Impact of Waste Cooking Oils Addition on Thermophilic Dry Co-Digestion of Wheat Straw and Horse Manure for Renewable Energy Production in Two Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Raw Materials and Mixtures Thereof
2.3. Inoculum
2.4. Analytical Methods
2.5. Cellulose
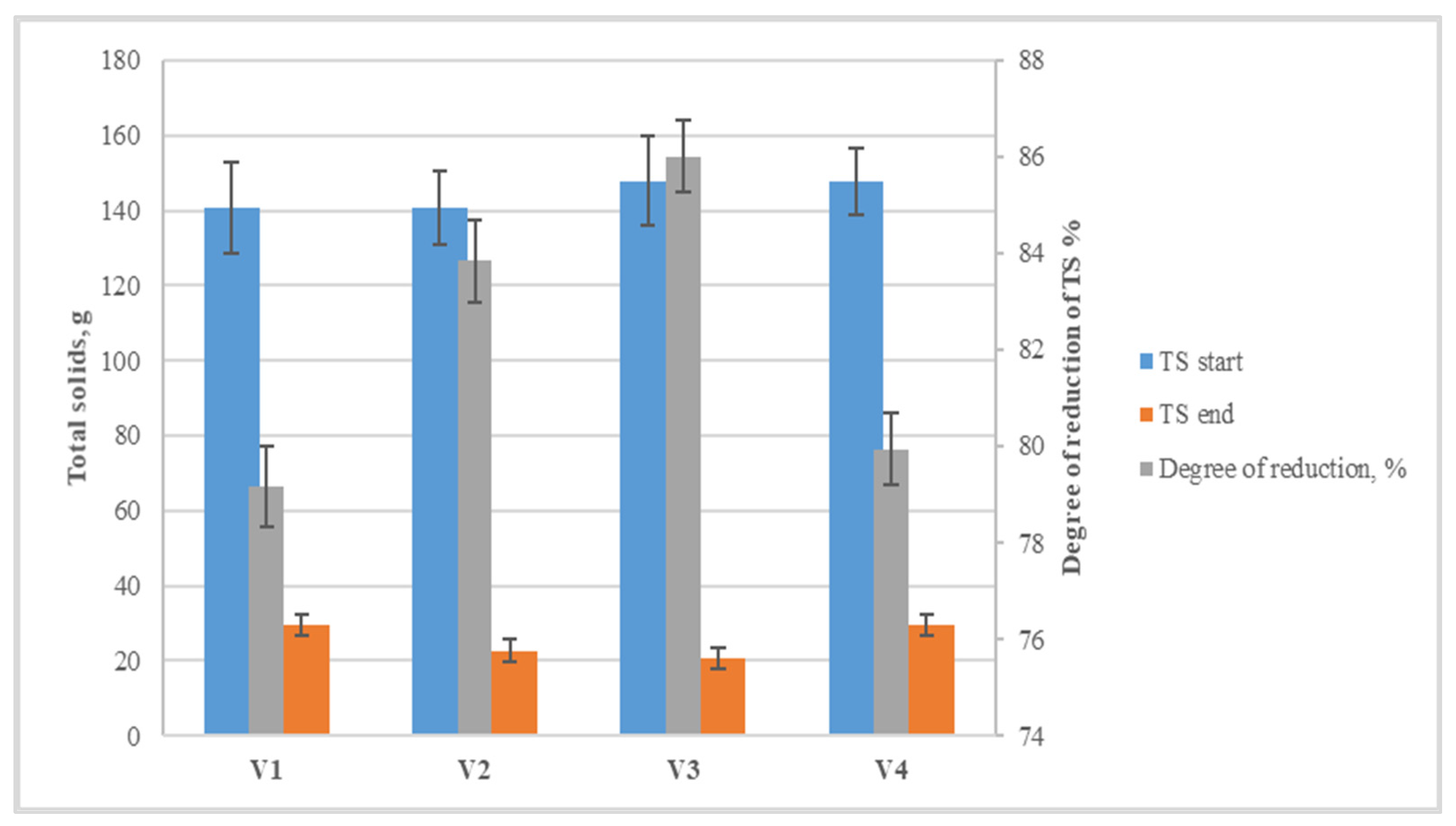
2.6. Total Solids and Volatile Solids
2.7. Volatile Fatty Acids Measurement
2.8. pH
2.9. Calculation of the Potential for Obtaining of Energy
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Subramanian, S. Sequential production of hydrogen and methane by anaerobic digestion of organic wastes: A review. Environ. Chem. Lett. 2021, 19, 1043–1063. [Google Scholar] [CrossRef]
- Akturk, A.S.; Demirer, G.N. Improved Food Waste Stabilization and Valorization by Anaerobic Digestion Through Supplementation of Conductive Materials and Trace Elements. Sustainability 2020, 12, 5222. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Lu, W.; Liu, B.; Hassanein, Z.; Mahmood, H.; Khalid, S. Exploring the Role of Fossil Fuels and Renewable Energy in Determining Environmental Sustainability: Evidence from OECD Countries. Sustainability 2023, 15, 2048. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Yeshanew, M.M.; Paillet, F.; Barrau, C.; Frunzo, L.; Lens, P.N.L.; Esposito, G.; Escudie, R.; Trably, E. Co-production of Hydrogen and Methane From the Organic Fraction of Municipal Solid Waste in a Pilot Scale Dark Fermenter and Methanogenic Biofilm Reactor. Front. Environ. Sci. 2018, 6, 41. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Lupki, F.B.; Campolina, G.A.; Nelson, D.L.; Molina, G. The colors of biotechnology: General overview and developments of white, green and blue areas. FEMS Microbiol. Lett. 2018, 365, 21. [Google Scholar] [CrossRef]
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Gao, Z.; Alshehri, K.; Li, Y.; Qian, H.; Sapsford, D.; Cleall, P.; Harbottle, M. Advances in biological techniques for sustainable lignocellulosic waste utilization in biogas production. Renew. Sustain. Energy Rev. 2022, 170, 112995. [Google Scholar] [CrossRef]
