | Poultry Cattle | 2023 | [49] |
| DON, isoDON, DON-3GlcA, DON-15GlcA, DOM-1, DOM-3GlcA, DOM-15GlcA | Serum Urine | Pig | 2023 | [113] |
| OTA, OTα | Plasma Feces/excreta, urine liver, kidney, muscle, skin, fat | Poultry Pig | 2022 | [114] |
| FB1, FB2, FB3 HFB1, HFB2, HFB3 | Excreta | Poultry | 2022 | [115] |
| DON, 3 and 15-ADON, DOM-1, ZEA, α-ZEL, β-ZEL, α-ZAL, β-ZAL, ZAN, OTA, AFB1, AFB2, AFG1, AFM1, T-2, HT-2, OTα | Feces Serum | Pig | 2021 | [39] |
| DON, 3-ADON, 15-ADON, DOM-1, ZEA, α-ZEL, β-ZEL, α-ZAL, β-ZAL, ZAN, OTA, AFB1, AFB2, AFG1, AFM1, T-2, HT-2, NIV, TEN, AOH, AME, ATX-I, CIT, DAS, FUS-X, STER, T-2 triol, OTα, HFB1, DH-CIT, ENNs and BEA | Urine | Pig | 2021 | [116] |
| DON, DOM-1, ZEA, α-ZEL, OTA, OTα, CIT, DH-CIT | Plasma Urine | Pig | 2021 | [117] |
| CIT, DH-CIT | Plasma | Poultry Pig | 2020 | [107,118] |
| FM1, HFB1 | Plasma | Poultry | 2020 | [110] |
| DON, DOM-1, 3/15ADON, AFB1, AFM1, ENNA, ENNA1, ENNB, ENNB1, BEA, FB1, FB2, OTA, ZEA, α-ZEL, β-ZEL, α-ZAL, β-ZAL, ZAN, TEA, AOH, AME, T-2 | Plasma Urine Feces/excreta | Poultry Pig | 2019 | [44] |
| AFB1, DON, DON-s, DON-GlcA, ZEA, ZEA-GlcA | Plasma Feces/excreta Urine | Poultry Pig | 2019 | [100] |
| DON, DOM1, 3/15ADON, AFB1, AFM1, ENNA, ENNA1, ENNB, ENNB1, BEA, FB1, FB2, OTA, ZEA, α-ZEL, β-ZEL, α-ZAL, β-ZAL, ZAN, TEA, AOH, AME, T-2 | Plasma DBS | Poultry Pig | 2019 | [119] |
| ZEA, α-ZEL, βZEL, ZAN, α-ZAL, β-ZAL, ZEN14G, ZEN14S, ZEA-14GlcA, α-ZEL-14GlcA, α-ZEL-7GlcA, β-ZEL-14GlcA, and β-ZEL-16GlcA | Plasma | Pig | 2019 | [48] |
| AFM1, DON, DOM-1, ZEA, α-ZEL, β-ZEL, FB1, OTA, DOM1, | Urine | Pig | 2019 | [120] |
| AFB1, AF2, AFG1, AFG2, AFM1, AFP1, AFQ1, AFB1-N7-guanine | Feces/excreta Ileal content | Poultry | 2019 | [121] |
| FB1, FB2, FB3, pHFB1, HFB1, pHFB2, HFB2, FB3, pHFB3, HFB3 | Feces Urine Serum | Pig | 2018 | [111] |
| FB1, pHFB1, HFB1 and FB2 | Plasma | Poultry | 2018 | [122] |
| ZEA, ZAN, β-ZAL, α-ZAL, β-ZEL, α-ZEL | Heart, liver, spleen and muscle | Pig | 2018 | [123] |
| FUS-X, NIV | Plasma Feces Urine | Goats | 2018 | [124] |
| AFB1 | Liver and gizzards | Poultry | 2017 | [125] |
| ZEA, α-ZEL, β-ZEL, Phase II metabolites | Urine Feces | Poultry | 2017 | [126] |
4.1. Analytical Methods
4.2. Biomarkers of Exposure
4.3. Biomarkers of Exposure According to the Animal Species
4.3.1. Cattle
4.3.2. Pigs
4.3.3. Poultry
4.4. Analysis of Animal Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 15-ADON | 15-acetyldeoxynivalenol |
| 3-ADON | 3-acetyldeoxynivalenol |
| ABM | Animal biomonitoring |
| ACN | Acetonitrile |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFL | Aflatoxicol |
| AFM1 | Aflatoxin M1 |
| AFP1 | Aflatoxin P1 |
| AFQ1 | Aflatoxin Q1 |
| AFs | Aflatoxins |
| AME | Alternariol monomethyl ether |
| AOH | Alternariol |
| BEA | Beauvericin |
| CIT | Citrinin |
| DAS | Diacetoxyscirpenol |
| DH-CIT | Dihydrocitrinone |
| DOM-1 | Deepoxidesoxynivalenol |
| DON | Deoxynivalenol |
| DON-3GlcA | DON-3-glucuronide |
| DON-15GlcA | DON-15-glucuronide |
| DON-3gluc | DON-3 glucoside |
| DON-s | DON sulfate |
| EFSA | European Food Safety Authority |
| ELISA | Enzyme-linked immunosorbent assay |
| ENNA | Enniatin A |
| ENNA1 | Enniatin A1 |
| ENNB | Enniatin B |
| ENNB1 | Enniatin B1 |
| ERGOT | ergot alkaloids |
| EtOAc | Ethyl acetate |
| EU | European Union |
| FB1 | Fumonisin B1 |
| FB2 | Fumonisin B2 |
| FB3 | Fumonisin B3 |
| FB4 | Fumonisin B3 |
| FBs | Fumonisins |
| FLD | Fluorescence detector |
| FUS-X | Fusarenon-X |
| HBM | Human biomonitoring |
| GlcA | Glucuronide |
| HFBx | Hydrolyzed FBx |
| HT-2 | HT-2 toxin |
| IAC | Immunoaffinity column |
| IARC | International Agency for Research on Cancer |
| LC | Liquid chromatography |
| LLE | Liquid-liquid extraction |
| LOQ | Limit of quantification |
| MeOH | Methanol |
| MON | Moniliformin |
| MS | Mass spectrometer |
| MS/MS | Tandem mass spectrometry |
| NEO | Neosolaniol |
| NIV | Nivalenol |
| OTA | Ochratoxin A |
| OTα | Ochratoxin α |
| OTB | Ochratoxin B |
| PAT | Patulin |
| pHFBx | Partially hydrolyzed FBx |
| QuEChERs | Quick, easy, cheap, effective, rugged, and safe |
| ROQC | Roquefortine C |
| SLE | Solid–liquid extraction |
| SPE | Solid phase extraction |
| STER | Sterigmatocystin |
| T-2 | T-2 toxin |
| TENT | Tentoxin |
| ZAL | Zearalanol |
| α-ZAL | α-zearalanol |
| β-ZAL | β-zearalanol |
| ZAN | Zearalanone |
| ZAN-14GlcA | ZAN-14-glucuronide |
| ZEA | Zearalenone |
| α-ZEL | α-zearalenol |
| β-ZEL | β-zearalenol |
| ZEL-14GlcA | ZEA-14-glucuronide |
| ZEL-16GlcA | ZEA-16-glucuronide |
References
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Buszewska-Forajta, M. Mycotoxins, Invisible Danger of Feedstuff with Toxic Effect on Animals. Toxicon 2020, 182, 34–53. [Google Scholar] [CrossRef]
- Alvito, P.; Assunção, R.M.; Bajard, L.; Martins, C.; Mengelers, M.J.B.; Mol, H.; Namorado, S.; van den Brand, A.D.; Vasco, E.; Viegas, S.; et al. Current Advances, Research Needs and Gaps in Mycotoxins Biomonitoring under the HBM4EU—Lessons Learned and Future Trends. Toxins 2022, 14, 826. [Google Scholar] [CrossRef]
- Falkauskas, R.; Bakutis, B.; Jovaišienė, J.; Vaičiulienė, G.; Gerulis, G.; Kerzienė, S.; Jacevičienė, I.; Jacevičius, E.; Baliukonienė, V. Zearalenone and Its Metabolites in Blood Serum, Urine, and Milk of Dairy Cows. Animals 2022, 12, 1651. [Google Scholar] [CrossRef]
- World Health Organization. Fact Sheets Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 29 April 2024).
