Low Red to Far-Red Light Ratio Promoted Growth and Fruit Quality in Salt-Stressed Tomato Plants Based on Metabolomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Experimental Indexes
2.2.1. Determination of Plant Growth Parameters
2.2.2. Determination of Tomato Fruit Quality Parameters
2.2.3. Metabolite Extraction and Profiling Analysis of Tomato Fruits
2.3. Data Analysis
3. Results
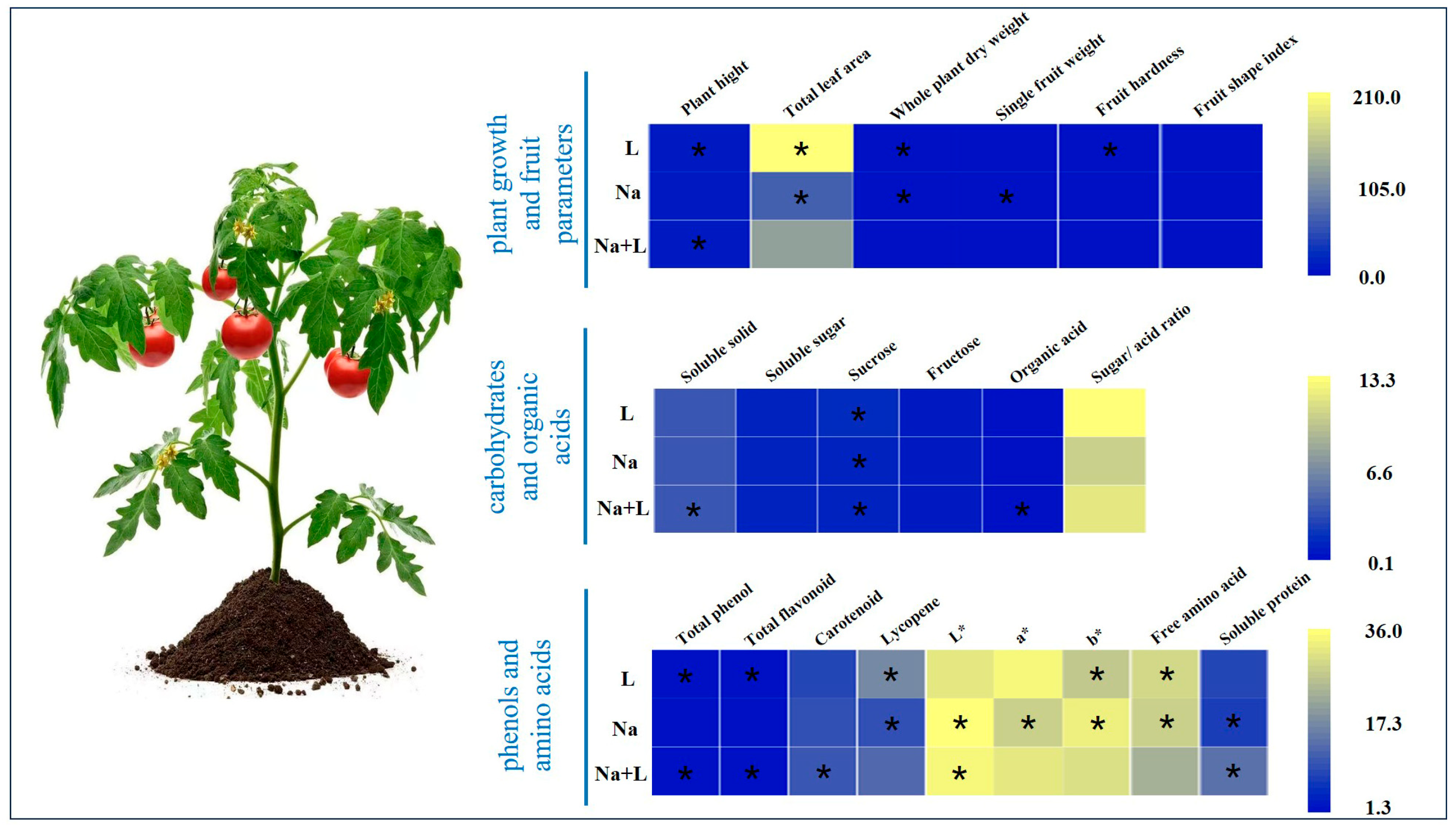
3.1. Low R/FR Alleviated the Adverse Effects of Salt Stress on Tomato Plant Growth and Fruit Quality
3.2. Metabolomics Analysis
3.2.1. Identification of Differentially Expressed Metabolites
3.2.2. Low R/FR Enhanced the Contents of Carbohydrates and Organic Acids in Salt-Stressed Tomato Fruits
3.2.3. Low R/FR Improved the Contents of Phenols and Amino Acids in Tomato Fruits under Salt Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ntagkas, N.; de Vos, R.C.H.; Woltering, E.J.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Modulation of the Tomato Fruit Metabolome by LED Light. Metabolites 2020, 10, 266. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.W.; Ai, K.Q.; Bao, E.C.; Zou, Z.R. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Cai, L.L.; Long, Z.D.; Zhang, X.; Zhao, F.Y. Effects of non-uniform salt stress on growth, yield, and quality of tomato. Soil Sci. Plant Nutr. 2021, 67, 545–556. [Google Scholar] [CrossRef]
- Shah, W.H.; Rasool, A.; Saleem, S.; Mushtaq, N.U.; Tahir, I.; Hakeem, K.R.; Rehman, R.U. Understanding the integrated pathways and mechanisms of transporters, protein kinases, and transcription factors in plants under salt stress. Int. J. Genom. 2021, 202, 5578727. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, K.N.; More, S.J.; Kumar, A.; Kumar, D.; Singh, B.; Bhardwaj, V.; Kumar, A.; Das, S.K.; Singh, R.K.; Zinta, G. Salinity responses and tolerance mechanisms in underground vegetable crops: An integrative review. Planta 2022, 255, 68. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effects of Salinity Stress at Different Growth Stages on Tomato Growth, Yield, and Water-Use Efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 624–634. [Google Scholar] [CrossRef]
- Martínez, J.P.; Fuentes, R.; Farías, K.; Lizana, C.; Alfaro, J.F.; Fuentes, L.; Calabrese, N.; Bigot, S.; Quinet, M.; Lutts, S. Effects of Salt Stress on Fruit Antioxidant Capacity of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum var. cerasiforme) Tomatoes. Agronomy 2020, 10, 1481. [Google Scholar] [CrossRef]
- Van Meulebroek, L.; Hanssens, J.; Steppe, K.; Vanhaecke, L. Metabolic Fingerprinting to Assess the Impact of Salinity on Carotenoid Content in Developing Tomato Fruits. Int. J. Mol. Sci. 2016, 17, 821. [Google Scholar] [CrossRef]
- Tan, T.T.; Li, S.L.; Fan, Y.F.; Wang, Z.L.; Ali Raza, M.; Shafiq, I.; Wang, B.B.; Wu, X.L.; Yong, T.W.; Wang, X.C.; et al. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop. J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Smith, H. Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Fernández-Milmanda, G.L.; Ballaré, C.L. Shade avoidance: Expanding the color and hormone palette. Trends Plant Sci. 2021, 26, 509–523. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, T.; Choi, S.; Wang, Y.J.; Lin, M.Y.; Liceaga, A.M. Supplemental intracanopy far-red radiation to red LED light improves fruit quality attributes of greenhouse tomatoes. Sci. Hortic. 2020, 261, 108985. [Google Scholar] [CrossRef]
