Nature of Perovskite Mineralization of Silicate-Carbonate Veins in the Margins of Kusinsko-Kopanskaya Layered Intrusion (South Urals, Russia)
Abstract
:1. Introduction
1.1. Historical Background
1.2. Modern Studies of Perovskite
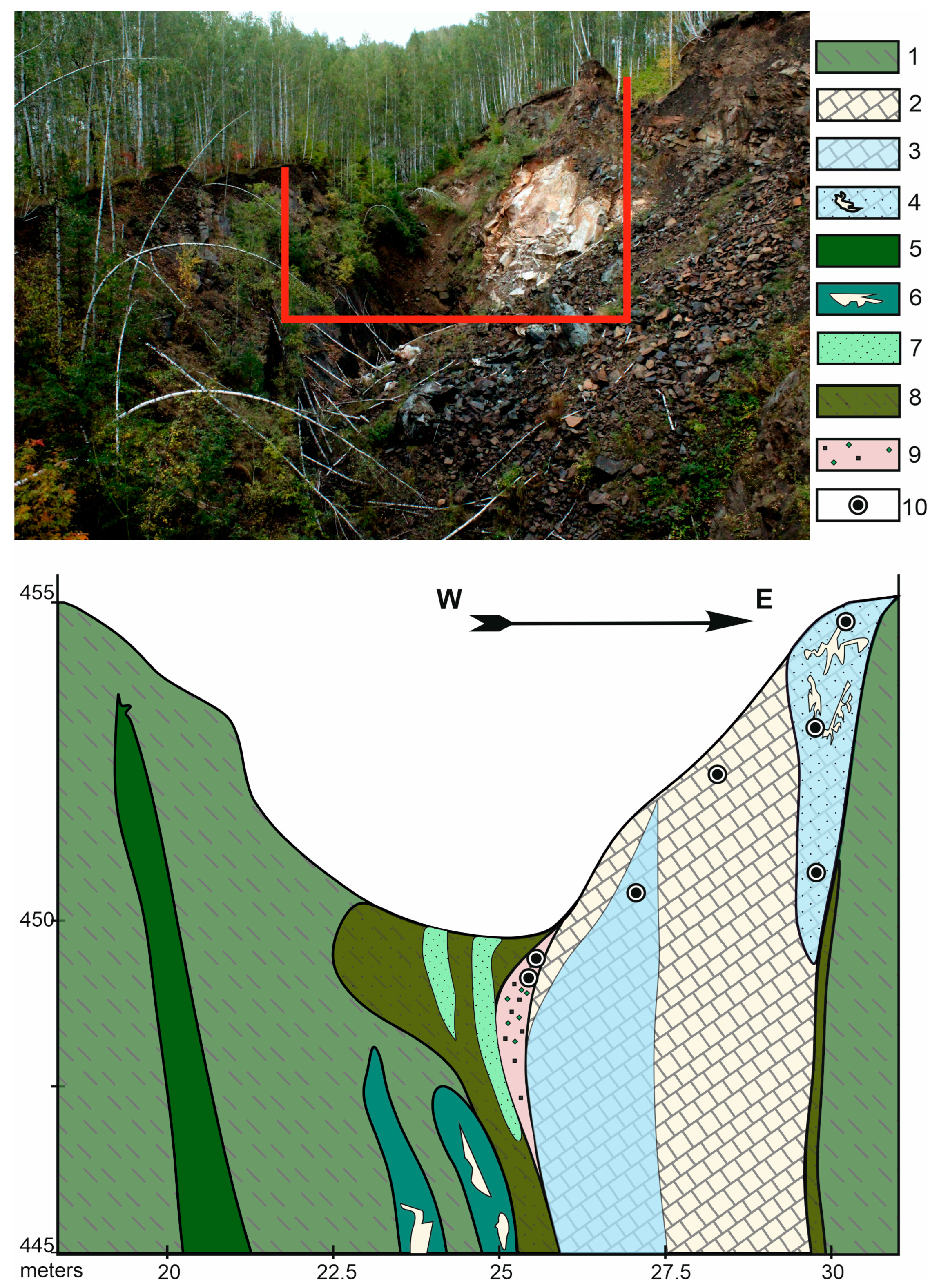
2. Geological Setting
3. Material and Methods
3.1. Sample Collection
3.2. Analytical Methods
4. Results
4.1. Parageneses with Perovskite

| № | MgO | Al2O3 | SiO2 | CaO | TiO2 | V2O5 | MnO | FeO | Fe2O3 | Y2O3 | ZrO2 | Ce2O3 | Nd2O3 | Sm2O3 | Σ | Mineral |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17.11 | 0.59 | – * | – | 0.05 | – | 1.39 | 19.73 | 63.90 | – | – | – | – | – | 102.77 | Mg0.79Fe0.18Mn0.04Fe1.98Al0.02O4.00 |
| 2 | – | – | – | 40.93 | 56.15 | 0.79 | – | 0.60 | – | – | – | – | – | – | 98.47 | Ca1.01Ti0.97V0.01Fe0.01O2.98 |
| 3 | – | – | – | 41.79 | 57.06 | 0.40 | 0.03 | 0.46 | – | – | – | – | – | – | 98.74 | Ca1.00Ti0.98V0.01Fe0.01O2.99 |
| 4 | 17.03 | 1.08 | – | – | 0.22 | 0.09 | 1.39 | 18.65 | 61.51 | – | – | – | – | – | 99.97 | Mg0.80Fe0.17Mn0.04Fe1.95Al0.04O4.00 |
| 5 | 0.38 | 1.55 | 36.60 | 34.78 | 2.37 | – | – | – | 27.08 | – | – | – | – | – | 102.76 | (Ca3.03Mg0.05)∑3.08(Fe1.65Al0.15Ti0.14)∑1.94 Si2.98O12.02 |
| 6 | – | 0.23 | – | 41.18 | 55.54 | 0.49 | – | 0.72 | – | – | 1.01 | 0.54 | 0.13 | – | 99.84 | Ca1.00Ti0.95V0.01Fe0.01Al0.01O2.97 |
| 7 | – | 0.21 | 0.36 | 40.25 | 56.31 | 0.54 | – | 0.54 | – | – | – | 0.59 | – | – | 98.80 | Ca0.99Ti0.97V0.01Fe0.01Al0.01Si0.01O2.99 * |
| 8 | – | – | 0.41 | 41.04 | 56.41 | 0.54 | – | 0.46 | – | – | – | 0.48 | – | 0.31 | 99.65 | Ca1.00Ti0.97V0.01Fe0.01Si0.01O2.98 * |
| 9 | – | 0.09 | – | 39.30 | 56.11 | 0.43 | – | 1.04 | – | – | – | 0.90 | 0.52 | – | 98.39 | Ca0.98Ti0.98Fe0.02V0.01Ce0.01O2.99 |
| 10 | 13.02 | – | – | 0.73 | – | – | 2.14 | 22.61 | 61.15 | – | – | – | – | – | 99.65 | Mg0.67Fe0.28Mn0.06Ca0.03Fe2.00O4.00 |
| 11 | – | – | – | 40.28 | 56.16 | – | – | 1.76 | – | – | – | – | – | – | 98.20 | Ca0.99Ti0.97Fe0.03O2.97 |
| 12 | 0.33 | 2.40 | 36.05 | 34.71 | 2.79 | – | – | – | 26.07 | – | – | – | – | – | 102.35 | (Ca3.03Mg0.04)∑3.07(Fe1.61Al0.17Ti0.14)∑1.92 Si2.94O12.04 |
| 13 | – | – | – | 40.81 | 56.01 | – | – | 0.27 | – | – | – | 0.32 | – | – | 97.41 | Ca1.01Ti0.98Fe0.01O2.98 |
| 14 | – | – | – | 40.60 | 58.04 | – | – | 0.42 | – | – | – | – | – | – | 99.06 | Ca0.99Ti1.00Fe0.01O3.00 |
