Production and Characterization of Nanocellulose from Maguey (Agave cantala) Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drying and Size Reduction for Maguey Fibers
2.2. Removal of Non-Cellulosic Components of Maguey Fibers
2.2.1. Alkali Treatment
2.2.2. Bleaching
2.3. Determination of the Chemical Composition of Cellulose
2.3.1. Determination of Holocellulose Content
2.3.2. Determination of α-Cellulose Content
2.3.3. Determination of Hemicellulose Content
2.3.4. Determination of Lignin and Other Extractive Contents
2.4. Strong Acid Hydrolysis of Cellulose
Calculation of Nanocellulose Yield from Maguey Cellulose
2.5. Characterization
2.5.1. Morphological Analysis via Scanning Electron Microscopy and Transmission Electron Microscopy
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.3. Zeta Potential Analysis
2.5.4. X-ray Diffraction (XRD)
2.5.5. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Removal of Non-Cellulosic Components of Maguey Fiber by Alkali Treatment and Bleaching
3.1.1. Cellulose Yield from Maguey Fiber
3.1.2. Holocellulose, α-Cellulose, Hemicellulose, and Lignin and Other Extractive Contents
3.2. Strong Acid Hydrolysis of Cellulose
3.2.1. Nanocellulose Yield from Strong Acid Hydrolysis of Cellulose
3.2.2. Morphological Analysis of Maguey Fiber, Cellulose, and Post-Hydrolyzed Cellulose
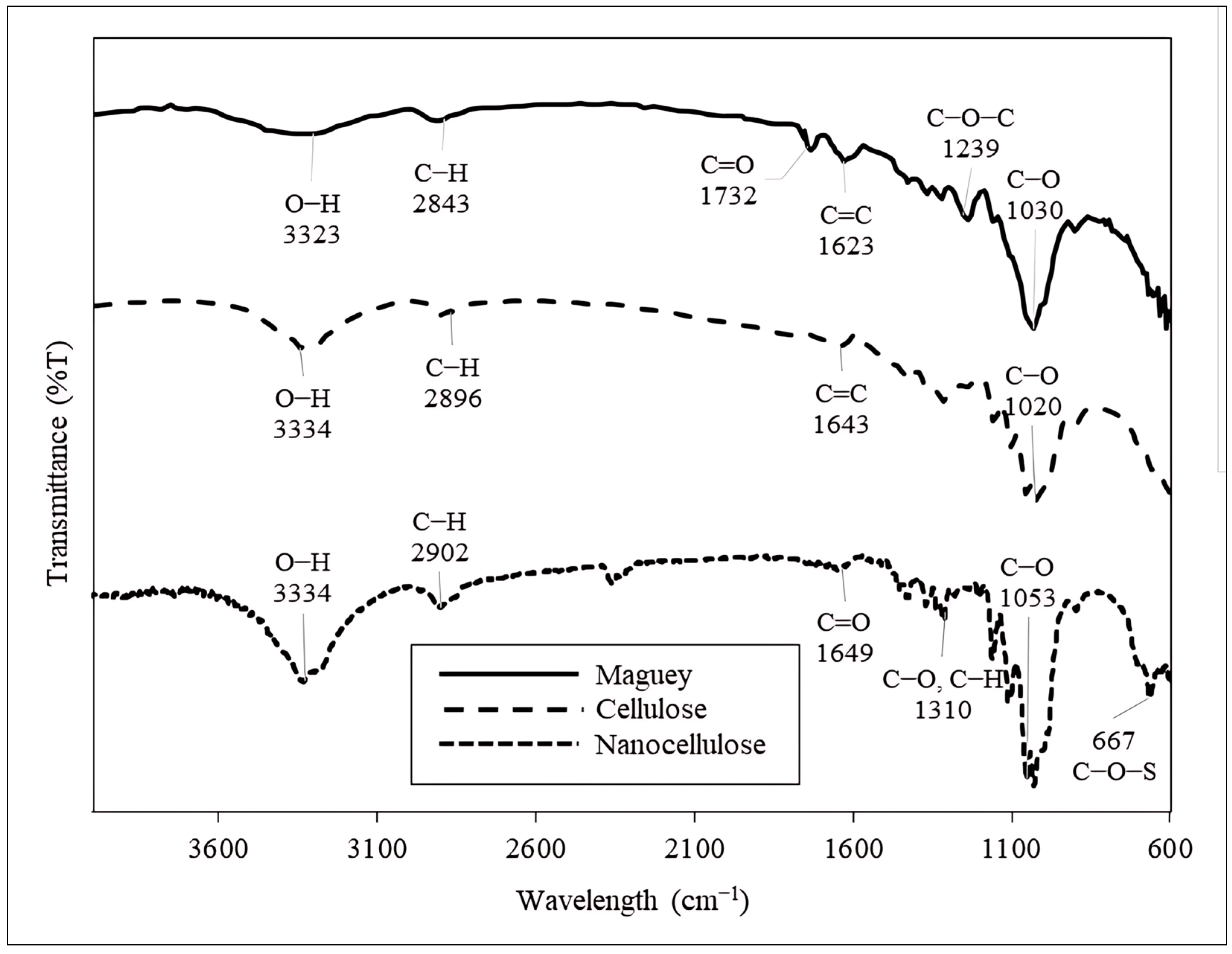
3.2.3. Fourier Transform Infrared (FTIR) Spectroscopy Analysis of Maguey Fiber, Cellulose, Nanocellulose
3.2.4. Zeta Potential Analysis of Nanocellulose
3.2.5. X-ray Diffraction Analysis of Nanocellulose
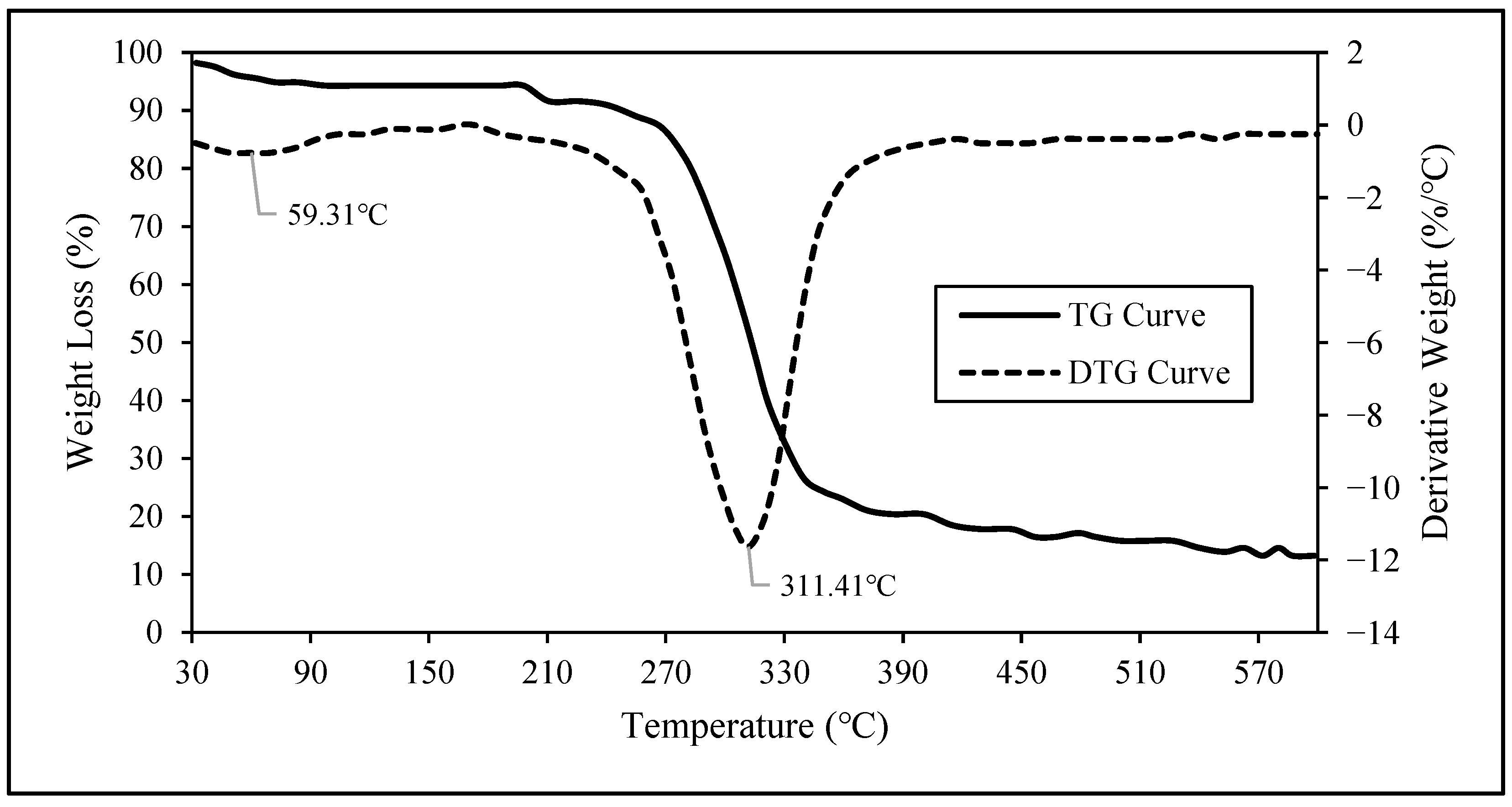
3.2.6. Thermogravimetric Analysis of Nanocellulose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-Based Nanocomposites for Sustainable Applications: A Review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Chen, P.; Re, G.; Berglund, L.; Wohlert, J. Surface Modification Effects on Nanocellulose—Molecular Dynamics Simulations Using Umbrella Sampling and Computational Alchemy. J. Mater. Chem. A 2020, 8, 23617–23627. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Balea, A.; Fuente, E.; Concepcion Monte, M.; Merayo, N.; Campano, C.; Negro, C.; Blanco, A. Industrial Application of Nanocelluloses in Papermaking: A Review of Challenges, Technical Solutions, and Market Perspectives. Molecules 2020, 25, 526. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Abdullah, I. Isolation and Characterization of Cellulose Nanocrystals from Agave angustifolia Fibre. BioRes 2013, 8, 1893–1908. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Kichigina, G.A.; Kushch, P.P.; Kiryukhin, D.P.; Krasnikova, S.S.; Badamshina, E.R.; Vasil’ev, S.G.; Soldatenkov, T.A.; et al. Cellulose from Annual Plants and Its Use for the Production of the Films Hydrophobized with Tetrafluoroethylene Telomers. Molecules 2022, 27, 6002. [Google Scholar] [CrossRef] [PubMed]
- Fauzee, S.N.; Othaman, R. Extraction and Dissolution of Cellulose from Nypa Fruit Husk for Nanofibers Fabrication. AIP Conf. Proc. 2013, 1571, 904–910. [Google Scholar] [CrossRef]
- Mustikaningrum, M.; Cahyono, R.B.; Yuliansyah, A.T. Effect of NaOH Concentration in Alkaline Treatment Process for Producing Nano Crystal Cellulose-Based Biosorbent for Methylene Blue. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012005. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y. Optimization of Bleaching Process for Cellulose Extraction from Apple and Kale Pomace and Evaluation of Their Potentials as Film Forming Materials. Carbohydr. Polym. 2021, 253, 117225. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Lv, K.; Sun, J.; Dai, C.; Liao, B.; Liu, C.; Mei, C.; Wu, Q.; Hubbe, M. Cellulose nanomaterials in oil and gas industry: Current status and future perspectives. Prog. Mater. Sci. 2023, 139, 101187. [Google Scholar] [CrossRef]
