A Novel Preparation Method of (Ti,Zr,Nb,Mo,W)B2-SiC Composite Ceramic Based on Reactive Sintering of Pre-Alloyed Metals
Abstract
:1. Introduction
2. Materials and Experimental Procedures
2.1. Materials Preparation
2.2. Fabrication of MeB2-SiC
2.3. Oxidation Tests
2.4. Characterizations
3. Results
3.1. Pre-Alloying Behavior of Multiple Metallic Elements
3.2. Phase Composition and Microstructure of the As-Sintered MeB2-SiC Ceramic
3.3. Mechanical and Oxidation Properties of the MeB2-SiC Ceramic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-high temperature ceramics: Materials for extreme environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Golla, B.R.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on ultra-high temperature boride ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- He, L.; Pan, L.; Zhou, W.; Niu, Z.; Chen, X.; Chen, M.; Zhang, Q.; Pan, W.; Xiao, P.; Li, Y. Thermal corrosion behavior of Yb4Hf3O12 ceramics exposed to calcium-ferrum-alumina-silicate (CFAS) at 1400 °C. J. Eur. Ceram. Soc. 2023, 43, 4114–4123. [Google Scholar] [CrossRef]
- Pan, L.; He, L.; Niu, Z.; Xiao, P.; Zhou, W.; Li, Y. Corrosion behavior of ytterbium hafnate exposed to water-vapor with Al(OH)3 impurities. J. Eur. Ceram. Soc. 2023, 43, 612–620. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Gudkova, S.A.; Starikov, A.Y.; Zherebtsov, D.A.; Kirsanova, A.A.; Häßner, M.; Niewa, R. High-entropy oxide phases with magnetoplumbite structure. Ceram. Int. 2019, 45, 12942–12948. [Google Scholar] [CrossRef]
- Anandkumar, M.; Trofimov, E. Synthesis, properties, and applications of high-entropy oxide ceramics: Current progress and future perspectives. J. Alloys Compd. 2023, 960, 170690. [Google Scholar] [CrossRef]