| 15 | 30.45 | – | – | – | 64.24 | 0.85 | 1.94 | 2.24 | – | – | – | – | – | – | 99.72 | Mg0.93Ti0.99Fe0.04 Mn0.03V0.01O3.00 |
| 16 | – | – | – | 41.21 | 59.10 | – | – | 0.49 | – | – | – | – | – | – | 100.80 | Ca0.99Ti1.00Fe0.01O3.00 |
| 17 | – | 1.09 | – | 12.39 | 36.16 | – | – | – | 6.28 | 2.59 | 34.36 | 1.01 | 1.55 | 0.34 | 95.77 | (Ca0.81Y0.08Nd0.03Ce0.02Sm0.01)∑0.95Zr1.02 (Ti1.66Fe0.29Al0.08)∑1.93O6.94 |
| 18 | – | – | – | 10.38 | 33.55 | – | – | 6.81 | – | 4.53 | 32.01 | 1.31 | 4.10 | 0.98 | 93.67 | (Ca0.71Y0.15Nd0.09Ce0.03Sm0.02)∑1.00Zr1.00 (Ti1.62Fe0.37)∑1.99O6.95 |
| 19 | 29.54 | – | – | – | 65.00 | – | 1.42 | 4.42 | – | – | – | – | – | – | 100.38 | Mg0.90Ti1.00Fe0.08Mn0.02O3.00 |
| 20 | – | – | – | 8.00 | 31.96 | – | – | 0.02 | 6.76 | 7.63 | 32.80 | 2.27 | 5.37 | 1.96 | 103.53 | (Ca0.56Y0.26Nd0.12Ce0.05Sm0.04Fe0.04)∑1.07 Zr1.04(Ti1.56Fe0.33)∑1.89O7.00 |
| 21 | – | – | – | 7.64 | 32.03 | – | – | 0.88 | 5.49 | 7.28 | 32.58 | 2.96 | 6.14 | 1.61 | 102.10 | (Ca0.54Y0.25Nd0.14Fe0.08Ce0.07Sm0.04)∑1.12 Zr1.04(Ti1.57Fe0.27)∑1.84O7.00 |

| № | MgO | SiO2 | CaO | TiO2 | MnO | FeO | Fe2O3 | Y2O3 | Nb2O5 | ZrO2 | La2O3 | Ce2O3 | Nd2O3 | Σ | Formulae |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.34 | – | – | – | 0.35 | 40.08 | 55.78 | – | – | – | – | – | – | 98.55 | Fe0.86Mg0.13Fe2.00O4.00 |
| 2 | – | – | 40.20 | 56.73 | – | 1.97 | – | – | – | – | – | – | 98.90 | Ca0.99Fe0.04Ti0.98O2.98 | |
| 3 | – | – | 41.31 | 57.84 | – | 0.33 | – | – | – | – | – | – | 99.48 | Ca1.01Fe0.01Ti0.99O2.99 | |
| 4 | – | – | 40.78 | 57.51 | 0.09 | 0.37 | – | – | 0.19 | – | – | – | 98.94 | Ca1.00Fe0.01Ti0.99O2.99 | |
| 5 | – | – | 40.63 | 56.95 | – | 0.36 | – | – | – | – | – | – | 97.94 | Ca1.00Fe0.01Ti0.99O2.99 | |
| 6 | – | – | 39.84 | 56.61 | – | 1.40 | – | – | – | – | – | – | 97.85 | Ca0.99Fe0.03Ti0.99O2.99 | |
| 7 | – | 2.08 | 11.48 | 31.81 | – | – | 6.38 | 3.74 | 32.85 | 2.26 | – | 3.12 | 1.96 | 95.68 | (Ca0.77Y0.12Ce0.07Nb0.06Nd0.4)∑1.06 Zr1.00(Ti1.50Fe0.30)∑1.80Si0.13O7.00 * |
| 8 | – | – | 40.46 | 58.24 | – | 0.38 | – | – | – | – | – | 99.08 | Ca0.99Fe0.01Ti1.00O3.00 | ||
| 9 | 0.66 | 1.30 | 11.73 | 31.92 | – | – | 7.21 | 3.87 | 31.70 | 1.87 | 0.54 | 2.85 | 1.17 | 94.82 | (Ca0.78Y0.13Ce0.06Nb0.05 Nd0.3La0.01Mg0.06)∑1.13 Zr0.96(Ti1.49Fe0.34)∑1.83Si0.08O6.90 * |
| 10 | 18.18 | 54.01 | 26.03 | 0.14 | – | 0.15 | – | – | – | – | – | – | 98.51 | Ca1.02Mg0.99Fe0.07Si1.98O5.98 | |