- Simeonov, I.S.; Denchev, D.D.; Kabaivanova, L.V.; Kroumova, E.T.Z.; Chorukova, E.Y.; Hubenov, V.N.; Mihailova, S.N. Different types of pre-treatment of lignocellulosic wastes for methane production. Bulg. Chem. Commun. 2017, 49, 430–435. [Google Scholar]
- Yue, Z.B.; Li, W.W.; Yu, H.Q. Application of rumen microorganisms for anaerobic bioconversion of lignocellulosic biomass. Bioresour. Technol. 2013, 128, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Panadare, D.C.; Rathod, V.K. Applications of Waste Cooking Oil Other than Biodiesel: A Review. Iran. J. Chem. Eng. 2015, 12, 55–76. [Google Scholar]
- Hanum, F.; Yuan, L.C.; Kamahara, H.; Aziz, H.A.; Atsuta, Y.; Yamada, T.; Daimon, H. Treatment of Sewage Sludge Using Anaerobic Digestion in Malaysia: Current State and Challenges. Front. Energy Res. 2019, 7, 19. [Google Scholar] [CrossRef]
- Rouches, E.; Escudié, R.; Latrille, E.; Carrère, H. Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot-scale fungal pretreatment. Waste Manag. 2019, 85, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Kabaivanova, L.; Hubenov, V.; Dimitrova, L.; Simeonov, I.; Wang, H.; Petrova, P. Archaeal and Bacterial Content in a Two-Stage Anaerobic System for Efficient Energy Production from Agricultural Wastes. Molecules 2022, 27, 1512. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, C.; Hattou, S.; Buffiere, P.; Benbelkacem, H. Temperature phased anaerobic digestion (TPAD) of organic fraction of municipal solid waste (OFMSW) and digested sludge (DS): Effect of different hydrolysis conditions. Waste Manag. 2021, 126, 21–29. [Google Scholar] [CrossRef]
- Denchev, D.; Hubenov, V.; Simeonov, I.; Kabaivanova, L. Biohydrogen production from lignocellulosic waste with anaerobic bacteria. In Proceedings of the 4th International Conference on Water, Energy and Environment (ICWEE), Burgas, Bulgaria, 1–3 June 2016; pp. 7–12. [Google Scholar]
- Updegraff, D. Semimicro determination of cellulose inbiological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Issue Date 7/17/2005. 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42619.pdf (accessed on 5 January 2024).
