The Winding Road from Origin to Emergence (of Life)
Abstract
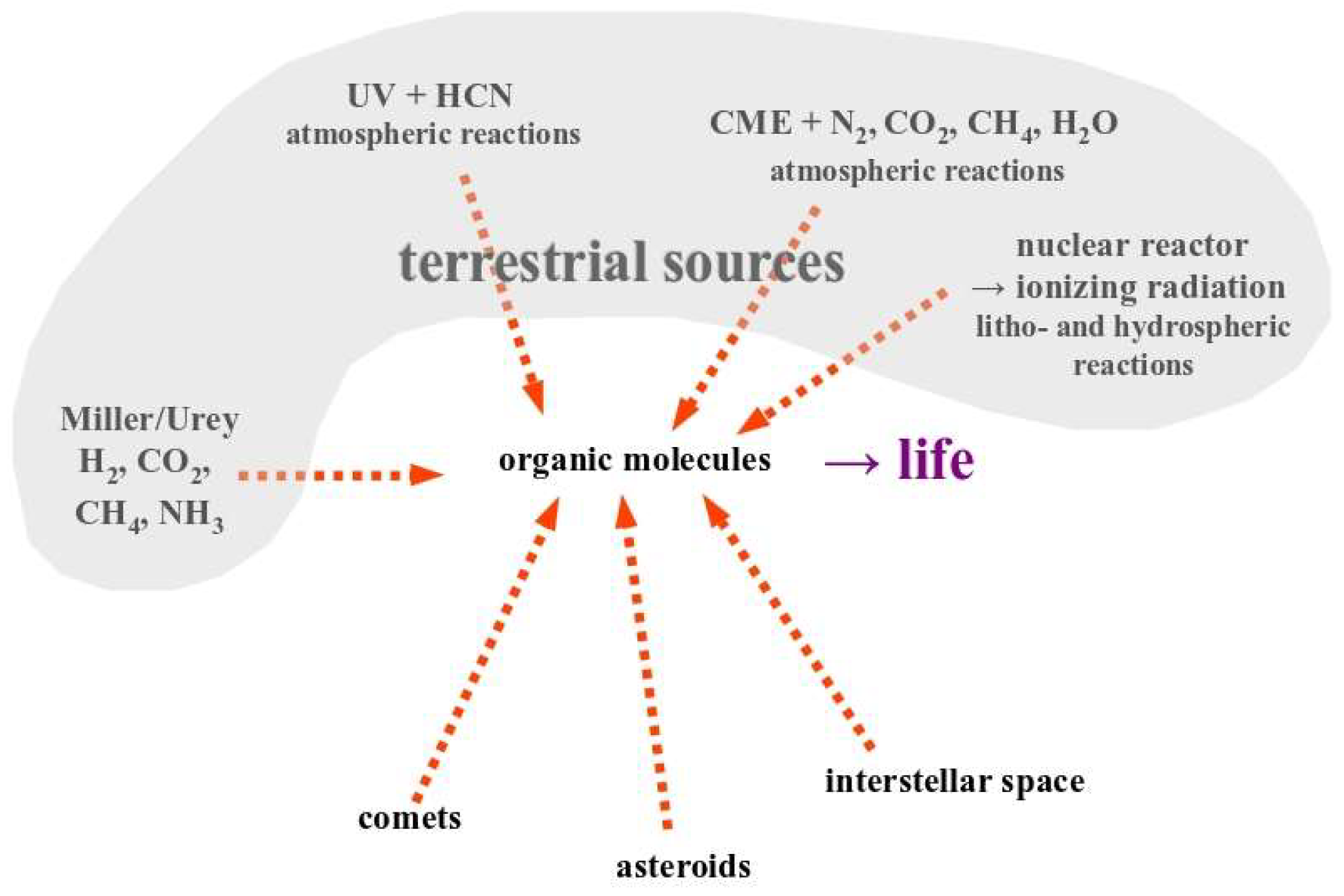
:1. The Prevailing Paradigm: It’s the Organics, Stupid!
2. Physics vs. Biology; from Sadi Carnot to Erwin Schrödinger and from Jöns Jacob Berzelius to Stanley Miller
2.1. The Century of Vitalism (~1800 to ~1900)
2.2. Organicism and Life’s Origin as a One-Off Event
- (i)
- According to statistical thermodynamics, the probability of fluctuation-induced increase in order decreases steeply with complexity of these systems and the degree of complexity required to arrive at even the simplest imaginable cellular structures is doubtlessly enormous. A number of biologists around the middle of the 20th century were perfectly aware of the vanishingly small probability for life to arise this way by mere chance prompting for example Jacques Monod to proclaim that “… man at last knows that he is alone in the unfeeling immensity of the universe, out of which he has emerged only by chance” [30]. It is in the framework of Monod’s thinking that the term “origin”, as describing life’s coming into being out of a soup of organics, unveils its profound sense: a freak event which obviously did happen (since we know for sure that there is life on Earth) but which was so unlikely that there is no point expecting it to have happened elsewhere in the cosmos. Needless to say that many citations listed in Section 1, while deeply rooted in the prebiotic soup paradigm, nonchalantly ignore the inevitability of Monod’s logic!
- (ii)
- To the post-19th-century biologists, the notion of anti-entropic properties inherent in organismal assemblies of organics may have been intuitively more acceptable than the idea of a vitalist force in hydrocarbons. We do observe this anti-entropic tendency in all living things, don’t we? Still, the order-generating properties of organismal assemblies as envisaged by the organicists are no less mystical then the vital force and relying on such properties takes the phenomenon “life” out of the explanatory framework of the physical sciences. In 1944, Erwin Schrödinger’s book “What is Life?” finally put an end to speculations about life’s specialness by rigorously treating life as part of the physical world [18].