| 11 | – | – | 38.43 | 56.25 | – | 1.56 | 0.16 | – | 0.12 | 1.96 | 2.22 | 0.58 | 101.28 | (Ca0.95Fe0.03Ce0.02La0.02)∑1.02Ti0.98O3.00 | |
| 12 | – | – | 40.84 | 58.08 | – | 0.81 | 0.32 | – | – | – | – | 0.37 | 100.42 | (Ca0.99Fe0.02)∑1.01Ti0.99O2.99 | |
| 13 | – | – | 38.21 | 54.20 | – | 1.13 | 0.26 | – | – | 2.48 | 2.86 | 0.82 | 99.96 | (Ca0.96Fe0.02Ce0.02La0.02Nd0.01)∑1.03 Ti0.96O2.99 | |
| 14 | 18.38 | 54.58 | 26.05 | – | – | 1.08 | – | – | – | – | – | – | 100.09 | Ca1.01Mg0.99Fe0.03Si1.97O5.97 | |
| 15 | – | – | 40.01 | 56.95 | – | 0.50 | – | – | – | 0.87 | 0.53 | 0.26 | 99.12 | (Ca0.99Fe0.01La0.01)∑1.01Ti0.99O2.99 | |
| 16 | 16.96 | 52.76 | 25.61 | – | – | 2.24 | – | – | – | – | – | – | 97.57 | Ca1.02Mg0.94Fe0.07Si1.97O5.97 |
4.2. Perovskite Crystal Shape Difference between Mines

4.3. Chemical Composition of Perovskites

| № | CaO | TiO2 | V2O3 | FeO | La2O3 | Ce2O3 | Nd2O3 | Sm2O3 | Σ | Formulae |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40.75 | 57.13 | 0.35 | 0.37 | – | 0.83 | – | – | 99.44 | (Ca1.00Fe0.01Ce0.01)∑1.01(Ti0.98V0.01) ∑0.99O2.99 |
| 2 | 37.82 | 54.33 | 0.62 | 2.06 | – | 2.42 | 2.03 | 0.23 | 99.51 | (Ca0.95Fe0.04Ce0.02Nd0.02)1.03(Ti0.96V0.01)0.97O2.98 |
| 3 | 38.16 | 54.61 | 0.54 | 1.76 | – | 2.88 | 1.74 | – | 99.69 | (Ca0.96Fe0.03Ce0.02Nd0.01)1.03(Ti0.96V0.01)0.97O2.99 |
| 4 | 40.53 | 57.40 | 0.50 | 0.68 | – | 0.73 | – | 0.21 | 100.05 | (Ca0.99Fe0.01Ce0.01)1.01(Ti0.98V0.01)0.99O2.98 |
| 5 | 38.79 | 55.63 | 0.32 | 1.57 | – | 1.42 | 0.26 | – | 98.15 | (Ca0.97Fe0.03Nb0.02Ce0.01)1.03(Ti0.98V0.01)0.99O2.98 |
| 6 | 39.40 | 56.05 | 0.37 | 1.43 | – | 1.52 | 0.65 | 0.19 | 99.60 | (Ca0.97Ce0.01Fe0.03Nd0.01)1.02(Ti0.97V0.01)0.98O2.98 |
| 7 | 39.39 | 56.55 | 0.62 | 0.66 | – | 0.71 | – | – | 97.92 | (Ca0.98Fe0.01Ce0.01)1.00(Ti0.99V0.01)1.00O2.98 |
| 8 | 40.80 | 57.31 | 0.38 | 0.41 | – | 0.75 | – | – | 99.66 | (Ca1.00Fe0.01Ce0.01)1.02(Ti0.98V0.01)0.99O2.99 |
| 9 | 39.53 | 56.77 | 0.84 | 0.67 | – | 0.66 | 0.69 | – | 99.15 | (Ca0.98Fe0.01Ce0.01Nd0.01)1.00(Ti0.98V0.02)1.00O2.98 |
| 10 | 37.29 | 55.38 | – | 1.75 | – | 2.35 | 1.26 | 0.16 | 98.19 | (Ca0.95Fe0.03Ce0.02Nd0.01)1.01(Ti0.99V0.00)0.99O2.98 |
| 11 | 40.38 | 57.50 | – | 0.66 | 0.36 | 0.43 | – | – | 99.33 | (Ca0.99Fe0.01)1.01(Ti0.99V0.00)0.99O2.98 |
| 12 | 39.85 | 56.72 | – | 0.77 | – | 0.75 | – | – | 98.09 | (Ca0.99Fe0.01Ce0.01)1.01(Ti0.99V0.00)0.99O2.98 |
| 13 | 40.12 | 56.61 | – | 0.48 | – | 0.27 | – | – | 97.48 | (Ca1.00Fe0.01)1.01(Ti0.99V0.00)0.99O2.99 |