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium Mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741. [Google Scholar] [CrossRef]
- Peng, W.-X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to Prevent and Reduce Mycotoxins for Compound Feed Manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and Medical Aspects of Aspergillus-Derived Mycotoxins Entering the Feed and Food Chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef]
- Spadaro, D.; Meloni, G.R.; Siciliano, I.; Prencipe, S.; Gullino, M.L. HPLC-MS/MS Method for the Detection of Selected Toxic Metabolites Produced by Penicillium Spp. in Nuts. Toxins 2020, 12, 307. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- EFSA Completes 30 Risk Assessments on Undesirable Substances in Animal Feed. Available online: https://www.efsa.europa.eu/en/press/news/efsa-completes-30-risk-assessments-undesirable-substances (accessed on 8 March 2024).
- Wheeler, T.; von Braun, J. Climate Change Impacts on Global Food Security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- Integrated and Innovative Key Actions for Mycotoxin Management in the Food and Feed Chain. Available online: https://cordis.europa.eu/project/id/678781 (accessed on 8 March 2024).
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-Mycotoxin Occurrence in Feed, Metabolism and Carry-over to Animal-Derived Food Products: A Review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.-J.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef]
- Viegas, S.; Osteresch, B.; Almeida, A.; Cramer, B.; Humpf, H.-U.; Viegas, C. Enniatin B and Ochratoxin A in the Blood Serum of Workers from the Waste Management Setting. Mycotoxin Res. 2018, 34, 85–90. [Google Scholar] [CrossRef]
- Viegas, S.; Assunção, R.; Martins, C.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Ribeiro, E.; Viegas, C. Occupational Exposure to Mycotoxins in Swine Production: Environmental and Biological Monitoring Approaches. Toxins 2019, 11, 78. [Google Scholar] [CrossRef]
- Viegas, S.; Veiga, L.; Malta-Vacas, J.; Sabino, R.; Figueredo, P.; Almeida, A.; Viegas, C.; Carolino, E. Occupational Exposure to Aflatoxin (AFB 1) in Poultry Production. J. Toxicol. Environ. Health A 2012, 75, 1330–1340. [Google Scholar] [CrossRef]
- Santos Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent Mycotoxins in Animal Feed: Occurrence and Analytical Methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef]
- World Health Organization & Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of Mycotoxin on Immune Response and Consequences for Pig Health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- International Agency for Research in Cancer (IARC). Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC, Ed.; International Agency for Research in Cancer: Lyon, France, 1993; Volume 56. [Google Scholar]
- Norbäck, D.; Hashim, J.H.; Cai, G.-H.; Hashim, Z.; Ali, F.; Bloom, E.; Larsson, L. Rhinitis, Ocular, Throat and Dermal Symptoms, Headache and Tiredness among Students in Schools from Johor Bahru, Malaysia: Associations with Fungal DNA and Mycotoxins in Classroom Dust. PLoS ONE 2016, 11, e0147996. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- International Agency for Research on Cancer (IARC). Chemical Agents and Related Occupations. A Review of Human Carcinogens; IARC, Ed.; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100 F, ISBN 978-92-832-1323-9. [Google Scholar]
- International Agency for Research on Cancer (IARC). Overal Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; International Agency for Research on Cancer: Lyon, France, 1987; Volume 7, Suppl. 7. [Google Scholar]
- Peyraud, J.-L.; MacLeod, M. Future of EU Livestock—How to Contribute to a Sustainable Agricultural Sector; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Logrieco, A.; Miller, J.; Eskola, M.; Krska, R.; Ayalew, A.; Bandyopadhyay, R.; Battilani, P.; Bhatnagar, D.; Chulze, S.; De Saeger, S.; et al. The Mycotox Charter: Increasing Awareness of, and Concerted Action for, Minimizing Mycotoxin Exposure Worldwide. Toxins 2018, 10, 149. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential Economic Losses to the US Corn Industry from Aflatoxin Contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wang, J.Q.; Li, S.L.; Zhang, Y.D.; Zheng, N. Aflatoxin M1 Cytotoxicity against Human Intestinal Caco-2 Cells Is Enhanced in the Presence of Other Mycotoxins. Food Chem. Toxicol. 2016, 96, 79–89. [Google Scholar] [CrossRef]
- Csenki, Z.; Garai, E.; Faisal, Z.; Csepregi, R.; Garai, K.; Sipos, D.K.; Szabó, I.; Kőszegi, T.; Czéh, Á.; Czömpöly, T.; et al. The Individual and Combined Effects of Ochratoxin A with Citrinin and Their Metabolites (Ochratoxin B, Ochratoxin C, and Dihydrocitrinone) on 2D/3D Cell Cultures, and Zebrafish Embryo Models. Food Chem. Toxicol. 2021, 158, 112674. [Google Scholar] [CrossRef]
- Pinhão, M.; Tavares, A.M.; Loureiro, S.; Louro, H.; Alvito, P.; Silva, M.J. Combined Cytotoxic and Genotoxic Effects of Ochratoxin A and Fumonisin B1 in Human Kidney and Liver Cell Models. Toxicol. In Vitro 2020, 68, 104949. [Google Scholar] [CrossRef]
- Commission of the European Communities. White Paper on Food Safety; The European Commission, Ed.; Commision of the European Communities: Brussels, Belgium, 2000. [Google Scholar]
- Weaver, A.C.; Adams, N.; Yiannikouris, A. Invited Review: Use of Technology to Assess and Monitor Multimycotoxin and Emerging Mycotoxin Challenges in Feedstuffs. Appl. Anim. Sci. 2020, 36, 19–25. [Google Scholar] [CrossRef]
- European Commission. European Parliament Directive 2002/32/EC of The European Parliament and of The Council of 7 May 2002 on Undesirable Substances in Animal Feed. Off. J. Eur. Union 2002, L140, 1–22. [Google Scholar]
- Tkaczyk, A.; Jedziniak, P. Mycotoxin Biomarkers in Pigs—Current State of Knowledge and Analytics. Toxins 2021, 13, 586. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risks for Animal Health Related to the Presence of Ergot Alkaloids in Feed. EFSA J. 2024, 22, e8496. [Google Scholar] [CrossRef]
- Degen, G.H.; Ali, N.; Gundert-Remy, U. Preliminary Data on Citrinin Kinetics in Humans and Their Use to Estimate Citrinin Exposure Based on Biomarkers. Toxicol. Lett. 2018, 282, 43–48. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Annual Call for Continuous Collection of Chemical Contaminants Occurrence Data in Food and Feed. Available online: https://www.efsa.europa.eu/en/call/annual-call-continuous-collection-chemical-contaminants-occurrence-data-food-and-feed (accessed on 4 March 2024).