- Van Wychen, S.; Laurens, L.M.L. Determination of Total Solids and Ash in Algal Biomass: Laboratory Analytical Procedure (LAP); No. NREL/TP-5100-60956; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016; p. 1118077. Available online: https://www.nrel.gov/docs/fy16osti/60956.pdf (accessed on 5 January 2024).
- Dell’Omo, P.P.; Spena, V.A. Mechanical pretreatment of lignocellulosic biomass to improve biogas production: Comparison of results for giant reed and wheat straw. Energy 2020, 203, 117798. [Google Scholar] [CrossRef]
- Mañunga, T.; Barrios-Pérez, J.D.; Zaiat, M.; Rodríguez-Victoria, J.A. Evaluation of pretreatment methods and initial pH on mixed inoculum for fermentative hydrogen production from cassava wastewater. Biofuels 2022, 13, 301–308. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Naji, A.; Rechdaoui, S.G.; Jabagi, E.; Lacroix, C.; Azimi, S.; Rocher, V. Horse Manure and Lignocellulosic Biomass Characterization as Methane Production Substrates. Fermentation 2023, 9, 580. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M.; Balajii, M. Chapter 10—Pretreatment of lignocellulosic sugarcane leaves and tops for bioethanol production. In Lignocellulosic Biomass to Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 301–324. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sar, T.; Gowd, S.C.; Rajendran, K.; Kumar, V.; Sarsaiya, S.; Li, Y.; Sindhu, R.; Binod, P.; Zhang, Z.; et al. A comprehensive review on thermochemical, and biochemical conversion methods of lignocellulosic biomass into valuable end product. Fuel 2023, 342, 127790. [Google Scholar] [CrossRef]
- Selvi, P.K.; Sharma, M.; Kamyotra, J.S. Spent oil management and its recycling potential in India inventory and issues. Procedia Environ. Sci. 2013, 18, 742–2013. [Google Scholar] [CrossRef]