| 14 | 39.90 | 57.20 | – | 0.48 | – | – | – | – | 97.57 | (Ca0.99Fe0.01)1.00(Ti1.00V0.00)1.00O2.98 |
| 15 | 38.43 | 56.85 | 0.31 | 1.70 | 0.70 | 2.51 | 0.40 | – | 100.89 | (Ca0.95Fe0.03Ce0.02La0.01)1.01(Ti0.98V0.01)0.99O2.98 |
| 16 | 38.38 | 56.68 | 0.32 | 1.66 | 0.62 | 2.55 | 0.36 | – | 100.58 | (Ca0.95Fe0.03Ce0.02La0.01)1.01(Ti0.98V0.01)0.99O2.98 |
| 17 | 37.33 | 54.91 | – | 1.39 | 1.04 | 3.06 | 0.38 | – | 98.12 | (Ca0.95Ce0.03Fe0.03La0.01)1.02(Ti0.98V0.00)0.98O2.98 |
| 18 | 39.76 | 58.60 | – | 0.66 | 0.15 | 0.28 | – | – | 99.45 | (Ca0.98Ce0.00Fe0.01)0.99(Ti1.01V0.00)1.01O2.98 |

| Element | Octahedral Perovskites of Perovskite Mine | Cubic Perovskites of Perovskite Mine | Cubic Perovskites of Zelentsov Mine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Fe | 2390 | 2800 | 3790 | 2620 | 3680 | 4240 | 3400 | 4070 | 2830 | 2410 | 2950 | 2550 |
| Sr | 57.0 | 57.70 | 55.7 | 53.20 | 52.1 | 62.6 | 52.0 | 53.0 | 29.2 | 22.2 | 20.5 | 39.80 |
| Y | 216 | 217.00 | 38.9 | 143.7 | 231 | 179 | 316 | 320 | 470 | 224 | 194 | 329 |
| Zr | 12.0 | 12.0 | 8.59 | 22.1 | 24.8 | 21.5 | 26.7 | 27.7 | 345 | 293 | 56.0 | 622 |
| Nb | 466 | 469 | 624 | 479 | 334 | 557 | 237 | 312 | 138.5 | 139 | 249 | 166 |
| La | 3320 | 3360 | 4760 | 2950 | 2260 | 3440 | 1770 | 2280 | 798 | 352 | 1550 | 510 |
| Ce | 5190 | 4720 | 8560 | 4000 | 3250 | 4370 | 2760 | 3430 | 1430 | 906 | 2530 | 493 |
| Pr | 463 | 480 | 735 | 341 | 327 | 407 | 292 | 322 | 153.6 | 116.6 | 251 | 45.9 |
| Nd | 895 | 903 | 1335 | 644 | 696 | 786 | 682 | 808 | 306 | 257 | 438 | 82.0 |
| Sm | 444 | 402.4 | 495 | 451 | 563 | 624 | 580 | 712 | 270 | 227 | 225 | 70.8 |
| Eu | 110.5 | 111.9 | 111.0 | 148.0 | 143 | 175 | 135 | 161 | 71.0 | 43.3 | 43.2 | 22.0 |
| Gd | 199.0 | 195 | 167.0 | 290.0 | 391 | 341 | 387 | 390 | 269 | 184 | 169 | 67.6 |
| Tb | 20.1 | 20.2 | 11.2 | 28.40 | 40.2 | 31.9 | 39.1 | 40.1 | 37.9 | 25.0 | 20.2 | 12.8 |
| Dy | 94.2 | 98.2 | 40.9 | 123.0 | 179 | 137 | 187 | 195 | 253 | 146.9 | 115 | 91.0 |
| Ho | 11.1 | 10.9 | 2.84 | 9.73 | 15.7 | 11.6 | 17.8 | 19.8 | 26.8 | 15.4 | 12.1 | 13.8 |
| Er | 35.8 | 36.3 | 8.60 | 36.2 | 64.2 | 42.5 | 73.8 | 72.4 | 138.5 | 76.1 | 59.3 | 93.0 |
| Tm | 2.18 | 2.19 | 0.37 | 1.60 | 2.37 | 1.69 | 3.03 | 3.39 | 7.52 | 3.75 | 3.12 | 6.44 |
| Yb | 10.6 | 11.0 | 11.3 | 7.17 | 11.5 | 7.34 | 14.9 | 16.1 | 44.1 | 22.3 | 14.7 | 45.7 |