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; De Baere, S.; Letor, B.; Rychlik, M.; Croubels, S.; Devreese, M. Multi LC-MS/MS and LC-HRMS Methods for Determination of 24 Mycotoxins Including Major Phase I and II Biomarker Metabolites in Biological Matrices from Pigs and Broiler Chickens. Toxins 2019, 11, 171. [Google Scholar] [CrossRef]
- den Hollander, D.; Croubels, S.; Lauwers, M.; Caekebeke, N.; Ringenier, M.; De Meyer, F.; Reisinger, N.; Van Immerseel, F.; Dewulf, J.; Antonissen, G. Applied Research Note: Biomonitoring of Mycotoxins in Blood Serum and Feed to Assess Exposure of Broiler Chickens. J. Appl. Poult. Res. 2021, 30, 100111. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks for Human and Animal Health Related to the Presence of Modified Forms of Certain Mycotoxins in Food and Feed. EFSA J. 2014, 12, 3916. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; De Mil, T.; Fraeyman, S.; De Baere, S.; De Saeger, S.; De Backer, P.; Croubels, S. Development and Validation of an LC–MS/MS Method for the Toxicokinetic Study of Deoxynivalenol and Its Acetylated Derivatives in Chicken and Pig Plasma. J. Chromatogr. B 2014, 971, 43–51. [Google Scholar] [CrossRef]
- Catteuw, A.; Broekaert, N.; De Baere, S.; Lauwers, M.; Gasthuys, E.; Huybrechts, B.; Callebaut, A.; Ivanova, L.; Uhlig, S.; De Boevre, M.; et al. Insights into In Vivo Absolute Oral Bioavailability, Biotransformation, and Toxicokinetics of Zearalenone, α-Zearalenol, β-Zearalenol, Zearalenone-14-Glucoside, and Zearalenone-14-Sulfate in Pigs. J. Agric. Food Chem. 2019, 67, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- De Baere, S.; Ochieng, P.E.; Kemboi, D.C.; Scippo, M.-L.; Okoth, S.; Lindahl, J.F.; Gathumbi, J.K.; Antonissen, G.; Croubels, S. Development of High-Throughput Sample Preparation Procedures for the Quantitative Determination of Aflatoxins in Biological Matrices of Chickens and Cattle Using UHPLC-MS/MS. Toxins 2023, 15, 37. [Google Scholar] [CrossRef]
- Dänicke, S.; Winkler, J. Invited Review: Diagnosis of Zearalenone (ZEN) Exposure of Farm Animals and Transfer of Its Residues into Edible Tissues (Carry Over). Food Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef]
- Martins, C.; Torres, D.; Lopes, C.; Correia, D.; Goios, A.; Assunção, R.; Alvito, P.; Vidal, A.; De Boevre, M.; De Saeger, S.; et al. Deoxynivalenol Exposure Assessment through a Modelling Approach of Food Intake and Biomonitoring Data—A Contribution to the Risk Assessment of an Enteropathogenic Mycotoxin. Food Res. Int. 2021, 140, 109863. [Google Scholar] [CrossRef] [PubMed]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Chinese Edition). J. Chin. Integr. Med. 2009, 7, 889–896. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; González-Peñas, E. Mycotoxin Determination in Animal Feed: An LC-FLD Method for Simultaneous Quantification of Aflatoxins, Ochratoxins and Zearelanone in This Matrix. Toxins 2020, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Jedziniak, P.; Panasiuk, Ł.; Pietruszka, K.; Posyniak, A. Multiple Mycotoxins Analysis in Animal Feed with LC-MS/MS: Comparison of Extract Dilution and Immunoaffinity Clean-up. J. Sep. Sci. 2019, 42, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, J.; Wu, C.; Hou, F.; Chang, S.; Wang, Z.; Pu, Q.; Guo, D.; Fu, H. Fast Screening of Aflatoxins in Dairy Cattle Feeds with CE-LIF Method Combined with Preconcentration Technique of Vortex Assisted Low Density Solvent–Microextraction. Electrophoresis 2019, 40, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Wang, S.; Fotina, H.; Wang, Z. A Novel Lateral Flow Immunochromatographic Assay for Rapid and Simultaneous Detection of Aflatoxin B1 and Zearalenone in Food and Feed Samples Based on Highly Sensitive and Specific Monoclonal Antibodies. Toxins 2022, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Biscoto, G.L.; Salvato, L.A.; Alvarenga, É.R.; Dias, R.R.S.; Pinheiro, G.R.G.; Rodrigues, M.P.; Pinto, P.N.; Freitas, R.P.; Keller, K.M. Mycotoxins in Cattle Feed and Feed Ingredients in Brazil: A Five-Year Survey. Toxins 2022, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Fumagalli, F.; Rizzi, N.; Grandi, E.; Vailati, S.; Manoni, M.; Ottoboni, M.; Cheli, F.; Pinotti, L. An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy. Toxins 2022, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Haleem, K.S.; Ghazanfar, S.; Tauseef, I.; Bano, N.; Adetunji, C.O.; Saleem, M.H.; Alshaya, H.; Paray, B.A. Quantitative Estimation of Aflatoxin Level in Poultry Feed in Selected Poultry Farms. Biomed. Res. Int. 2022, 2022, 5397561. [Google Scholar] [CrossRef]
- Awapak, D.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Co-Occurrence and Toxicological Relevance of Secondary Metabolites in Dairy Cow Feed from Thailand. Food Addit. Contam. Part A 2021, 38, 1013–1027. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Ochieng, P.E.; Antonissen, G.; Croubels, S.; Scippo, M.-L.; Okoth, S.; Kangethe, E.K.; Faas, J.; Doupovec, B.; Lindahl, J.F.; et al. Multi-Mycotoxin Occurrence in Dairy Cattle and Poultry Feeds and Feed Ingredients from Machakos Town, Kenya. Toxins 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Bani Ismail, Z.; Al-Nabulsi, F.; Abu-Basha, E.; Hananeh, W. Occurrence of On-Farm Risk Factors and Health Effects of Mycotoxins in Dairy Farms in Jordan. Trop. Anim. Health Prod. 2020, 52, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Beyene, A.M.; Du, X.; Schrunk, D.E.; Ensley, S.; Rumbeiha, W.K. High-Performance Liquid Chromatography and Enzyme-Linked Immunosorbent Assay Techniques for Detection and Quantification of Aflatoxin B1 in Feed Samples: A Comparative Study. BMC Res. Notes 2019, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Akinmusire, O.O.; El-Yuguda, A.-D.; Musa, J.A.; Oyedele, O.A.; Sulyok, M.; Somorin, Y.M.; Ezekiel, C.N.; Krska, R. Mycotoxins in Poultry Feed and Feed Ingredients in Nigeria. Mycotoxin Res. 2019, 35, 149–155. [Google Scholar] [CrossRef]