- Castle, E.; Csanádi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and properties of high-entropy ultra-high temperature carbides. Sci. Rep. 2018, 8, 8609. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E.; Zhou, Y. Synthesis of single-phase high-entropy carbide powders. Scr. Mater. 2019, 162, 90–93. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Xu, Y.; Liu, Z.; Qian, B.; Xuan, F. Selective laser melting of CoCrFeNiMn high entropy alloy powder modified with nano-TiN particles for additive manufacturing and strength enhancement: Process, particle behavior and effects. Powder Technol. 2020, 360, 509–521. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188, 193–200. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y. High-entropy transparent fluoride laser ceramics. J. Am. Ceram. Soc. 2020, 103, 750–756. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Gucci, F.; Zhu, H.; Chen, K.; Reece, M.J. Data-driven design of ecofriendly thermoelectric high-entropy sulfides. Inorg. Chem. 2018, 57, 13027–13033. [Google Scholar] [CrossRef]
- Gild, J.; Braun, J.; Kaufmann, K.; Marin, E.; Harrington, T.; Hopkins, P.; Vecchio, K.; Luo, J. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater. 2019, 5, 337–343. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, J.X.; Li, F.; Wei, X.F.; Wu, H.Z.; Zhang, G.J. A high entropy silicide by reactive spark plasma sintering. J. Adv. Ceram. 2019, 8, 148–152. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Shao, G.; Wang, H.; Zhu, J.; Liu, W.; Fan, B.; Xu, H.; Lu, H.; Zhou, Y.; et al. Oscillatory pressure sintering of high entropy (Zr0.2Ta0.2Nb0.2Hf0.2Mo0.2)B2 ceramic. Ceram. Int. 2021, 47, 8707–8710. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.M.; Jiang, Z.B.; Zhu, Q.Q.; Sun, S.K.; You, Y.; Plucknett, K.; Lin, H.T. Dense high-entropy boride ceramics with ultra-high hardness. Scr. Mater. 