| Lu | 0.81 | 0.78 | 0.13 | 0.59 | 0.89 | 0.54 | 1.06 | 1.18 | 2.91 | 1.65 | 0.97 | 3.94 |
| Pb | 18.0 | 18.0 | 24.0 | 16.1 | – | 16.0 | 12.3 | 15.2 | 1.32 | 0.77 | 3.51 | 4.69 |
| La/Yb | 313 | 305 | 422 | 411 | 197 | 469 | 119 | 142 | 18 | 16 | 105 | 11 |
4.4. U-Pb Age of Perovskite Mineralization
5. Discussion
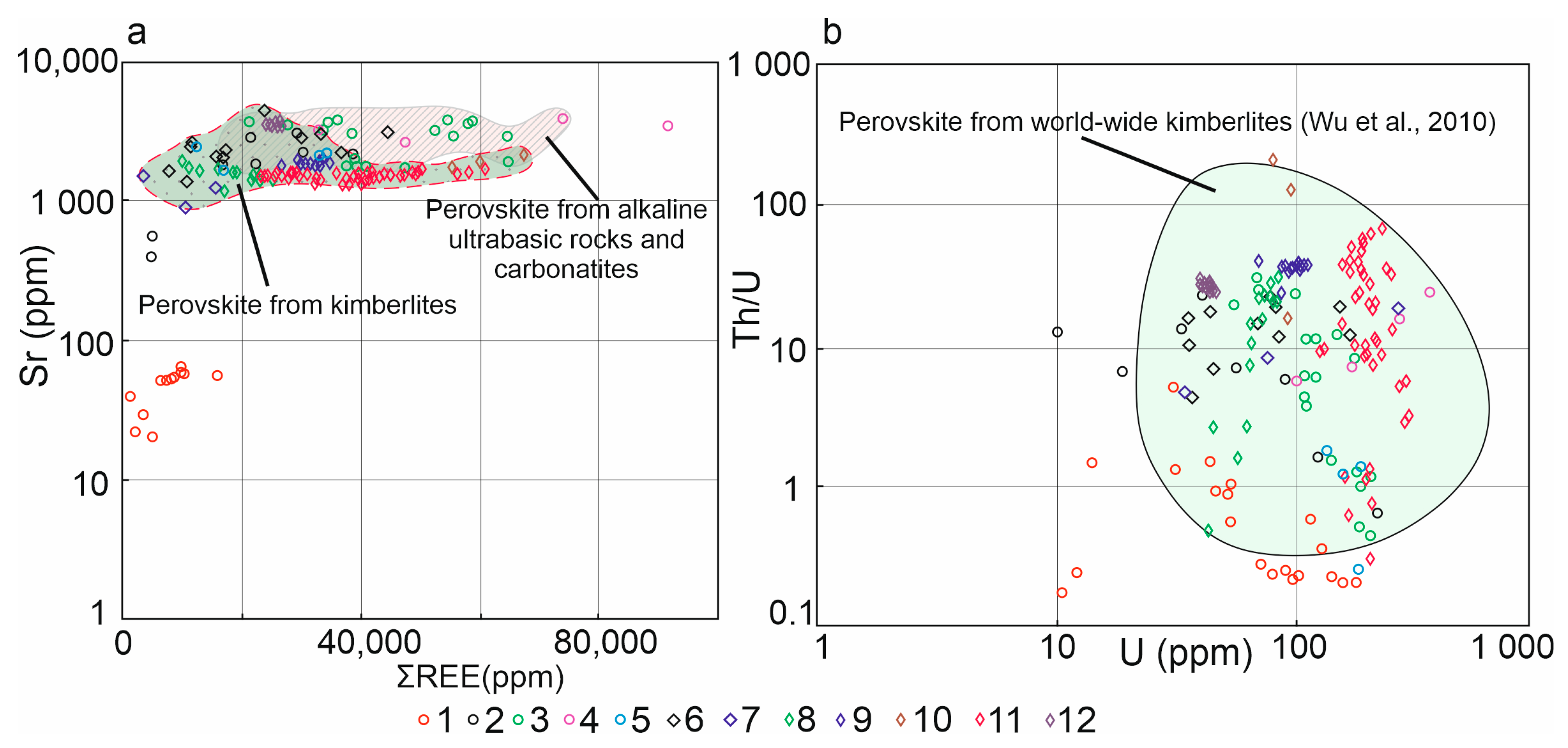
5.1. Comparison of Perovskite from Calcite-Silicate Veins and Ultramafic Alkaline Rocks
5.2. The Nature of Perovskite Mineralization and Its Geological Implications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, G. Beschreibung einiger neuen Mineralien des Urals. Ann. Der Phys. Und Chem. 1839, 48, 551–573. [Google Scholar] [CrossRef]
- Mushketov, I.V. Materials for the Study of the Geonostic Structure and Ore Resources of the Zlatoust Mining District in the Southern Urals; Printing House of the Imperial Academy of Sciences: St. Petersburg, Russia, 1887; 231p. (In Russian) [Google Scholar]
- Pekov, I. Minerals First Discovered on the Territory of the Former Soviet Union; Ocean Pictures: Moscow, Russia, 1998; 369p. [Google Scholar]
- Popov, V.A.; Rassomakhin, M.A. Mineral assemblages of the Shishim mine in the Southern Urals. Mineralogy 2023, 9, 23–44. (In Russian) [Google Scholar] [CrossRef]
- Plat, G.G. Perovskite, loparite and Ba-Fe hollandite from the Schryburt Lake carbonatite complex, northwestern Ontario, Canada. Mineral. Mag. 1994, 58, 49–57. [Google Scholar] [CrossRef]
- Mitchell, R.H. Perovskites: Modern and Ancient; Almaz Press Inc.: Thunder Bay, ON, Canada, 2002; 316p. [Google Scholar]
- Batumike, J.M.; Griffin, W.L.; Belousova, E.A.; Pearson, N.J.; O’Reilly, S.Y.; Shee, S.R. LAM-ICP-MS U–Pb dating of kimberlitic perovskite: Eocene-Oligocene kimberlites from the Kundelungu Plateau, D.R. Congo. Earth Planet. Sci. Lett. 2008, 267, 609–619. [Google Scholar] [CrossRef]
- Wu, F.; Yang, Y.; Mitchell, R.H.; Li, Q.; Yang, J.; Zhang, Y. In situ U-Pb age determination and Nd isotopic analysis of perovskites from kimberlites in southern Africa and Somerset Island, Canada. Lithos 2010, 115, 205–222. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Kamenetsky, V.S.; Sharygin, V.V.; Golovin, A.V. Trace element partitioning in perovskite: Implications for the geochemistry of kimberlites and other mantle-derived under saturated. Rocks Chem. Geol. 2013, 353, 112–131. [Google Scholar] [CrossRef]