- Nishimwe, K.; Bowers, E.; Ayabagabo, J.d.D.; Habimana, R.; Mutiga, S.; Maier, D. Assessment of Aflatoxin and Fumonisin Contamination and Associated Risk Factors in Feed and Feed Ingredients in Rwanda. Toxins 2019, 11, 270. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-Occurrence of Mycotoxins in Maize Food and Maize-Based Feed from Small-Scale Farms in Brazil: A Pilot Study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mokubedi, S.M.; Phoku, J.Z.; Changwa, R.N.; Gbashi, S.; Njobeh, P.B. Analysis of Mycotoxins Contamination in Poultry Feeds Manufactured in Selected Provinces of South Africa Using UHPLC-MS/MS. Toxins 2019, 11, 452. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Rodríguez-Estévez, V.; Arenas-Fernández, P.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of Mycotoxins in Swine Feeding from Spain. Toxins 2019, 11, 342. [Google Scholar] [CrossRef]
- Yang, C.-K.; Cheng, Y.-H.; Tsai, W.-T.; Liao, R.-W.; Chang, C.-S.; Chien, W.-C.; Jhang, J.-C.; Yu, Y.-H. Prevalence of Mycotoxins in Feed and Feed Ingredients between 2015 and 2017 in Taiwan. Environ. Sci. Pollut. Res. 2019, 26, 23798–23806. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Girgin, G.; Baydar, T. Mycotoxin Detection in Maize, Commercial Feed, and Raw Dairy Milk Samples from Assiut City, Egypt. Vet. Sci. 2019, 6, 57. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, M.; Ramos, A.J.; Prim, M.; Sanchis, V.; Marín, S. Usefulness of the Analytical Control of Aflatoxins in Feedstuffs for Dairy Cows for the Prevention of Aflatoxin M1 in Milk. Mycotoxin Res. 2020, 36, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhanshetty, M.; Banerjee, K. Development and Validation of a Method for Direct Analysis of Aflatoxins in Animal Feeds by Ultra-High-Performance Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2020, 103, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Vaičiulienė, G.; Bakutis, B.; Jovaišienė, J.; Falkauskas, R.; Gerulis, G.; Kerzienė, S.; Baliukonienė, V. Prevalence of Mycotoxins and Endotoxins in Total Mixed Rations and Different Types of Ensiled Forages for Dairy Cows in Lithuania. Toxins 2021, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Patyal, A.; Gill, J.P.S.; Bedi, J.S.; Aulakh, R.S. Assessment of Aflatoxin Contamination in Dairy Animal Concentrate Feed from Punjab, India. Environ. Sci. Pollut. Res. 2021, 28, 37705–37715. [Google Scholar] [CrossRef] [PubMed]
- Bervis, N.; Lorán, S.; Juan, T.; Carramiñana, J.J.; Herrera, A.; Ariño, A.; Herrera, M. Field Monitoring of Aflatoxins in Feed and Milk of High-Yielding Dairy Cows under Two Feeding Systems. Toxins 2021, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Nasaruddin, N.; Jinap, S.; Samsudin, N.I.P.; Kamarulzaman, N.H.; Sanny, M. Prevalence of Mycotoxigenic Fungi and Assessment of Aflatoxin Contamination: A Multiple Case Study along the Integrated Corn-based Poultry Feed Supply Chain in Malaysia. J. Sci. Food Agric. 2021, 101, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, Deoxynivalenol and Zearalenone in Feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Kassaw, T.S.; Megerssa, Y.C.; Woldemariyam, F.T. Occurrence of Aflatoxins in Poultry Feed in Selected Chicken Rearing Villages of Bishoftu Ethiopia. Vet. Med. Res. Rep. 2022, 13, 277–286. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Y.; Guo, Q.; Wang, X.; Luo, S.; Yang, W.; Li, J.; Chen, Y. Immunoaffinity Cleanup and Isotope Dilution-Based Liquid Chromatography Tandem Mass Spectrometry for the Determination of Six Major Mycotoxins in Feed and Feedstuff. Toxins 2022, 14, 631. [Google Scholar] [CrossRef]
- Mackay, N.; Marley, E.; Leeman, D.; Poplawski, C.; Donnelly, C. Analysis of Aflatoxins, Fumonisins, Deoxynivalenol, Ochratoxin A, Zearalenone, HT-2, and T-2 Toxins in Animal Feed by LC–MS/MS Using Cleanup with a Multi-Antibody Immunoaffinity Column. J. AOAC Int. 2022, 105, 1330–1340. [Google Scholar] [CrossRef]
- Changwa, R.; De Boevre, M.; De Saeger, S.; Njobeh, P.B. Feed-Based Multi-Mycotoxin Occurrence in Smallholder Dairy Farming Systems of South Africa: The Case of Limpopo and Free State. Toxins 2021, 13, 166. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; González-Peñas, E. Co-Occurrence of Mycotoxins in Feed for Cattle, Pigs, Poultry, and Sheep in Navarra, a Region of Northern Spain. Toxins 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Guan, S.; Li, A.; Wang, J.; An, G.; Hofstetter, U.; Schatzmayr, G. Mycotoxin Occurrence in Feeds and Raw Materials in China: A Five-Year Investigation. Toxins 2023, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Hao, W.; Guan, S.; Wang, J.; An, G. Mycotoxin Contamination in Feeds and Feed Materials in China in Year 2020. Front. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, A.; Wang, J.; An, G.; Guan, S. Mycotoxin Contamination of Feeds and Raw Materials in China in Year 2021. Front. Vet. Sci. 2022, 9, 929904. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Oueslati, S.; Mañes, J.; Berrada, H. Multimycotoxin Determination in Tunisian Farm Animal Feed. J. Food Sci. 2019, 84, 3885–3893. [Google Scholar] [CrossRef] [PubMed]