2019, 164, 135–139. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.Q.; Zou, J.; Wang, W.M.; Wang, X.G.; Fu, Z.Y. Strong high-entropy diboride ceramics with oxide impurities at 1800 °C. Sci. China Mater. 2023, 66, 2061–2070. [Google Scholar] [CrossRef]
- Yang, Y.; Bi, J.Q.; Sun, K.N.; Qiao, L.J.; Liang, G.D.; Wang, H.Y.; Yuan, J.L.; Chen, Y.G. The effect of chemical element on hardness in high-entropy transition metal diboride ceramics. J. Eur. Ceram. Soc. 2023, 43, 5774–5781. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E. Processing of dense high-entropy boride ceramics. J. Eur. Ceram. Soc. 2020, 40, 3815–3823. [Google Scholar] [CrossRef]
- Hoque, M.S.B.; Milich, M.; Akhanda, M.S.; Shivakumar, S.; Hoglund, E.R.; Staicu, D.; Qin, M.; Quiambao-Tomko, K.F.; Tomko, J.A.; Braun, J.L. Thermal and ablation properties of a high-entropy metal diboride: (Hf0.2Zr0.2Ti0.2Ta0.2Nb0.2)B2. J. Eur. Ceram. Soc. 2023, 43, 4581–4587. [Google Scholar] [CrossRef]
- Zhang, S.C.; Hilmas, G.E.; Fahrenholtz, W.G. pressure less Densification of Zirconium Diboride with Boron Carbide Additions. J. Am. Ceram. Soc. 2006, 89, 1544–1550. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Binner, J.; Zou, J. Synthesis of ultra-refractory transition metal diboride compounds. J. Mater. Res. 2016, 31, 2757–2772. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Chen, S.Y.; Tong, Y.; Tseng, K.K.; Yeh, J.W.; Poplawsky, J.D.; Wen, J.G.; Gao, M.C.; Kim, G.; Chen, W.; Ren, Y.; et al. Phase transformations of HfNbTaTiZr high-entropy alloy at intermediate temperatures. Scr. Mater. 2019, 158, 50–56. [Google Scholar] [CrossRef]
- ASTM E-399-90; Standard Test Method for Plane-Strain Fracture Toughness of Metallic Materials. Annual Book of ASTM Standards, Section 3, Metal Test Methods and Analytical Procedures, 1990; ASTM: Philadelphia, PA, USA, 1992; pp. 519–554.