- Potter, N.J.; Ferguson, M.R.M.; Kamenetsky, V.S.; Chakhmouradian, A.R.; Sharygin, V.V.; Thompson, J.M.; Goemann, K. Textural evolution of perovskite in the Afrikanda alkaline–ultramafic complex, Kola Peninsula, Russia. Contrib. Mineral. Petrol. 2018, 137, 100. [Google Scholar] [CrossRef]
- Myasnikov, V.S. Mineral mines of the Shishim and Nazyam mountains. Mineral. Ural. 1954, 1, 250–268. (In Russian) [Google Scholar]
- Gekimyants, V.M. Mineralogy of Titanium and Zirconium in Skarns, Rodingites, and Rodingite-Like Formations of the Western Urals; MGU: Moscow, Russia, 2002; 20p. (In Russian) [Google Scholar]
- Belkovsky, A.I.; Loktina, I.N.; Nesterov, A.R. Mineral mines of the Shishimsky-Nazyamsky mountains: Pre-carbonatite skarns and early (oreless) carbonatites. Probl. Petrog. Ore Form. 1998, 12–14. (In Russian) [Google Scholar]
- Popov, V.A. The Akhmatov Mine in the South Urals: Notes on Mineralogy. Mineral. Alm. 2012, 17, 8–46. [Google Scholar]
- Kholodnov, V.V.; Shagalov, E.S. The Upper and Lower Age Boundaries of the Middle Riphean (Ti-Fe-V) Intrusions of the Kusinsko-Kopanskaya Complex in the South Urals: U-Pb Dating of Zircons from the Medvedevskoe Deposite. Dokl. Earth Sci. 2012, 446, 1171–1175. [Google Scholar] [CrossRef]
- Kinny, P.D.; Griffin, B.J.; Heaman, L.M.; Brakhfogel, F.F.; Spetsius, Z.V. SHRIMP U–Pb Ages of Perovskite from Yakutian Kimberlites. Russ. Geol. Geophys. 1997, 38, 97–105. [Google Scholar]
- Ireland, T.R.; Compston, W.; Williams, I.S.; Wendt, I. U-Pb systematics of individual perovskite grains from the Allende and Murchison carbonaceous chondrites. Earth Planet. Sci. Lett. 1990, 101, 379–387. [Google Scholar] [CrossRef]
- Stepanov, S.Y.; Sharpenok, L.N.; Palamarchuk, R.S.; Glazov, A.I.; Palamarchuk, R.S. Distribution of trace elements in perovskite from skarns and calcite veins of the Chernaya rechka and Nazyam ridges (South Urals). Mineralogy 2017, 3, 61–70. [Google Scholar]
- Arakcheeva, A.V.; Lubman, G.U.; Pushcharovsky, D.Y.; Gekimyants, V.M.; Popov, V.A. Crystal structure and microtwinning of natural orthorhombic perovskite CaTiO3. Crystallogr. Rep. 1997, 42, 46–54. [Google Scholar]
- Goldschmidt, V. Atlas der Krystallformen; University of Toronto: Toronto, ON, Canada, 1913. (In German) [Google Scholar]
- Stepanov, S.Y.; Puchkov, V.N.; Palamarchuk, R.S.; Popov, V.A.; Lepehina, E.N.; Sharpenok, L.N.; Antonov, A.V. The first evidence for Paleozoic endogenous activity on the Western slope of the Southern Urals. Dokl. Earth Sci. 2020, 493, 499–503. [Google Scholar] [CrossRef]