- Nualkaw, K.; Poapolathep, S.; Zhang, Z.; Zhang, Q.; Giorgi, M.; Li, P.; Logrieco, A.F.; Poapolathep, A. Simultaneous Determination of Multiple Mycotoxins in Swine, Poultry and Dairy Feeds Using Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Jang, S.; Jo, H.; Kim, H.; Lee, S.; Yun, H.; Jeong, M.; Moon, J.; Na, T.; Cho, H. Optimization of the QuEChERS-Based Analytical Method for Investigation of 11 Mycotoxin Residues in Feed Ingredients and Compound Feeds. Toxins 2021, 13, 767. [Google Scholar] [CrossRef]
- Kövesi, B.; Cserháti, M.; Erdélyi, M.; Zándoki, E.; Mézes, M.; Balogh, K. Long-Term Effects of Ochratoxin A on the Glutathione Redox System and Its Regulation in Chicken. Antioxidants 2019, 8, 178. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nielsen, E.; et al. Risks for Animal Health Related to the Presence of Ochratoxin A (OTA) in Feed. EFSA J. 2023, 21, 1–89. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding (2006/576/EC). Off. J. Eur. Union 2006, 49, 7–9. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the Risks for Public Health Related to the Presence of Zearalenone in Food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for Animal Health Related to the Presence of Zearalenone and Its Modified Forms in Feed. EFSA J. 2017, 15, e04851. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to Human and Animal Health Related to the Presence of Deoxynivalenol and Its Acetylated and Modified Forms in Food and Feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk Assessment of Aflatoxins in Food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.; Nielsen, E.; Ntzani, E.; et al. Assessment of Information as Regards the Toxicity of Fumonisins for Pigs, Poultry and Horses. EFSA J. 2022, 20, e07534. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; González-Peñas, E. An LC–MS/MS Method for Multi-Mycotoxin Quantification in Cow Milk. Food Chem. 2017, 218, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Lucke, A.; Menke, B.; EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of T-2 and HT-2 Toxin in Food and Feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- Devreese, M.; De Baere, S.; De Backer, P.; Croubels, S. Quantitative Determination of Several Toxicological Important Mycotoxins in Pig Plasma Using Multi-Mycotoxin and Analyte-Specific High Performance Liquid Chromatography–Tandem Mass Spectrometric Methods. J. Chromatogr. A 2012, 1257, 74–80. [Google Scholar] [CrossRef]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for Exposure as A Tool for Efficacy Testing of A Mycotoxin Detoxifier in Broiler Chickens and Pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef]
- Habschied, K.; Kanižai Šarić, G.; Krstanović, V.; Mastanjević, K. Mycotoxins—Biomonitoring and Human Exposure. Toxins 2021, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and Metabolism of Deoxynivalenol in Animals and Humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Barański, W.; Gajęcka, M.; Zielonka, Ł.; Mróz, M.; Onyszek, E.; Przybyłowicz, K.E.; Nowicki, A.; Babuchowski, A.; Gajęcki, M.T. Occurrence of Zearalenone and Its Metabolites in the Blood of High-Yielding Dairy Cows at Selected Collection Sites in Various Disease States. Toxins 2021, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Devreese, M.; Croubels, S.; De Baere, S.; Gehring, R.; Antonissen, G. Comparative Toxicokinetics and Plasma Protein Binding of Ochratoxin A in Four Avian Species. J. Agric. Food Chem. 2018, 66, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Laurain, J.; Tardieu, D.; Matard-Mann, M.; Rodriguez, M.A.; Guerre, P. Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay. Toxins 2021, 13, 701. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Dumanović, J.; Alomar, S.Y.; Resanović, R.; Milovanović, Z.; Nepovimova, E.; Wu, Q.; Franca, T.C.C.; Wu, W.; Kuča, K. Research Update on Aflatoxins Toxicity, Metabolism, Distribution, and Detection: A Concise Overview. Toxicology 2023, 492, 153549. [Google Scholar] [CrossRef] [PubMed]
- Meerpoel, C.; Vidal, A.; Tangni, E.K.; Huybrechts, B.; Couck, L.; De Rycke, R.; De Bels, L.; De Saeger, S.; Van den Broeck, W.; Devreese, M.; et al. A Study of Carry-Over and Histopathological Effects after Chronic Dietary Intake of Citrinin in Pigs, Broiler Chickens and Laying Hens. Toxins 2020, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Catteuw, A.; Devreese, M.; De Baere, S.; Antonissen, G.; Huybrechts, B.; Ivanova, L.; Uhlig, S.; Martens, A.; De Saeger, S.; De Boevre, M.; et al. Toxicokinetic Studies in Piglets Reveal Age-Related Differences in Systemic Exposure to Zearalenone, Zearalenone-14-Glucoside, and Zearalenone-14-Sulfate. J. Agric. Food Chem. 2020, 68, 7757–7764. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Streit, B.; Gruber, C.; Gonaus, C. Enzymatic Degradation of Ochratoxin A in the Gastrointestinal Tract of Piglets. J. Anim. Sci. 2023, 101, skad171. [Google Scholar] [CrossRef]
- Antonissen, G.; De Baere, S.; Novak, B.; Schatzmayr, D.; den Hollander, D.; Devreese, M.; Croubels, S. Toxicokinetics of Hydrolyzed Fumonisin B1 after Single Oral or Intravenous Bolus to Broiler Chickens Fed a Control or a Fumonisins-Contaminated Diet. Toxins 2020, 12, 413. [Google Scholar] [CrossRef]
- Schertz, H.; Kluess, J.; Frahm, J.; Schatzmayr, D.; Dohnal, I.; Bichl, G.; Schwartz-Zimmermann, H.; Breves, G.; Dänicke, S. Oral and Intravenous Fumonisin Exposure in Pigs—A Single-Dose Treatment Experiment Evaluating Toxicokinetics and Detoxification. Toxins 2018, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Solano, B.; González-Peñas, E. Biomonitoring of 19 Mycotoxins in Plasma from Food-Producing Animals (Cattle, Poultry, Pigs, and Sheep). Toxins 2023, 15, 295. [Google Scholar] [CrossRef] [PubMed]