- Ortner, M.E.; Müller, W.; Schneider, I.; Bockreis, A. Environmental assessment of three different utilization paths of waste cooking oil from households. Resour. Conserv. Recycl. 2016, 106, 59–67. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Belo, I. Microbial valorization of waste cooking oils for valuable compounds production—A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2583–2616. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Effect of temperature on biohydrogen and biomethane production using a biochemical potential test with different mixtures of sewage sludge, vinasse and poultry manure. J. Clean. Prod. 2023, 382, 135237. [Google Scholar] [CrossRef]
- Zerback, T.; Schumacher, B.; Weinrich, S.; Hülsemann, B.; Nelles, M. Hydrothermal Pretreatment of Wheat Straw—Evaluating the Effect of Substrate Disintegration on the Digestibility in Anaerobic Digestion. Processes 2022, 10, 1048. [Google Scholar] [CrossRef]
- Qu, X.; Zeng, H.; Gao, Y.; Mo, T.; Li, Y. Bio-hydrogen production by dark anaerobic fermentation of organic wastewater. Front. Chem. 2022, 10, 978907. [Google Scholar] [CrossRef]
- Dubrovskis, V.; Plume, I.; Straume, I. Anaerobic co-fermentation of molasses and oil with straw pellets. Agron. Res. 2018, 16, 688–695. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Buyukkamaci, N.; Filibeli, A. Volatile fatty acid formation in an anaerobic hybrid reactor. Process Biochem. 2004, 39, 1491–1494. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A. The impacts of combustion emissions on air quality and climate—From coal to biofuels and beyond. Atmos. Environ. 2009, 43, 23–36. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef]
- Hubenov, V.; Miteva-Staleva, J.; Eneva, R.; Boteva, N.; Kabaivanova, L. Two-stage anaerobic digestion of wheat straw using immobilized microbial consortia. Ecol. Eng. Environ. Prot. 2021, 3, 35–44. [Google Scholar] [CrossRef]