- Woolley, A.R. Alkaline Rocks and Carbonatites of the World—Part 3: Africa; The Geological Society: London, UK, 2001. [Google Scholar]
- Arzamastsev, A.; Arzamastseva, L.; Bea, F.; Montero, P. Rare earth elements in rocks and minerals from alkaline plutons of the Kola Peninsula, NW Russia, as indicators of alkaline magma evolution. Russ. J. Earth Sci. 2002, 4, 187–209. [Google Scholar] [CrossRef]
- Alexandrov, S.M.; Troneva, M.A. Composition, mineral assemblages and genesis of serendibite-bearing magnesian skarns. Geochem. Int. 2006, 44, 665–680. [Google Scholar] [CrossRef]
- Starikova, A.E.; Sklyarov, E.V.; Kotov, A.B.; Sal’nikova, E.B.; Fedorovskii, V.S.; Lavrenchuk, A.V.; Mazukabzov, A.M. Vein calciphyre and contact Mg skarn from the Tazheran massif (Western Baikal area, Russia): Age and genesis. Dokl. Earth Sciense 2014, 457, 1003–1007. [Google Scholar] [CrossRef]
- Sklyarov, E.V.; Karmanov, N.S.; Lavrenckuk, A.V.; Starikova, A.E. Perovskites of the Tazheran Massif (Baikal, Russia). Minerals 2019, 9, 323. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. Compositional variation of perovskite group minerals from the carbonatite complexes of the Kola Alkaline Province, Russia. Can. Mineral. 1997, 35, 1293–1310. [Google Scholar]
- Arzamastsev, A.; Arzamastseva, L. Paleozoic Tholeiite Magmatism in the Kola Province, Russia: Relations to Alkaline Magmatism. In Proceedings of the Goldshmidt, Prague, Czech Republic, 15–19 August 2011. [Google Scholar]
- Mitchell, R.H. Kimberlites, Orangeites and Related Rocks; Plenium Press: New York, NY, USA, 1995; 410p. [Google Scholar]
- Chakhmouradian, A.R.; Mitchell, R.H. Occurrence, alteration patterns and compositional variation of perovskite in kimberlites. Can. Mineral. 2000, 38, 975–994. [Google Scholar] [CrossRef]
- Reguir, E.P.; Camacho, A.; Yang, P.; Chakhmouradian, A.R.; Kamenetsky, V.S.; Halden, N.M. Trace-element study and uranium–lead dating of perovskite from the Afrikanda plutonic complex, Kola Peninsula (Russia) using LA-ICP-MS. Mineral. Petrol. 2010, 100, 95–103. [Google Scholar] [CrossRef]
- Chen, W.; Simonetti, A. Evidence for the Multi-Stage Petrogenetic History of the Oka Carbonatite Complex (Quebec, Canada) as Recorded by Perovskite and Apatite. Minerals 2014, 4, 437–476. [Google Scholar] [CrossRef]