- Panisson, J.C.; Wellington, M.O.; Bosompem, M.A.; Nagl, V.; Schwartz-Zimmermann, H.E.; Columbus, D.A. Urinary and Serum Concentration of Deoxynivalenol (DON) and DON Metabolites as an Indicator of DON Contamination in Swine Diets. Toxins 2023, 15, 120. [Google Scholar] [CrossRef]
- Streit, B.; Czabany, T.; Weingart, G.; Marchetti-Deschmann, M.; Prasad, S. Toolbox for the Extraction and Quantification of Ochratoxin A and Ochratoxin Alpha Applicable for Different Pig and Poultry Matrices. Toxins 2022, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, S.; Yu, S.; Zhao, Y.; Wu, Y.; Wu, A. LC-MS/MS Analysis of Fumonisin B1, B2, B3, and Their Hydrolyzed Metabolites in Broiler Chicken Feed and Excreta. Toxins 2022, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Jedziniak, P. Development of a Multi-Mycotoxin LC-MS/MS Method for the Determination of Biomarkers in Pig Urine. Mycotoxin Res. 2021, 37, 169–181. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Jedziniak, P.; Zielonka, Ł.; Dąbrowski, M.; Ochodzki, P.; Rudawska, A. Biomarkers of Deoxynivalenol, Citrinin, Ochratoxin A and Zearalenone in Pigs after Exposure to Naturally Contaminated Feed Close to Guidance Values. Toxins 2021, 13, 750. [Google Scholar] [CrossRef] [PubMed]
- Meerpoel, C.; Vidal, A.; Huybrechts, B.; Tangni, E.K.; De Saeger, S.; Croubels, S.; Devreese, M. Comprehensive Toxicokinetic Analysis Reveals Major Interspecies Differences in Absorption, Distribution and Elimination of Citrinin in Pigs and Broiler Chickens. Food Chem. Toxicol. 2020, 141, 111365. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; Croubels, S.; De Baere, S.; Sevastiyanova, M.; Romera Sierra, E.M.; Letor, B.; Gougoulias, C.; Devreese, M. Assessment of Dried Blood Spots for Multi-Mycotoxin Biomarker Analysis in Pigs and Broiler Chickens. Toxins 2019, 11, 541. [Google Scholar] [CrossRef]
- Gambacorta, L.; Olsen, M.; Solfrizzo, M. Pig Urinary Concentration of Mycotoxins and Metabolites Reflects Regional Differences, Mycotoxin Intake and Feed Contaminations. Toxins 2019, 11, 378. [Google Scholar] [CrossRef]
- Jurišić, N.; Schwartz-Zimmermann, H.E.; Kunz-Vekiru, E.; Moll, W.D.; Schweiger, W.; Fowler, J.; Berthiller, F. Determination of Aflatoxin Biomarkers in Excreta and Ileal Content of Chickens. Poult. Sci. 2019, 98, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- De Baere, S.; Croubels, S.; Novak, B.; Bichl, G.; Antonissen, G. Development and Validation of a UPLC-MS/MS and UPLC-HR-MS Method for the Determination of Fumonisin B1 and Its Hydrolysed Metabolites and Fumonisin B2 in Broiler Chicken Plasma. Toxins 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M.; et al. A QuEChERS-Based Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Determination of Nine Zearalenone-Like Mycotoxins in Pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Phruksawan, W.; Poapolathep, S.; Giorgi, M.; Imsilp, K.; Sakulthaew, C.; Owen, H.; Poapolathep, A. Toxicokinetic Profile of Fusarenon-X and Its Metabolite Nivalenol in the Goat (Capra Hircus). Toxicon 2018, 153, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sineque, A.; Macuamule, C.; Dos Anjos, F. Aflatoxin B1 Contamination in Chicken Livers and Gizzards from Industrial and Small Abattoirs, Measured by ELISA Technique in Maputo, Mozambique. Int. J. Environ. Res. Public Health 2017, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, H.; Sun, F.; De Ruyck, K.; Zhang, J.; Jin, Y.; Li, Y.; Wang, Z.; Zhang, S.; De Saeger, S.; et al. Metabolic Profile of Zearalenone in Liver Microsomes from Different Species and Its in Vivo Metabolism in Rats and Chickens Using Ultra High-Pressure Liquid Chromatography-Quadrupole/Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 11292–11303. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-J.; Yang, H.-I.; Wu, H.-C.; Liu, J.; Wang, L.-Y.; Lu, S.-N.; Lee, M.-H.; Jen, C.-L.; You, S.-L.; Santella, R.M.; et al. Aflatoxin B 1 Exposure Increases the Risk of Cirrhosis and Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers. Int. J. Cancer 2017, 141, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Arce-López, B.; Lizarraga, E.; Flores-Flores, M.; Irigoyen, Á.; González-Peñas, E. Development and Validation of a Methodology Based on Captiva EMR-Lipid Clean-up and LC-MS/MS Analysis for the Simultaneous Determination of Mycotoxins in Human Plasma. Talanta 2020, 206, 120193. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef]
- Kang, R.; Qu, H.; Guo, Y.; Zhang, M.; Fu, T.; Huang, S.; Zhao, L.; Zhang, J.; Ji, C.; Ma, Q. Toxicokinetics of a Single Oral Dose of OTA on Dezhou Male Donkeys. Toxins 2023, 15, 88. [Google Scholar] [CrossRef]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Meerpoel, C.; Vidal, A.; Andjelkovic, M.; De Boevre, M.; Tangni, E.K.; Huybrechts, B.; Devreese, M.; Croubels, S.; De Saeger, S. Dietary Exposure Assessment and Risk Characterization of Citrinin and Ochratoxin A in Belgium. Food Chem. Toxicol. 2021, 147, 111914. [Google Scholar] [CrossRef] [PubMed]
- Schelstraete, W.; Devreese, M.; Croubels, S. Comparative Toxicokinetics of Fusarium Mycotoxins in Pigs and Humans. Food Chem. Toxicol. 2020, 137, 111140. [Google Scholar] [CrossRef]
- Alnaemi, H.; Dawood, T.; Algwari, Q. Plasma-Activated Water Application for Detoxification of Aflatoxin B1, Ochratoxin A, and Fumonisin B1 in Poultry Feeds. Open Vet. J. 2023, 13, 1654. [Google Scholar] [CrossRef]











| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| Animal * feed | 810 | 2–34.3 | 87.8 | 2016–2021 | Brazil [57,66], China [84] |