- Kabaivanova, L.; Petrova, P.; Hubenov, V.; Simeonov, I. Biogas Production Potential of Thermophilic Anaerobic Biodegradation of Organic Waste by a Microbial Consortium Identified with Metagenomics. Life 2022, 12, 702. [Google Scholar] [CrossRef]
- Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability 2023, 15, 1859. [Google Scholar] [CrossRef]
- Börjesson, P.; Mattiasson, B. Biogas as a resource-efficient vehicle fuel. Trends Biotechnol. 2008, 26, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A Review of Anaerobic Digestion Systems for Biodegradable Waste: Configurations, Operating Parameters, and Current Trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef]







| Variant | Pretreatment | Added Waste Cooking Oil, % |
|---|---|---|
| V1 | No | No |
| V2 | 4% (wt.) Ca(OH)2, 55 °C/24 h | No |
| V3 | No | 5 |
| V4 | 4% (wt.) Ca(OH)2, 55 °C/24 h | 5 |
| Indicator | Type of Raw Material Used | |
|---|---|---|
| Wheat Straw | Horse Manure | |
| Moisture content, % | 7.64 ± 0.2 | 63.29 ± 1.6 |
| Density, kg/m3 | 152.0 ± 5.7 | 518.9 ± 28.1 |
| Total solids, % | 92.36 ± 1.8 | 36.71 ± 3.8 |
| Volatile solids, % TS | 89.58 ± 1.3 | 47.87 ± 1.7 |
| Cellulose, % TS | 37.6 ± 2.4 | 28.7 ± 2.2 |
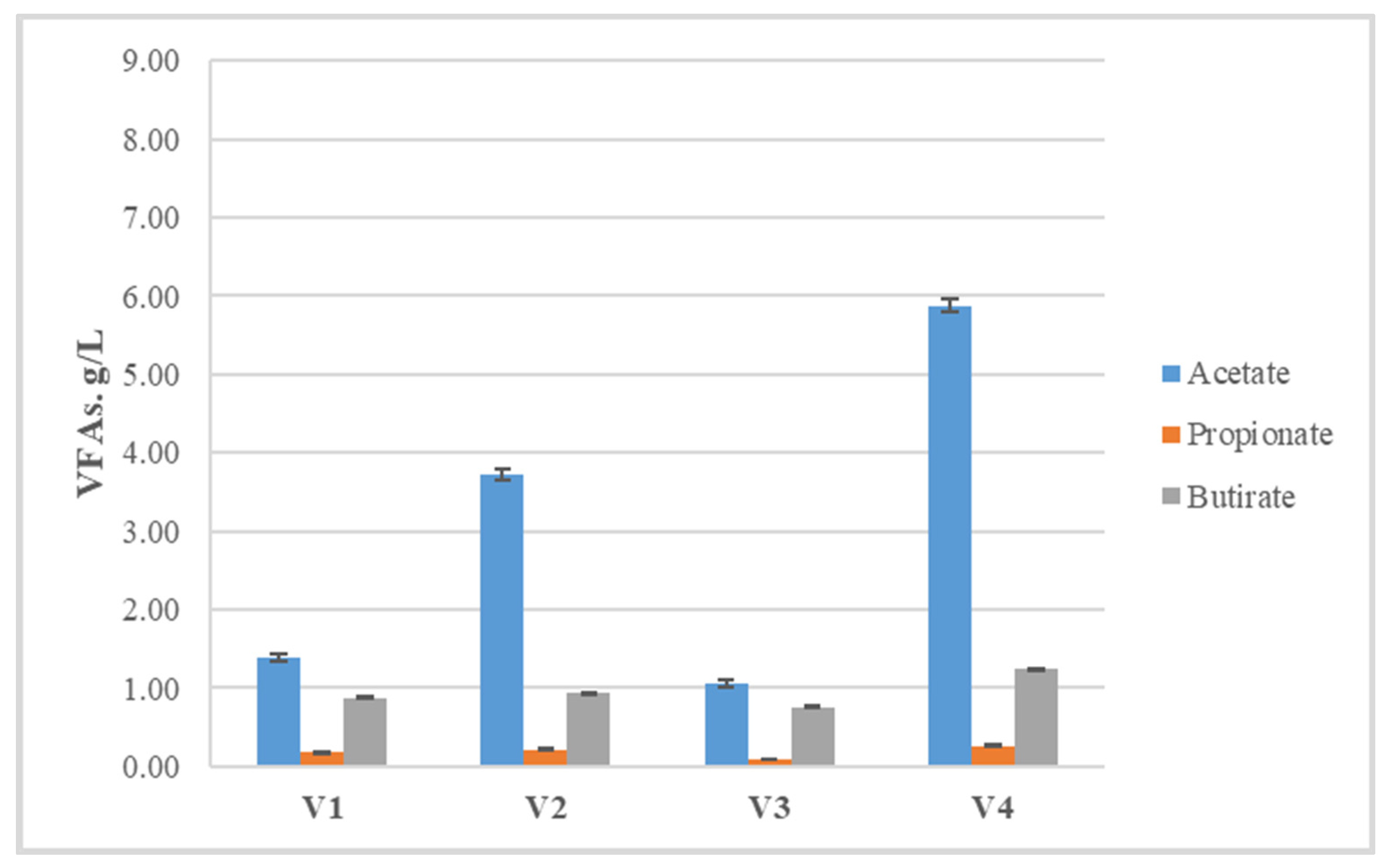
| Volatile Fatty Acids, g/L | |||||
|---|---|---|---|---|---|
| Variant | i-Butyrate | i-Valerate | Valerate | Caproate | Propionate/Acetate |
| V1 | 0 | 0 | 0 | 0.15 ± 0.01 | 0.13 |
| V2 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.18 ± 0.01 | 0.06 |
| V3 | 0 | 0 | 0 | 0.09 ± 0.01 | 0.09 |
| V4 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.24 ± 0.01 | 0.05 |
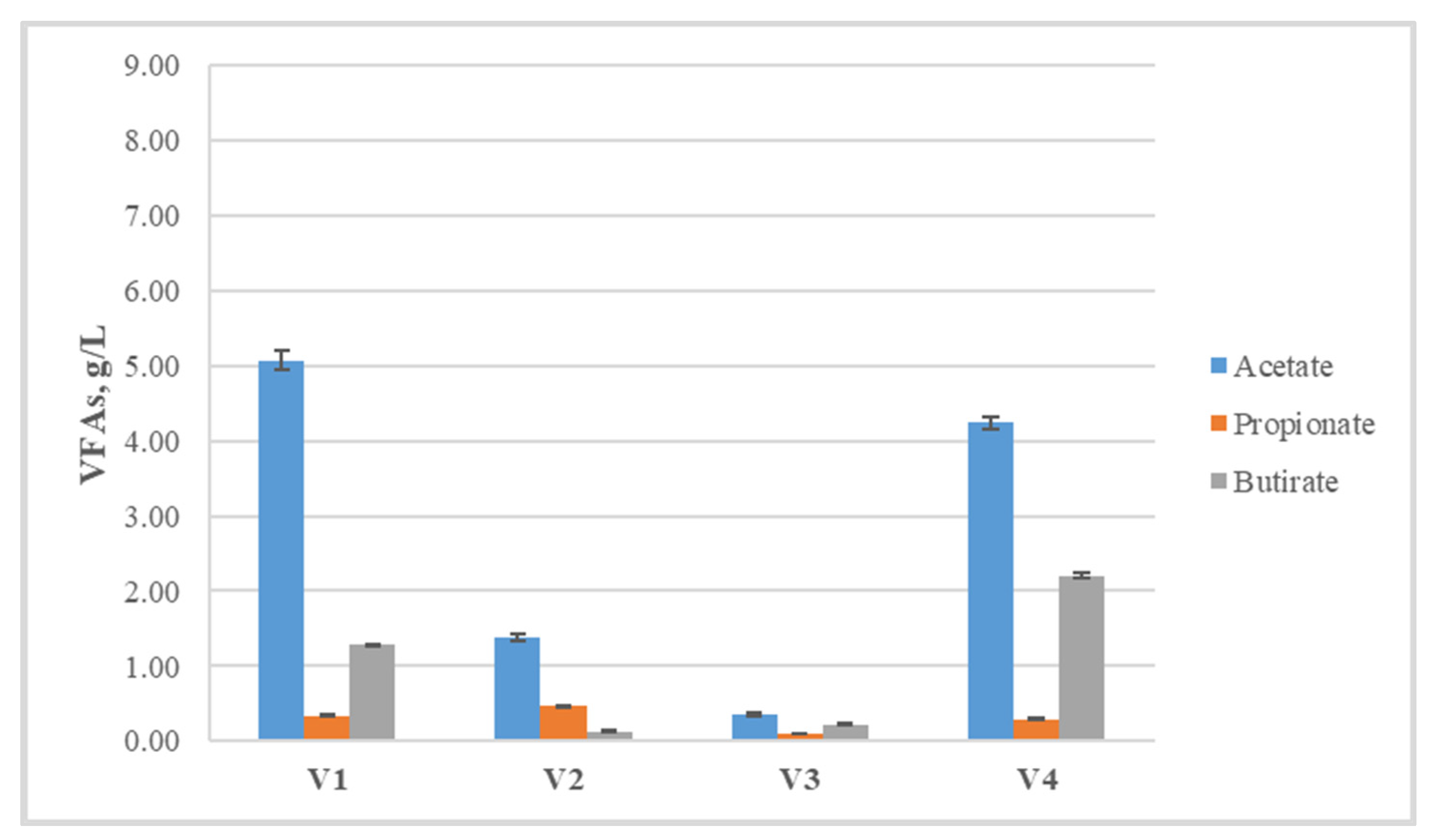
| Volatile Fatty Acids, g/L | |||||
|---|---|---|---|---|---|