- Chalapathi Rao, N.V.; Fu-Yuan, R.H.; Li, Q.-L.; Lehmann, B. Mesoproterozoic U–Pb ages, trace element and Sr–Nd isotopic composition of perovskite from kimberlites of the Eastern Dharwar craton, southern India: Distinct mantle sources and a widespread 1.1 Ga tectonomagmatic event. Chem. Geol. 2013, 353, 48–64. [Google Scholar]
- Ghobadi, M.; Gerdes, A.; Kogarko, L.; Hoefer, H.; Brey, G. In situ LA-ICP-MS Isotopic and Geochronological Studies on Carbonatites and Phoscorites from the Guli Massif, Maymecha-Kotuy, Polar Siberia. Geochem. Int. 2018, 56, 766–783. [Google Scholar] [CrossRef]
- Rass, I.T. Fractionation of microcomponents in coexisting high- and low-calcium alkaline-ultrabasic series of the Odikhincha massif (Polar Siberia). Geochemistry 2004, 8, 852–863. (In Russian) [Google Scholar]
- Chernoostrovets, A.N. The history of discovery and study of Zelentsov mineral mine (mine of the 3-rd magnetic bald peak) of the Nazyam mountains, Southern Urals. Ural. Geol. J. 2014, 3, 42–52. (In Russian) [Google Scholar]
- Puchkov, V.N.; Kozlov, V.I.; Krasnobaev, A.A. Paleozoic U-Pb SHRIMP dating of magmatic rocks of the Bashkir megaanticlinorium. Geol. Collect. 2011, 9, 36–43. (In Russian) [Google Scholar]
- Krasnobaev, A.A.; Puchkov, V.N.; Sergeeva, N.D. Polychronous zirconology of navysh volcanics of the Ai formation (Southern Urals). Dokl. Earth Sci. 2018, 478, 56–61. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanov, S.; Palamarchuk, R.; Kutyrev, A.; Lepekhina, E.; Sharpenok, L.; Shagalov, E.; Minervina, E. Nature of Perovskite Mineralization of Silicate-Carbonate Veins in the Margins of Kusinsko-Kopanskaya Layered Intrusion (South Urals, Russia). Minerals 2024, 14, 478. https://doi.org/10.3390/min14050478
Stepanov S, Palamarchuk R, Kutyrev A, Lepekhina E, Sharpenok L, Shagalov E, Minervina E. Nature of Perovskite Mineralization of Silicate-Carbonate Veins in the Margins of Kusinsko-Kopanskaya Layered Intrusion (South Urals, Russia). Minerals. 2024; 14(5):478. https://doi.org/10.3390/min14050478
Chicago/Turabian StyleStepanov, Sergey, Roman Palamarchuk, Anton Kutyrev, Elena Lepekhina, Ludmila Sharpenok, Evgeniy Shagalov, and Elena Minervina. 2024. "Nature of Perovskite Mineralization of Silicate-Carbonate Veins in the Margins of Kusinsko-Kopanskaya Layered Intrusion (South Urals, Russia)" Minerals 14, no. 5: 478. https://doi.org/10.3390/min14050478