| Cattle feed | 345 | 3.9–56 | 187.9 | 2014–2020 | Tunisia [86], Egypt [70], Thailand [87], South Africa [81], Spain [82], Kenya [61] |
| Pig feed | 552 | 0–7 | 65.5 | 2017–2020 | China [84], Spain [68,82], Thailand [87]; |
| Poultry feed | 675 | 0–26.7 | 27 | 2013–2020 | China [84], South Africa [67], Tunisia [86], Nigeria [64], Thailand [87], Spain [82] and Kenya [61] |
| Sheep feed | 116 | 8–31 | 45.3 | 2016–2020 | Tunisia [86], Spain [82] |
| TOTAL | 2498 | 0–56 | 187.9 | 2013–2021 |
| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| Animal * feed | 1612 | 29–91 | 2503.9 | 2016–2021 | Brazil [57,66], China [83,84,85], Lithuania [73], Thailand [60] |
| Cattle feed | 658 | 3–100 | 1793.7 | 2014–2020 | China [77], Egypt [70], Jordan [62], Kenya [61], South Africa [81], Spain [82], Thailand [87], Tunisia [86] |
| Pig feed | 3762 | 7.0–99.4 | 7681 | 2015–2021 | China [77,83,84,85], Spain [82], Thailand [87], Taiwan [69] |
| Poultry feed | 3537 | 56–100 | 1490 | 2013–2021 | China [77,83,84,85], Kenya [61], Nigeria [64], South Africa [67], Spain [82], Thailand [87], Tunisia [86] |
| Sheep feed | 116 | 3–52 | 658 | 2016–2020 | Tunisia [86], Spain [82] |
| TOTAL | 9685 | 3–100 | 7681 | 2013–2021 |
| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| Animal * feed | 871 | 26.5–87.9 | 4969.1 | 2016–2021 | Brazil [57,66], Lithuania [73], Thailand [60] |
| Cattle feed | 641 | 37–99.3 | 2490 | 2016–2020 | China [77], Jordan [62], Kenya [61], South Africa [81], Spain [82], Thailand [87], Tunisia [86] |
| Pig feed | 1871 | 4.4–99.6 | >5000 | 2015–2020 | China [77], Spain [68,82], Taiwan [69], Thailand [87] |
| Poultry feed | 946 | 31–100 | 2970.1 | 2015–2020 | China [77], Kenya [61], South Africa [67], Spain [82], Thailand [87] Tunisia [86] |
| Sheep feed | 116 | 6.0–72 | 887 | 2016–2020 | Tunisia [86], Spain [82] |
| TOTAL | 4445 | 4.4–100 | >5000 | 2015–2021 |
| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| 15-ADON | |||||
| Animal * feed | 0 | ||||
| Cattle feed | 212 | 17–36 | 858.8 | 2016–2019 | South Africa [81], Thailand [87], Tunisia [86] |
| Pig feed | 100 | 16 | 83.2 | n.i. ** | Thailand [87] |
| Poultry feed | 248 | 5–35 | 840.7 | 2015–2017 | South Africa [67], Thailand [87], Tunisia [86] |
| Sheep feed | 16 | 25 | 19 | 2016–2017 | Tunisia [86] |
| TOTAL | 576 | 5–36 | 858.8 | 2015–2019 | |
| 3-ADON | |||||
| Animal Feed | 0 | ||||
| Cattle feed | 212 | 3–16.9 | 300.0 | 2016–2019 | South Africa [81], Thailand [87], Tunisia [86] |
| Pig feed | 100 | n.i. | n.i. | n.i. | Thailand [87] |
| Poultry feed | 248 | 1–95 | 167.9 | 2015–2017 | South Africa [67], Thailand [87], Tunisia [86] |
| Sheep feed | 16 | n.i. | n.i. | n.i. | Tunisia [86] |
| TOTAL | 576 | 1–95 | 300 | 2015–2019 | |
| DON-3gluc | |||||
| Animal Feed | 34 | 26.5 | 28.8 | 2018–2019 | Thailand [60] |
| Cattle feed | 16 | 88 | 61.7 | 2018–2019 | Kenya [61] |
| Pig feed | 0 | ||||
| Poultry feed | 27 | 100 | 45.7 | 2019 | Kenya [61] |
| Sheep feed | 0 | ||||
| TOTAL | 77 | 26.5–100 | 61.7 | 2018–2019 | |
| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| AFB1 | |||||
| Animal * feed | 130 | 13–61 | 390 | 2016–2020 | Brazil [66], Lithuania [73], Thailand [60] |
| Cattle feed | 1012 | 3.9–100 | 374.6 | 2014–2020 | China [77], Tunisia [86], Egypt [70], Thailand [87], Spain [71,75,82], South Africa [81], Kenya [61], India [74] |
| Pig feed | 1048 | 3.1–100 | 59.7 | 2017–2020 | China [77], Spain [68,82], Thailand [87] |
| Poultry feed | 1036 | 13–99.9 | 760 | 2013–2020 | China [77], Ethiopia [78], South Africa [67], Tunisia [86], Nigeria [64], Thailand [87], Spain [82], Kenya [61], Malaysia [76] |
| Sheep feed | 116 | 12 | 6.1 | 2016–2020 | Tunisia [86], Spain [82] |
| TOTAL | 3342 | 3.1–100 | 760 | 2013–2020 | |
| AFB2 | |||||
| Animal feed | 45 | 4 | 5.4 | 2016 | Brazil [66] |
| Cattle feed | 727 | 5–81 | 31.5 | 2014–2020 | Tunisia [86], Egypt [70], Thailand [87], Spain [71,82], Shout Africa [81], Kenya [61], India [74] |
| Pig feed | 428 | 1.3–14 | 4.1 | 2017–2020 | Spain [68,82], Thailand [87] |
| Poultry feed | 465 | 11–100 | 188 | 2013–2020 | South Africa [67], Tunisia [86], Nigeria [64], Thailand [87], Spain [82], Kenya [61], Malaysia [76], Ethiopia [78] |
| Sheep feed | 116 | 15 | 4.9 | 2016–2020 | Tunisia [86], Spain [82] |
| TOTAL | 1781 | 1.3–100 | 188 | 2013–2020 | |
| AFG1 | |||||
| Animal * feed | 45 | 4 | 12 | 2016 | Brazil [66] |
| Cattle feed | 727 | 2.6–88 | 123 | 2014–2020 | Tunisia [86], Egypt [70], Thailand [87], Spain [71,82], Shout Africa [81], Kenya [61], India [74] |
| Pig feed | 428 | 0.9–10 | 6 | 2017–2020 | Spain [68,82], Thailand [87] |
| Poultry feed | 465 | 7–97 | 921.4 | 2013–2020 | South Africa [67], Tunisia [86], Nigeria [64], Thailand [87], Spain [82], Kenya [61], Malaysia [76], Ethiopia [78] |
| Sheep feed | 116 | 10 | 6.5 | 2016–2020 | Tunisia [86], Spain [82] |
| TOTAL | 1781 | 0.9–97 | 921.4 | 2013–2020 | |
| AFG2 | |||||
| Animal feed | 45 | 0 | 2016 | Brazil [66] | |
| Cattle feed | 727 | 1.3–44 | 28.5 | 2014–2020 | Tunisia [86], Egypt [70], Thailand [87], Spain [71,82], Shout Africa [81], Kenya [61], India [74] |
| Pig feed | 428 | 0–17 | 4.4 | 2017–2020 | Spain [68,82], Thailand [87] |
| Poultry feed | 465 | 2–82 | 221.4 | 2013–2020 | South Africa [67], Tunisia [86], Nigeria [64], Thailand [87], Spain [82], Kenya [61], Malaysia [76], Ethiopia [78] |
| Sheep feed | 116 | 16 | 4 | 2016–2020 | Tunisia [86], Spain [82] |
| TOTAL | 1781 | 0–82 | 221.4 | 2013–2020 | |
| Total AFs | |||||
| Animal * feed | 1850 | 1–65.7 | 66.7 | 2017–2021 | Brazil [57], China [83,84,85], Rwanda [65] |
| Cattle feed | 1750 | 2.4–59 | 406.1 | 2013–2021 | India [74], Italy [58], Jordan [62], Rwanda [65] |