- Wang, D.Y.; Li, X.; Wang, Y.X. First-principles investigation of hexagonal WB2 surfaces. J. Phys. Soc. Jpn. 2012, 81, 044712. [Google Scholar] [CrossRef]
- Zhao, E.J.; Meng, J.A.; Ma, Y.M.; Wu, Z.J. Phase stability and mechanical properties of tungsten borides from first principles calculations. Phys. Chem. Chem. Phys. 2010, 12, 13158–13165. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.F.; Xu, Y.H.; Wu, Z.J.; Zhou, D.F.; Liu, X.J.; Cao, X.Q.; Meng, J. Low-compressibility and hard materials ReB2 and WB2: Prediction from first-principles study. Phys. Rev. B 2006, 74, 224112. [Google Scholar] [CrossRef]
- Qin, M.D.; Gild, J.; Wang, H.R.; Harrington, T.; Vecchio, K.S.; Luo, J. Dissolving and stabilizing soft WB2 and MoB2 phases into high-entropy borides via boron-metals reactive sintering to attain higher hardness. J. Eur. Ceram. Soc. 2020, 40, 4348–4353. [Google Scholar] [CrossRef]
- Chen, X.Q.; Fu, C.L.; Krcmar, M.; Painter, G.S. Electronic and structural origin of ultraincompressibility of 5d transition-metal diborides MB2 (M=W, Re, Os). Phys. Rev. Lett. 2008, 100, 196403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shi, L.; Li, S.; Duan, Y.; Xia, W.; Wang, Y. Influence of uniaxial strains on the mechanical properties of transition metal borides X2B, XB and XB2 (X=Cr, Mo, W). Phys. B 2018, 550, 100–111. [Google Scholar] [CrossRef]
- Liu, J.X.; Shen, X.Q.; Wu, Y.; Li, F.; Liang, Y.; Zhang, G.J. Mechanical properties of hot-pressed high-entropy diboride-based ceramics. J. Adv. Ceram. 2020, 9, 503–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.B.; Sun, S.K.; Guo, W.M.; Chen, Q.S.; Qiu, J.X.; Plucknett, K.; Lin, H.T. Microstructure and mechanical properties of high-entropy borides derived from boro/carbothermal reduction. J. Eur. Ceram. Soc. 2019, 39, 3920–3924. [Google Scholar] [CrossRef]