- Kim, H.J.; Lin, M.Y.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Ahres, M.; Pálmai, T.; Gierczik, K.; Dobrev, P.; Vanková, R. The impact of far-red light supplementation on hormonal responses to cold acclimation in barley. Biomolecules 2021, 11, 450. [Google Scholar] [CrossRef]
- Wang, Y.L.; Bian, Z.H.; Pan, T.H.; Cao, K.; Zou, Z.R. Improvement of tomato salt tolerance by the regulation of photosynthetic performance and antioxidant enzyme capacity under a low red to far-red light ratio. Plant Physiol. Biochem. 2021, 167, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.X.; Gao, X.X.; Li, B.; Wang, W.J.; Bai, L.Q. Low red to far-red light ratio promotes salt tolerance by improving leaf photosynthetic capacity in cucumber. Front. Plant Sci. 2023, 13, 1053780. [Google Scholar] [CrossRef]
- Hayes, S.; Pantazopoulou, C.K.; van Gelderen, K.; Reinen, E.; Tween, A.L.; Sharma, A.; de Vries, M.; Prat, S.; Schuurink, R.C.; Testerink, C. Soil salinity limits plant shade avoidance. Curr. Biol. 2019, 29, 1669–1676. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- Yang, D.S.; Zhang, J.; Li, M.X.; Shi, L.X. Metabolomics analysis reveals the salt-tolerant mechanism in Glycine soja. J. Plant Growth Regul. 2017, 36, 460–471. [Google Scholar] [CrossRef]
- Kotilainen, T.; Aphalo, P.J.; Brelsford, C.C.; Böök, H.; Devraj, S.; Heikkilä, A.; Hernández, R.; Kylling, A.; Lindfors, A.; Robson, T.M. Patterns in the spectral composition of sunlight and biologically meaningful spectral photon ratios as affected by atmospheric factors. Agric. For. Meteorol. 2020, 291, 108041. [Google Scholar] [CrossRef]
- Li, H. Experimental Principles and Techniques of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Mellidou, I.; Ainalidou, A.; Papadopoulou, A.; Leontidou, K.; Genitsaris, S.; Karagiannis, E.; Van de Poel, B.; Karamanoli, K. Comparative Transcriptomics and Metabolomics Reveal an Intricate Priming Mechanism Involved in PGPR-Mediated Salt Tolerance in Tomato. Front. Plant Sci. 2021, 12, 713984. [Google Scholar] [CrossRef] [PubMed]
- Abooshahab, R.; Hooshmand, K.; Razavi, S.A.; Gholami, M.; Sanoie, M.; Hedayati, M. Plasma metabolic profiling of human thyroid nodules by gas chromatography-mass spectrometry (GC-MS)-based untargeted metabolomics. Front. Cell Dev. Biol. 2020, 8, 385. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.L.; Cheng, Y.J.; Feng, L.Y.; Wu, X.L.; Fan, Y.F.; Raza, M.A.; Wang, X.C.; Yong, T.W.; Liu, W.G.; et al. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 2020, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Monostori, I.; Heilmann, M.; Kocsy, G.; Rakszegi, M.; Ahres, M.; Altenbach, S.B.; Szalai, G.; Pál, M.; Toldi, D.; Simon-Sarkadi, L.; et al. LED lighting—Modification of growth, metabolism, yield and flour composition in wheat by spectral quality and intensity. Front. Plant Sci. 2018, 9, 605. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Xie, E.; Wei, X.J.; Ding, A.Z.; Zheng, L.; Wu, X.N.; Anderson, B. Short-term effects of salt stress on the amino acids of Phragmites australis root exudates in constructed wetlands. Water 2020, 12, 569. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Nicolás-Almansa, M.; Razi, M.; Rubio, M.; Ruiz, D.; Martínez-Gómez, P. Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective. Int. J. Mol. Sci. 2021, 22, 333. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.Z.; Li, F.; Yan, C.R.; Zhong, X.L.; Liu, Q.; Xia, X.; Li, H.R.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Liang, W.J.; Ma, X.L.; Wan, P.; Liu, L.Y. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Yang, D.; Jia, X.; Xie, H. Heptyl vicianoside and methyl caramboside from sour star fruit. Nat. Prod. Res. 2019, 33, 1233–1236. [Google Scholar] [CrossRef]
- Su, B.N.; Pawlus, A.D.; Jung, H.A.; Keller, W.J.; McLaughlin, J.L.; Kinghorn, A.D. Chemical Constituents of the Fruits of Morinda citrifolia (Noni) and Their Antioxidant Activity. J. Nat. Prod. 2005, 68, 592–595. [Google Scholar] [CrossRef]
- Seckin, B.; Sekmen, A.H.; Türkan, I. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regul. 2009, 28, 12–20. [Google Scholar] [CrossRef]
- Johnson, L.; Verraest, D.L.; van Haveren, J.; Hakala, K.; Peters, J.A.; van Bekkum, H. Methyl α-D-fructofuranoside: Synthesis and conversion into carboxylates. Tetrahedron Asymmetry 1994, 5, 2475–2484. [Google Scholar] [CrossRef]
- Gerschenson, L.N. The production of galacturonic acid enriched fractions and their functionality. Food Hydrocoll. 2017, 68, 23–30. [Google Scholar] [CrossRef]
- Duan, H.R.; Tiika, R.J.; Tian, F.P.; Lu, Y.; Zhang, Q.; Hu, Y.; Cui, G.X.; Yang, H.S. Metabolomics analysis unveils important changes involved in the salt tolerance of Salicornia europaea. Front. Plant Sci. 2023, 13, 1097076. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, S.Y.; Oh, M.M. Growth and phenolic compounds of Crepidiastrum denticulatum under various blue light intensities with a fixed phytochrome photostationary state using far-red light. Hortic. Environ. Biotechnol. 2019, 60, 199–206. [Google Scholar] [CrossRef]
- Tegelberg, R.; Julkunen Tiitto, R.; Aphalo, P.J. Red: Far-red light ratio and UV-B radiation: Their effects on leaf phenolics and growth of silver birch seedlings. Plant Cell Environ. 2004, 27, 1005–1013. [Google Scholar] [CrossRef]
- Naliwajski, M.; Skłodowska, M. The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress. Cells 2021, 10, 609. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Turi, C.E.; Saxena, P.K. Serotonin: An ancient molecule and an important regulator of plant processes. Biotechnol. Adv. 2016, 34, 1347–1361. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Li, R.; Li, C.; Zhou, X.; Xu, X.; Sun, M.; Bai, L.; Hou, L. Low Red to Far-Red Light Ratio Promoted Growth and Fruit Quality in Salt-Stressed Tomato Plants Based on Metabolomic Analysis. Agronomy 2024, 14, 983. https://doi.org/10.3390/agronomy14050983
Miao Y, Li R, Li C, Zhou X, Xu X, Sun M, Bai L, Hou L. Low Red to Far-Red Light Ratio Promoted Growth and Fruit Quality in Salt-Stressed Tomato Plants Based on Metabolomic Analysis. Agronomy. 2024; 14(5):983. https://doi.org/10.3390/agronomy14050983
Chicago/Turabian StyleMiao, Yanxiu, Ruochan Li, Caixia Li, Xiaolin Zhou, Xinxin Xu, Meihua Sun, Longqiang Bai, and Leiping Hou. 2024. "Low Red to Far-Red Light Ratio Promoted Growth and Fruit Quality in Salt-Stressed Tomato Plants Based on Metabolomic Analysis" Agronomy 14, no. 5: 983. https://doi.org/10.3390/agronomy14050983