| Pig feed | 2715 | 21–58 | 245.0 | 2015–2021 | China [83,84,85], Taiwan [69] |
| Poultry feed | 4414 | 7.4–97.5 | 1919.8 | 2017–2021 | China [83,84,85], Ethiopia [78], Kenya [61], Malaysia [76], Pakistan [59], Rwanda [65] |
| Sheep feed | 0 | ||||
| TOTAL | 10,729 | 1–97.5 | 1919.8 | 2013–2021 | |
| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| FB1 | |||||
| Animal * feed | 79 | 41.2–9 | 53,000 | 2016–2019 | Brazil [66], Thailand [60] |
| Cattle feed | 193 | 23.4–100 | 1494 | 2018–2019 | Tunisia [86], Thailand [87], Kenya [61] |
| Pig feed | 328 | 50–85 | 3959 | 2017 | Spain [68], Thailand [87] |
| Poultry feed | 262 | 96–100 | 7125.3 | 2013–2019 | South Africa [67], Nigeria [64], Thailand [87], Kenya [61] |
| Sheep feed | 0 | Tunisia [86] | |||
| TOTAL | 862 | 23.4–100 | 53,000 | 2013–2019 | |
| FB2 | |||||
| Animal feed | 79 | 14.7–87 | 2800 | 2016–2019 | Brazil [66], Thailand [60] |
| Cattle feed | 193 | 19.5–94 | 677.3 | 2018–2019 | Tunisia [86], Thailand [87], Kenya [61] |
| Pig feed | 328 | 29.8–77 | 961 | 2017 | Spain [68], Thailand [87] |
| Poultry feed | 262 | 91–100 | 728.8 | 2013–2019 | South Africa [67], Nigeria [64], Thailand [87], Kenya [61] |
| sheep feed | 0 | Tunisia [86] | |||
| TOTAL | 862 | 14.7–100 | 2800 | 2013–2019 | |
| Total FBs | |||||
| Animal feed | 1860 | 45.4–93.4 | 17,490 | 2017–2021 | Brazil [57], China [83,84,85], Rwanda [65] |
| Cattle feed | 1284 | 100 | 11,638.2 | 2017–2019 | Jordan [62], Kenya [61], Rwanda [65] |
| Pig feed | 2715 | 50.4–99 | 13,254 | 2015–2021 | China [83,84,85], Taiwan [69] |
| Poultry feed | 4314 | 91–100 | 17,052 | 2017–2021 | China [83,84,85], Kenya [61], Rwanda [65] |
| Sheep feed | 0 | ||||
| TOTAL | 10,173 | 45.4–100 | 17,490 | 2015–2021 | |
| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| T-2 | |||||
| Animal * feed | 817 | 21.3–31.3 | 246.7 | 2016–2021 | Brazil [57], China [84], Lithuania [73] |
| Cattle feed | 239 | 2–13 | 1734.6 | 2016–2019 | Tunisia [86], Thailand [87], Kenya [61], Jordan [62] |
| Pig feed | 452 | 0.9–1 | 35.9 | 2017–2020 | China [84], Spain [68], Thailand [87] |
| Poultry feed | 545 | 4–100 | 956.5 | 2015–2020 | China [84], South Africa [67], Tunisia [86], Thailand [87], Kenya [61] |
| Sheep feed | 16 | n.i. ** | n.i. | 2016–2017 | Tunisia [86] |
| TOTAL | 2069 | 0.9–100 | 1734.6 | 2015–2021 | |
| HT-2 | |||||
| Animal feed | 0 | ||||
| Cattle feed | 151 | 1–37 | 173.4 | 2016–2019 | Tunisia [86], Thailand [87], Kenya [61] |
| Pig feed | 328 | 0.9–7 | 123 | 2017 | Spain [68], Thailand [87] |
| Poultry feed | 275 | 4–100 | 119.8 | 2015–2019 | South Africa [67], Tunisia [86], Thailand [87], Kenya [61] |
| Sheep feed | 16 | 13 | 13.1 | 2016–2017 | Tunisia [86] |
| TOTAL | 770 | 0.9–100 | 173.4 | 2015–2019 | |
| Matrix | n | Prevalence (%) | Maximum Level (µg/kg) | Collection | Countries |
|---|---|---|---|---|---|
| NIV | |||||
| Animal * feed | 0 | ||||
| Cattle feed | 228 | 5.2–94 | 117.5 | 2016–2019 | Tunisia [86], Thailand [87], South Africa [81], Kenya [61] |
| Pig feed | 100 | 18 | 165.4 | n.i. ** | Thailand [87] |
| Poultry feed | 200 | 23.3–96 | 647 | 2013–2019 | Tunisia [86], Nigeria [64], Thailand [87], Kenya [61] |
| Sheep feed | 16 | n.i. | n.i. | 2016–2017 | Tunisia [86] |
| TOTAL | 544 | 5.2–96 | 647 | 2013–2019 | |
| NEO | |||||
| Animal feed | 0 | ||||
| Cattle feed | 135 | n.i. | n.i. | 2016–2017 | Tunisia [86], Thailand [87] |
| Pig feed | 100 | n.i. | n.i. | n.i. | Thailand [87] |
| Poultry feed | 143 | n.i. | n.i. | 2016–2017 | Tunisia [86], Thailand [87] |
| Sheep feed | 16 | n.i. | n.i. | 2016–2017 | Tunisia [86] |
| TOTAL | 394 | n.i. | n.i. | 2016–2017 | |
| DAS | |||||
| Animal feed | 0 | ||||
| Cattle feed | 212 | 1 | 4.4 | 2016–2019 | Tunisia [86], Thailand [87] |
| Pig feed | 100 | 2 | 5.1 | n.i. | Thailand [87] |
| Poultry feed | 143 | 3–14 | 219.2 | 2016–2017 | Tunisia [86], Thailand [87] |
| Sheep feed | 16 | n.i. | n.i. | 2016–2017 | Tunisia [86] |
| TOTAL | 471 | 1–14 | 219.2 | 2016–2019 | |
| STER | |||||
| Animal feed | 0 | ||||
| Cattle feed | 177 | 6–45.5 | 139.1 | 2018–2020 | South Africa [81], Spain [82] |
| Pig feed | 328 | 2.2–10 | 308 | 2017–2020 | Spain [68,82] |
| Poultry feed | 100 | 7 | 5.1 | 2019–2020 | Spain [82] |
| Sheep feed | 100 | 5 | 5.6 | 2019–2021 | Spain [82] |
| TOTAL | 705 | 2.2–45.5 | 308 | 2017–2021 | |
| Poultry | Pigs | |||||||
|---|---|---|---|---|---|---|---|---|
| Mycotoxin | Plasma Serum or Blood | Urine | Feces | Excreta | Plasma Serum or Blood | Urine | Feces | Excreta |
| AFB1 | AFB1 AFL | AFB1-N7-Gua | AFB1 | AFB1, AFM1, AFB2 | ||||
| OTA | OTA, OTα | OTA, OTα | OTA | OTA, OTα | OTA, OTα | OTA, OTα | ||
| DON | DON-s | DON-s | DON, DOM-1 DON-GlcA | DON, DON-GlcA | ||||
| ZEA | ZEA, α-ZEL, β-ZEL, ZEA-GlcA | ZEA, α-ZEL, β-ZEL, ZEA-GlcA | ZEA, α-ZEL, β-ZEL, ZEA-GlcA | ZEA, ZEA-GlcA | ZEA, ZEA-GlcA | ZEA, α-ZEL | ||
| FB1 | FB1, pHFB1 | FB1 HFB1 | FB1 HFB1 | FB1 HFB1, pHFB1 | ||||
| CIT | CIT, DH-CIT | CIT, DH-CIT | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Solano, B.; Lizarraga Pérez, E.; González-Peñas, E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins 2024, 16, 218. https://doi.org/10.3390/toxins16050218
Muñoz-Solano B, Lizarraga Pérez E, González-Peñas E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins. 2024; 16(5):218. https://doi.org/10.3390/toxins16050218
Chicago/Turabian StyleMuñoz-Solano, Borja, Elena Lizarraga Pérez, and Elena González-Peñas. 2024. "Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep)" Toxins 16, no. 5: 218. https://doi.org/10.3390/toxins16050218