- Kovalčíková, A.; Tatarko, P.; Sedlák, R.; Medveď, D.; Chlup, Z.; Múdra, E.; Dusza, J. Mechanical and tribological properties of TiB2-SiC and TiB2-SiC-GNPs ceramic composites. J. Eur. Ceram. Soc. 2020, 40, 4860–4871. [Google Scholar] [CrossRef]
- Orlovskaya, N.; Stadelmann, R.; Lugovy, M.; Subbotin, V.; Subhash, G.; Neubert, M.; Aneziris, C.G.; Graule, T.; Kuebler, J. Mechanical properties of ZrB2–SiC ceramic composites: Room temperature instantaneous behaviour. Adv. Appl. Ceram. 2013, 112, 9–16. [Google Scholar] [CrossRef]
- Akin, I.; Ocak, B.C.; Sahin, F.; Goller, G. Effects of SiC and SiC-GNP additions on the mechanical properties and oxidation behavior of NbB2. J. Asian. Ceram. Soc. 2019, 7, 170–182. [Google Scholar] [CrossRef]
- Bhaumik, S.K.; Divakar, C.; Singh, A.K.; Upadhyaya, G.S. Synthesis and sintering of TiB2 and TiB2–TiC composite under high pressure. Mater. Sci. Eng. A 2000, 279, 275–281. [Google Scholar] [CrossRef]
- Zhang, G.J.; Deng, Z.Y.; Kondo, N.; Yang, J.F.; Ohji, T. Reactive hot pressing of ZrB2-SiC composites. J. Am. Ceram. Soc. 2000, 83, 2330–2332. [Google Scholar] [CrossRef]
- Sairam, K.; Sonber, J.K.; Murthy, T.S.R.C.; Subramanian, C.; Fotedar, R.K.; Hubli, R.C. Reaction spark plasma sintering of niobium diboride. Int. J. Refract. Met. Hard Mat. 2014, 43, 259–262. [Google Scholar] [CrossRef]
- Tao, Q.; Zhao, X.P.; Chen, Y.L.; Li, J.; Li, Q.; Ma, Y.M.; Li, J.J.; Cui, T.; Zhu, P.W.; Wang, X. Enhanced Vickers hardness by quasi-3D boron network in MoB2. RSC. Adv. 2013, 3, 18317–18322. [Google Scholar] [CrossRef]