- Kandhola, G.; Djioleu, A.; Rajan, K.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.W. Maximizing Production of Cellulose Nanocrystals and Nanofibers from Pre-Extracted Loblolly Pine Kraft Pulp: A Response Surface Approach. Bioresour. Bioprocess. 2020, 7, 19. [Google Scholar] [CrossRef]
- Huntley, C.J.; Crews, K.D.; Abdalla, M.A.; Russell, A.E.; Curry, M.L. Influence of Strong Acid Hydrolysis Processing on the Thermal Stability and Crystallinity of Cellulose Isolated from Wheat Straw. Int. J. Chem. Eng. 2015, 2015, 658163. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose Prepared by Acid Hydrolysis of Isolated Cellulose from Sugarcane Bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.Y. Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Molecules 2019, 24, 4273. [Google Scholar] [CrossRef]
- Bacha, E.G. Response Surface Methodology Modeling, Experimental Validation, and Optimization of Acid Hydrolysis Process Parameters for Nanocellulose Extraction. S. Afr. J. Chem. Eng. 2022, 40, 176–185. [Google Scholar] [CrossRef]
- Hulle, A.; Kadole, P.; Katkar, P. Agave americana Leaf Fibers. Fibers 2015, 3, 64–75. [Google Scholar] [CrossRef]
- Kolte, P.P.; Kolte, M.P.P.; Daberao, M.A.M. Agave americana: The Natural Leaf Fiber. Text. Rev. 2012. Available online: https://www.researchgate.net/publication/312583870 (accessed on 25 October 2021).
- Anwar Hossain, M.; Abul Hossain Head, M.; Abdullah AI-Mamun Head, S.M.; Rahman, M.; Shahabuddin, M.; Shariful Alam Treasurer, K. Isolation and Characterisation of Agave Cantala (Dakatia) Fibre. J. Sci. Technol. 2009, 1, 43–56. [Google Scholar]
- Palacios Hinestroza, H.; Hernández Diaz, J.A.; Esquivel Alfaro, M.; Toriz, G.; Rojas, O.J.; Sulbarán-Rangel, B.C. Isolation and Characterization of Nanofibrillar Cellulose from Agave tequilana Weber Bagasse. Adv. Mater. Sci. Eng. 2019, 2019, 1342547. [Google Scholar] [CrossRef]
- Ortega, Z.; Castellano, J.; Suárez, L.; Paz, R.; Díaz, N.; Benítez, A.N.; Marrero, M.D. Characterization of Agave americana L. Plant as Potential Source of Fibres for Composites Obtaining. SN Appl. Sci. 2019, 1, 987. [Google Scholar] [CrossRef]