| Variant | i-Butyrate | i-Valerate | Valerate | Caproate | Propionate/Acetate |
| V1 | 0 | 0 | 0.09 ± 0.01 | 0.31 ± 0.02 | 0.07 |
| V2 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0 | 0 | 0.34 |
| V3 | 0.34 ± 0.02 | 0.18 ± 0.01 | 0.11 ± 0.01 | 0.51 ± 0.02 | 0.29 |
| V4 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.15 ± 0.01 | 0.57 ± 0.02 | 0.07 |
| Energy Carrier | Total Yield, N cm3 | Y, cm3·g−1 | Lower Heating Value, KWh·N cm3 | Total Energy for Corresponding Energy Carrier, kWh·t−1 | Total Energy for the System, kWh·t−1 | |
|---|---|---|---|---|---|---|
| V1 | Hydrogen | 22.5 | 0.08 | 2.99 | 0.17 | 0.37 |
| Methane | 7.51 | 0.03 | 9.94 | 0.20 | ||
| V2 | Hydrogen | 73.34 | 0.29 | 2.99 | 0.56 | 30.85 |
| Methane | 1072.73 | 4.20 | 9.94 | 30.29 | ||
| V3 | Hydrogen | 25.87 | 0.10 | 2.99 | 0.19 | 7.11 |
| Methane | 257.16 | 0.98 | 9.94 | 6.92 | ||
| V4 | Hydrogen | 96.42 | 0.37 | 2.99 | 0.71 | 0.83 |
| Methane | 4.28 | 0.02 | 9.94 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubenov, V.; Varbacheva, I.; Kabaivanova, L. Impact of Waste Cooking Oils Addition on Thermophilic Dry Co-Digestion of Wheat Straw and Horse Manure for Renewable Energy Production in Two Stages. Life 2024, 14, 312. https://doi.org/10.3390/life14030312
Hubenov V, Varbacheva I, Kabaivanova L. Impact of Waste Cooking Oils Addition on Thermophilic Dry Co-Digestion of Wheat Straw and Horse Manure for Renewable Energy Production in Two Stages. Life. 2024; 14(3):312. https://doi.org/10.3390/life14030312
Chicago/Turabian StyleHubenov, Venelin, Iva Varbacheva, and Lyudmila Kabaivanova. 2024. "Impact of Waste Cooking Oils Addition on Thermophilic Dry Co-Digestion of Wheat Straw and Horse Manure for Renewable Energy Production in Two Stages" Life 14, no. 3: 312. https://doi.org/10.3390/life14030312