- Nastic, A.; Merati, A.; Bielawski, M.; Bolduc, M.; Fakolujo, O.; Nganbe, M. Instrumented and Vickers indentation for the characterization of stiffness, hardness and toughness of zirconia toughened Al2O3 and SiC armor. J. Mater. Sci. Technol. 2015, 31, 773–783. [Google Scholar] [CrossRef]


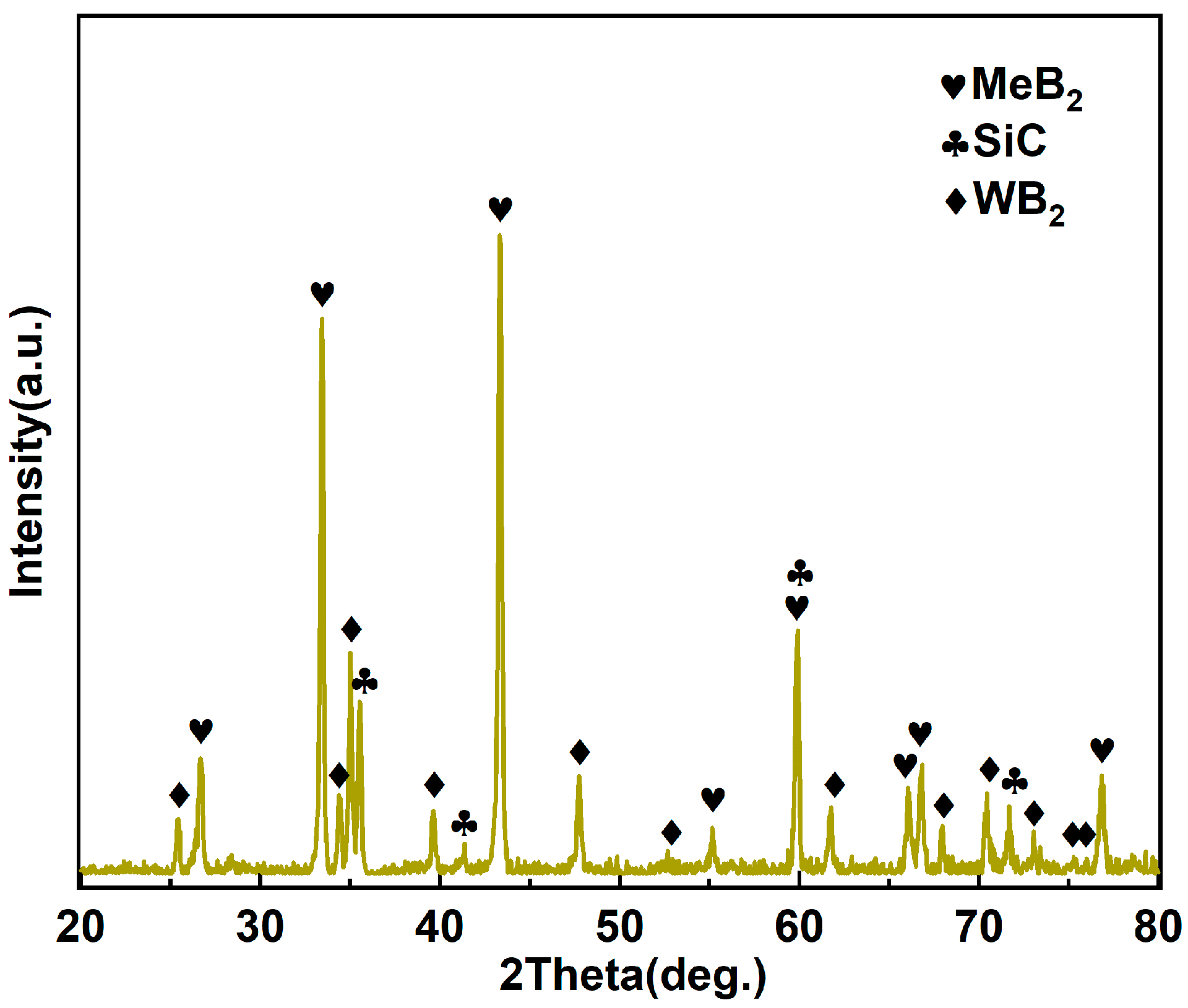



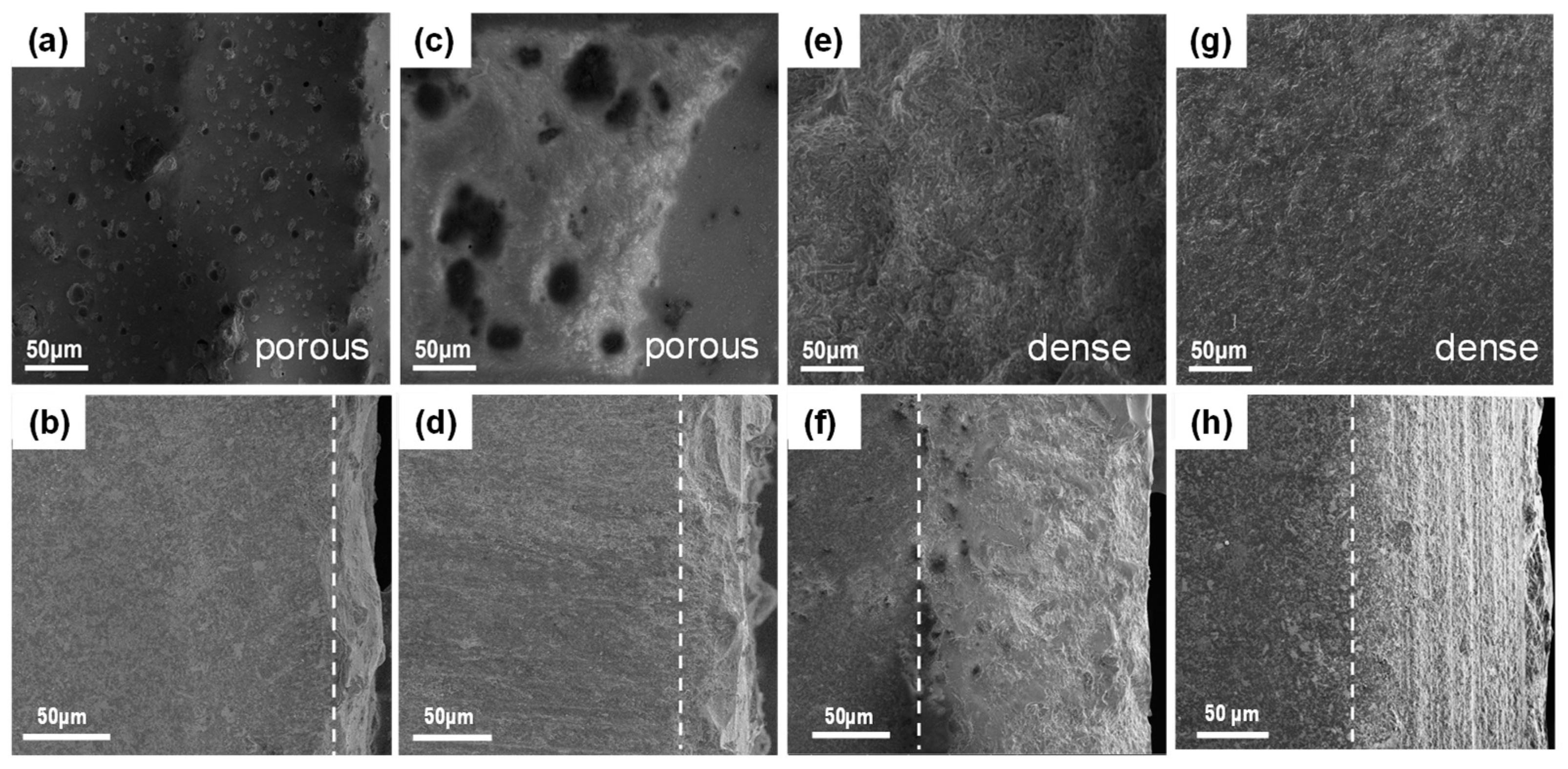
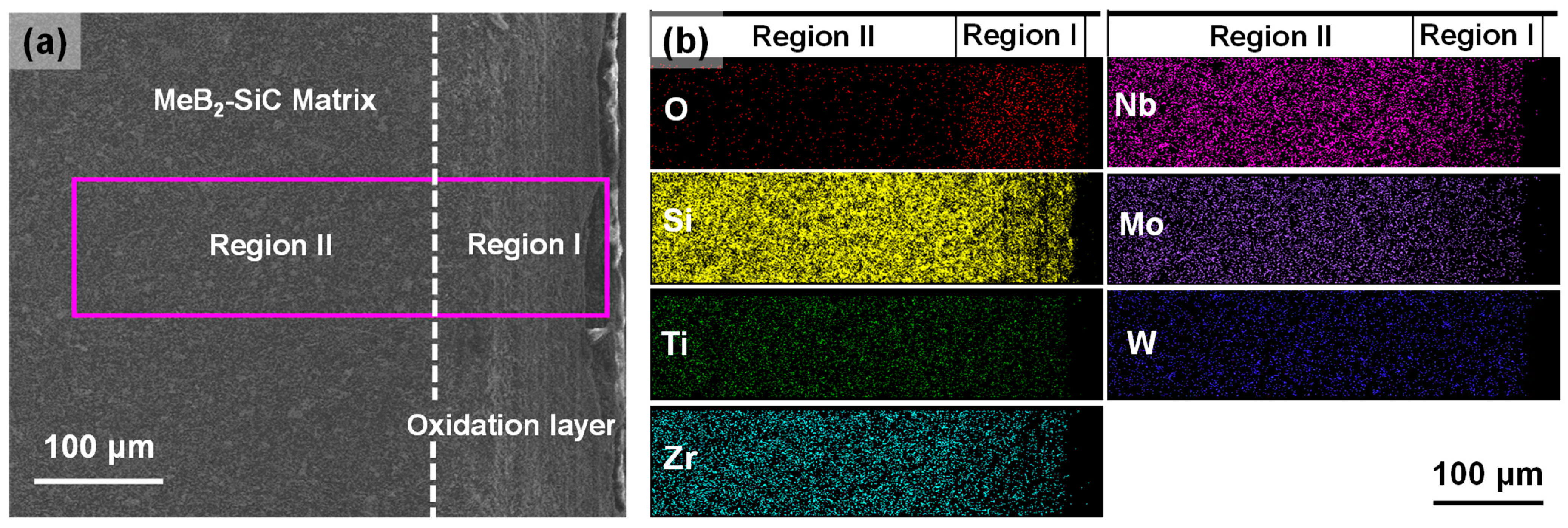
| Diborides | Pearson Symbol | Space Group | a = b (Å) | c (Å) | Standard Card (PDF #) |
|---|---|---|---|---|---|
| TiB2 | hP3 | P6/mmm (191) | 3.036 | 3.238 | 65-8698 |
| ZrB2 | hP3 | P6/mmm (191) | 3.165 | 3.530 | 89-3930 |
| NbB2 | hP3 | P6/mmm (191) | 3.102 | 3.321 | 75-1048 |
| MoB2 | hP3 | P6/mmm (191) * | 3.005 | 3.173 | 86-1097 |
| hR6 | R-3m (166) | 3.015 | 20.971 | 73-0704 | |
| WB2 | hP3 | P6/mmm (191) * | 3.020 | 3.050 | 89-3928 |
| hP12 | P63/mmc (194) | 2.983 | 13.879 | 43-1386 | |
| Calculation of MeB2 by average method | hP3 | P6/mmm (191) | 3.066 | 3.262 | This work |
| Experimental results of MeB2 from XRD | hP3 | P6/mmm (191) | 3.078 | 3.384 | This work |
| Phases | Ti | Zr | Nb | Mo | W | B | C | Si |
|---|---|---|---|---|---|---|---|---|
| MeB2 (Gray) | 6.39 | 6.43 | 6.65 | 5.61 | 5.08 | 69.84 | - | - |
| SiC (Black) | - | - | - | - | - | - | 53.89 | 46.11 |
| WB2 (White) | - | - | - | 12.66 | 36.77 | 50.57 | - | - |
| Material | Relative Density (%) | Vickers Hardness (GPa) | Flexure Strength (MPa) | Fracture Toughness (MPa·m½) | Ref. |
|---|---|---|---|---|---|
| (Ti,Zr,Nb,Mo,W)B2-SiC | 97.8 | 19.74 ± 0.8 | 533 ± 38 | 6.01 ± 0.77 | This work |
| (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)B2 | 99.8 | 22.0 ± 0.9 | 339 ± 17 | 3.81 ± 0.40 | [37] |
| (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)B2 | 96.3 | 21.7 ± 1.1 | - | 4.06 ± 0.35 | [38] |
| (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)B2-SiC | 99 | 22.4 ± 0.7 | 447 ± 45 | 4.85 ± 0.33 | [37] |