- Yang, X.; Berglund, L.A. Structural and Ecofriendly Holocellulose Materials from Wood: Microscale Fibers and Nanoscale Fibrils. Adv. Mater. 2021, 33, 2001118. [Google Scholar] [CrossRef]
- Trindade, W.G.; Hoareau, W.; Megiatto, J.D.; Razera, I.A.T.; Castellan, A.; Frollini, E. Thermoset Phenolic Matrices Reinforced with Unmodified and Surface-Grafted Furfuryl Alcohol Sugar Cane Bagasse and Curaua Fibers: Properties of Fibers and Composites. Biomacromolecules 2005, 6, 2485–2496. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum Alkaline Treatment Parameters for the Extraction of Cellulose and Production of Cellulose Nanocrystals from Apple Pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Krishnadev, P.; Subramanian, K.S.; Janavi, G.J.; Ganapathy, S.; Lakshmanan, A. Synthesis and Characterization of Nano-Fibrillated Cellulose Derived from Green Agave americana L. Fiber. BioRes 2020, 15, 2442–2458. [Google Scholar] [CrossRef]
- Syafri, E.; Jamaluddin, S.N.; Mahardika, M.; Amanda, P.; Ilyas, R. Isolation and Characterization of Cellulose Nanofibers from Agave gigantea by Chemical-Mechanical Treatment. Int. J. Biol. Macromol. 2022, 200, 25–33. [Google Scholar] [CrossRef]
- Subramanian, P.; Sriramajayam, S.; Vijayakumary, P.; Raja, K.; Reddy, M.; Research, P.G. Extraction of Cellulose from Banana Sheath and Its Characterization. Pharma Innov. J. 2022, 11, 1861–1867. [Google Scholar]
- Fareez, I.M.; Ibrahim, N.A.; Wan Yaacob, W.M.H.; Mamat Razali, N.A.; Jasni, A.H.; Abdul Aziz, F. Characteristics of Cellulose Extracted from Josapine Pineapple Leaf Fibre after Alkali Treatment Followed by Extensive Bleaching. Cellulose 2018, 25, 4407–4421. [Google Scholar] [CrossRef]
- Fitriana, N.E.; Suwanto, A.; Jatmiko, T.H.; Mursiti, S.; Prasetyo, D.J. Cellulose Extraction from Sugar Palm (Arenga Pinnata) Fibre by Alkaline and Peroxide Treatments. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012053. [Google Scholar] [CrossRef]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; Peres, D.; Dufresne, A. Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2010, 17, 1147–1158. [Google Scholar] [CrossRef]
- Espino, E.; Cakir, M.; Domenek, S.; Román-Gutiérrez, A.D.; Belgacem, N.; Bras, J. Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind. Crops Prod. 2014, 62, 552–559. [Google Scholar] [CrossRef]
- Gallardo-Sánchez, M.A.; Diaz-Vidal, T.; Navarro-Hermosillo, A.B.; Figueroa-Ochoa, E.B.; Ramirez Casillas, R.; Anzaldo Hernández, J.; Rosales-Rivera, L.C.; Martinez, A.; Enríques, S.; Macías-Balleza, E.R. Optimization of the Obtaining of Cellulose Nanocrystals from Agave tequilana Weber Var. Azul Bagasse by Acid Hydrolysis. Nanomaterials 2021, 11, 520. [Google Scholar] [CrossRef]
- Ramírez Casillas, R.; del Carmen López López, M.; Becerra Aguilar, B.; Dávalos Olivares, F.; Satyanarayana, K.G. Preparation and Characterization of Cellulose Nanocrystals using Soluble Grade Cellulose from Acid Hydrolysis of Huizache (Acacia farnesiana L. Willd.). BioResources 2019, 14, 3319–3338. [Google Scholar] [CrossRef]
- Mueller, S.; Weder, C.; Foster, E.J. Isolation of cellulose nanocrystals from pseudostems of banana plants. RSC Adv. 2014, 4, 907–915. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Ishak, W.H.W.; Ahmad, I.; Ramli, S.; Amin, M.C.I.M. Gamma Irradiation-Assisted Synthesis of Cellulose Nanocrystal-Reinforced Gelatin Hydrogels. Nanomaterials 2018, 8, 749. [Google Scholar] [CrossRef]
- IR Spectrum Table & Charts. Available online: https://www.sigmaaldrich.com/PH/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 5 April 2023).
- Lopes, J.d.O.; Garcia, R.A.; de Souza, N.D. Infrared Spectroscopy of the Surface of Thermally-Modified Teak Juvenile Wood. Maderas Cienc. Tecnol. 2018, 20, 737–746. [Google Scholar] [CrossRef]
- Merlic, C.; Fam, B.; The Regents of University of California. Problems in NMR and IR Spectroscopy. Available online: https://webspectra.chem.ucla.edu/index.html (accessed on 7 April 2024).