| (Hf0.2Zr0.2Mo0.2Nb0.2Ti0.2)B2 | 98.1 | 26.3 ± 1.8 | - | 3.64 ± 0.36 | [38] |
| (Hf0.2Mo0.2Ta0.2Nb0.2Ti0.2)B2 | 98.5 | 27.0 ± 0.4 | - | 4.47 ± 0.40 | [38] |
| (Ti0.2Zr0.2Hf0.2Ta0.2Mo0.2)B2 | 92.4 | 19.1 ± 1.8 | - | - | [12] |
| (Ti0.2Zr0.2Hf0.2Ta0.2Cr0.2)B2 | 92.2 | 19.9 ± 2.6 | - | - | [12] |
| TiB2-SiC | 98.1–98.7 | 23.7–23.8 | 349–601 | 4.8–5.3 | [39] |
| ZrB2–SiC | 99.1 | 20–21 | - | 3.8 ± 0.1 | [40] |
| NbB2–SiC | 97.5 | 17.9–18.9 | - | 4.16–4.62 | [41] |
| MoB2–SiC * | - | - | - | - | - |
| WB2–SiC * | - | - | - | - | - |
| Materials | Vickers Hardness (GPa) | Ref. |
|---|---|---|
| TiB2 (191) | 23.6 | [42] |
| ZrB2 (191) | 22.0 | [43] |
| NbB2 (191) | 20.25 | [44] |
| MoB2 (191) | 15.2 | [45] |
| WB2 (191) | 11.5 | [36] |
| Calculated value of MeB2 (191) * | 18.6 | / |
| SiC | 23.0 | [46] |
| WB2 (194) | 26.8 | [33] |
| Calculated value of MeB2-SiC * | 21.26 | / |
| Measured value of MeB2-SiC | 19.74 ± 0.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, Y.; Wang, Z.; Tian, H.; Wu, F.; Fu, L.; Dai, J.; Sha, J. A Novel Preparation Method of (Ti,Zr,Nb,Mo,W)B2-SiC Composite Ceramic Based on Reactive Sintering of Pre-Alloyed Metals. Crystals 2024, 14, 14. https://doi.org/10.3390/cryst14010014
Zu Y, Wang Z, Tian H, Wu F, Fu L, Dai J, Sha J. A Novel Preparation Method of (Ti,Zr,Nb,Mo,W)B2-SiC Composite Ceramic Based on Reactive Sintering of Pre-Alloyed Metals. Crystals. 2024; 14(1):14. https://doi.org/10.3390/cryst14010014
Chicago/Turabian StyleZu, Yufei, Zi Wang, Hongliang Tian, Fan Wu, Lianshen Fu, Jixiang Dai, and Jianjun Sha. 2024. "A Novel Preparation Method of (Ti,Zr,Nb,Mo,W)B2-SiC Composite Ceramic Based on Reactive Sintering of Pre-Alloyed Metals" Crystals 14, no. 1: 14. https://doi.org/10.3390/cryst14010014