- Nacos, M.K.; Katapodis, P.; Pappas, C.; Daferera, D.; Tarantilis, P.A.; Christakopoulos, P.; Polissiou, M. Kenaf Xylan—A Source of Biologically Active Acidic Oligosaccharides. Carbohydr. Polym. 2006, 66, 126–134. [Google Scholar] [CrossRef]
- Fitriani, F.; Aprilia, S.; Arahman, N.; Bilad, M.R.; Amin, A.; Huda, N.; Roslan, J. Isolation and Characterization of Nanocrystalline Cellulose Isolated from Pineapple Crown Leaf Fiber Agricultural Wastes Using Acid Hydrolysis. Polymers 2021, 13, 4188. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Lai, C.W.; Hamid, S.B.A. Facile Preparation of Highly Crystalline Nanocellulose by Using Ionic Liquid. Adv. Mater. Res. 2015, 1087, 106–110. [Google Scholar]
- Dos Reis, R.R.; Effting, C.; Schackow, A. Cellulose Nanofibrils on Lightweight Mortars for Improvement of the Performance of Cement Systems. Carbohydr. Polym. Technol. Appl. 2023, 5, 100303. [Google Scholar] [CrossRef]
- Orrabalis, C.; Rodríguez, D.; Pampillo, L.G.; Londoño-Calderón, C.; Trinidad, M.; Martínez-García, R. Characterization of Nanocellulose Obtained from Cereus Forbesii (a South American Cactus). Mater. Res. 2019, 22, e20190243. [Google Scholar] [CrossRef]
- Robles-García, M.Á.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; Barrera-Rodríguez, A.; Aguilar, J.; Aguilar, J.A.; Reynoso-Marín, F.J.; Ceja, I.; Dórame-Miranda, R.; Rodríguez-Félix, F. Nanofibers of Cellulose Bagasse from Agave tequilana Weber Var. Azul by Electrospinning: Preparation and Characterization. Carbohydr. Polym. 2018, 192, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Whba, F.; Mohamed, F.; Idris, M.; Yahya, M. Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI. Appl. Sci. 2023, 13, 6316. [Google Scholar] [CrossRef]
- Jirathampinyo, S.; Chumchoochart, W.; Tinoi, J. Integrated Biobased Processes for Nanocellulose Preparation from Rice Straw Cellulose. Processes 2023, 11, 1006. [Google Scholar] [CrossRef]




| Fiber Sources | Process Parameters | Lignocellulose Yield, % | Components, % | Reference | |||
|---|---|---|---|---|---|---|---|
| Alkali Treatment | Bleaching | α-Cellulose | Hemicellulose | Lignin and Other Extractives | |||
| Agave cantala | 4 wt. % NaOH, 75 °C, 2 h * | 5 v/v % NaClO, 70 °C to room temp., 2 h | 53.56 | 89.45 ± 2.64 | 2.96 ± 0.13 | 7.59 ± 2.77 | Present study |
| Agave americana | 2–4 w/v % NaOH, 80 °C, 2 h | 2 w/v % NaClO2, 80 °C, 4 h | 65.00 ± 2.00 | 90.00 ± 5.00 | 9.00 ± 5.00 | 0.04 ± 0.10 | [28] |
| Agave angustifolia | 4% NaOH, 70–80 °C, 2 h * | 1.7 w/v % NaClO2, 70–80 °C, 4 h | 67.01 ± 0.15 | 97.31 ± 0.02 | 3.14 ± 0.35 | 0.23 ± 0.04 | [7] |
| Agave gigantea | 5 w/v % NaOH, 80 °C, 2 h | 1.7 wt. % NaClO2, 80 °C, 1 h * | n.a. | 89.39 | 3.73 | 0.53 | [29] |
| Apple pomace | 6% NaOH, 60 °C, 30 min | 1% NaClO, 95 °C, 1 h | 33.17 | 84.72 | n.a. | n.a. | [26] |
| Banana sheath | 2% NaOH, room temp., 6 h | 5% NaClO2, 50 °C, 1 h | 28.40 | 46.50 | 9.34 | 11.62 | [30] |
| Pineapple leaf | 2 w/v % NaOH | 4 w/v % NaClO, 85 ± 5 °C, 4 h | 70.90 ± 1.10 | 85.53 ± 2.30 | 0.30 ± 0.90 | 0.40 ± 0.30 | [31] |
| Sugar palm | 10 w/w % NaOH, 150 °C, 2 h | 15% H2O2, 60 °C, 1.5 h | n.a. | 86.99 ± 1.98 | 9.95 ± 0.87 | 2.90 ± 1.38 | [32] |
| Fiber Sources | Process Parameters | Nanocellulose Yield, % | Characteristics | Reference | |||
|---|---|---|---|---|---|---|---|
| Acid Hydrolysis | Diameter (nm) | Length (nm) | Crystallinity Index (%) | Thermal Stability (°C) | |||
| Agave cantala | 50 wt. % H2SO4, 50 °C, 45 min | 81.58 ± 0.36 (43.50–43.89 *) | 8–75 | 72–866 | 74.80 | 311.41 | Present study |
| Agave americana | 70% HNO3 and 80% CH3COOH, 100 °C, 30 min | 27.0 * | n.a | 18.2 ± 10.14 | 64.11 | 374.70 | [28] |
| Agave angustifolia | 60 wt. % H2SO4, 45 °C, 45 min | n.a | 8–15 | 170–500 | 82.40 | 361 | [7] |
| Agave sisalana | 55 wt. % H2SO4, 45–60 °C, 20–30 min | n.a | 5.9 ± 1.0–10.5 ± 2.9 | 177 ± 56–433 ± 132 | n.a | n.a | [33] |
| Agave tequilana | 65 wt. % H2SO4, 50 °C, 60 min | n.a | 11 ± 4 | 323 ± 113 | 71 | 324 | [34] |
| Agave tequilana Weber var. Azul | 60–65 wt. % H2SO4, 40–60 °C, 40–70 min | 4.2–96 | 8.6–9.1 | 216–829 | 88.4–90.1 | n.a | [35] |
| Apple pomace | 45% H2SO4, 50 °C, 45 min | n.a | 7.9 ± 1.25 | 28 ± 2.03 | 78 | 187 | [26] |
| Acacia fornesiana L. Willd | 60–65 wt. % H2SO4, 45–55 °C, 45–65 min | n.a | n.a | 100–260 | n.a | n.a | [36] |
| Banana pseudostem | 11 M H2SO4, 50 °C, 30–240 min | 10 * | 13 ± 4– 19 ± 6 | 319 ± 68–466 ± 159 | 69–74 | n.a | [37] |
| Barley | 65 wt. % H2SO4, 50 °C, 60 min | n.a | 10 ± 4 | 329 ± 123 | 66 | 357 | [34] |
| Sugarcane bagasse | 60 w/v % H2SO4, 50 °C, 5 h | n.a | 35 | 170 | n.a | 345 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumarago, E.C.; dela Cerna, M.F.M.; Leyson, A.K.B.; Tan, N.P.B.; Magsico, K.F. Production and Characterization of Nanocellulose from Maguey (Agave cantala) Fiber. Polymers 2024, 16, 1312. https://doi.org/10.3390/polym16101312
Sumarago EC, dela Cerna MFM, Leyson AKB, Tan NPB, Magsico KF. Production and Characterization of Nanocellulose from Maguey (Agave cantala) Fiber. Polymers. 2024; 16(10):1312. https://doi.org/10.3390/polym16101312
Chicago/Turabian StyleSumarago, Erwin C., Mary Frahnchezka M. dela Cerna, Andrea Kaylie B. Leyson, Noel Peter B. Tan, and Kendra Felizimarie Magsico. 2024. "Production and Characterization of Nanocellulose from Maguey (Agave cantala) Fiber" Polymers 16, no. 10: 1312. https://doi.org/10.3390/polym16101312