3. The Demystification of Life and Its Integration into the Physical Sciences
3.1. Schrödinger and the Crux of the 2nd Law
3.2. Non-Living Systems Can Display Entropy-Decreasing Features; the End of the Life/Non-Life-Dichotomy
4. Reassessing the Organo-Centric View of Living Entities
4.1. CHONPS vs. FeNiMoWCo+; Giant vs. Dwarf?
4.2. CHONPS vs. FeNiMoWCo+; Which One Does the Work?
5. A 2nd Law-Compliant Emergence of Life; from Generalities to Specifics
5.1. The 2nd Law Meets Prebiotic Chemistry
5.2. Extant Life’s Universal Steam Engine: Chemiosmosis
5.3. Chemiosmosis, a Physical Phenomenon in Biological Disguise
6. Free Energy Conversion Prior to Chemiosmosis?
7. Towards Scenarios Featuring Prebiotic Chemiosmotic Processes
8. The Other “What Is Life” Question
Funding
Acknowledgments
Conflicts of Interest
References
- Horneck, G.; Walter, N.; Westall, F.; Grenfell, J.L.; Martin, W.F.; Gomez, F.; Leuko, S.; Lee, N.; Onofri, S.; Tsiganis, K.; et al. AstRoMap European Astrobiology Roadmap. Astrobiology 2016, 16, 201–243. [Google Scholar] [CrossRef]
- Available online: https://solarsystem.nasa.gov/news/517/why-bennu-10-reasons/ (accessed on 28 April 2024).
- Available online: https://www.isas.jaxa.jp/en/missions/spacecraft/current/hayabusa2.html (accessed on 28 April 2024).
- Available online: https://www.esa.int/Science_Exploration/Space_Science/Rosetta/Rosetta_s_comet_contains_ingredients_for_life (accessed on 28 April 2024).
- Sasselov, D.D.; Grotzinger, J.P.; Sutherland, J.D. The origin of life as a planetary phenomenon. Sci. Adv. 2022, 6, eaax3419. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. Letter to Joseph Dalton Hooker on February 1, 1871. In The Life and Letters of Charles Darwin, Including an Autobiographical Chapter; Darwin, F., Ed.; John Murray: London, UK, 1887; Volume 3. [Google Scholar]
- Miller, S.L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.C. Organic compound synthesis on the primitive Earth: Several questions about the origin of life have been answered, but much remains to be studied. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Rimmer, P.; Cassone, G.; Knížek, A.; Civiš, S.; Šponer, J.E.; Ivanek, O.; Šponer, J.; Saeidfirozeh, H.; Kubelík, P.; et al. One-Pot Hydrogen Cyanide-Based Prebiotic Synthesis of Canonical Nucleobases and Glycine Initiated by High-Velocity Impacts on Early Earth. Astrobiology 2020, 20, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Brown, A.W. Novel Apparatuses for Incorporating Natural Selection Processes into Origins-of-Life Experiments to Produce Adaptively Evolving Chemical Ecosystems. Life 2022, 12, 1508. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ise, J.-I.; Aoki, R.; Kinoshita, M.; Naito, K.; Udo, T.; Kunwar, B.; Takahashi, J.-I.; Shibata, H.; Mita, H.; et al. Formation of Amino Acids and Carboxylic Acids in Weakly Reducing Planetary Atmospheres by Solar Energetic Particles from the Young Sun. Life 2023, 13, 1103. [Google Scholar] [CrossRef]
- Ebisuzaki, T.; Maruyama, S. Nuclear geyser model of the origin of life: Driving force to promote the synthesis of building blocks of life. Geosci. Front. 2017, 8, 275–298. [Google Scholar] [CrossRef]
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.-J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.R.; Cottin, H.; et al. Prebiotic chemicals—Amino acid and phosphorus—In the coma of comet 67P/Churyumov-Gerasimenko. Sci. Adv. 2016, 2, e1600285. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef]
- Oba, Y.; Koga, T.; Takano, Y.; Ogawa, N.O.; Ohkouchi, N.; Sasaki, K.; Sato, H.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H.; et al. Uracil in the carbonaceous asteroid (162173) Ryugu. Nat. Commun. 2023, 14, 1292. [Google Scholar] [CrossRef] [PubMed]
- Potiszil, C.; Yamanaka, M.; Sakaguchi, C.; Ota, T.; Kitagawa, H.; Kunihiro, T.; Tanaka, R.; Kobayashi, K.; Nakamura, E. Organic Matter in the Asteroid Ryugu: What We Know So Far. Life 2023, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, S.S.; Aponte, J.C.; Bhattacharjee, S.; Dong, G.; Hofmann, A.E.; Dworkin, J.P.; Glavin, D.P.; Elsila, J.E.; Graham, H.V.; Naraoka, H.; et al. Polycyclic aromatic hydrocarbons in samples of Ryugu formed in the interstellar medium. Science 2023, 382, 1411–1416. [Google Scholar] [CrossRef]
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell and Mind; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures; Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Nicolis, G.; Prigogine, I. Self-Organization in Non-Equilibrium Systems; John Wiley and Sons: New York, NY, USA, 1977. [Google Scholar]
- Branscomb, E.; Russell, M.J. Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Branscomb, E.; Biancalani, T.; Goldenfeld, N.; Russell, M. Escapement mechanisms and the conversion of disequilibria; the engines of creation. Phys. Rep. 2017, 677, 1–60. [Google Scholar] [CrossRef]
- Aristotle, On the History of Animals, Book V, Part 1. Available online: http://demonax.info/doku.php?id=text:history_of_animals_book_1-5#book_v (accessed on 28 April 2024).
- Driesch, H. Philosophy of Vitalism. Nature 1913, 92, 400. [Google Scholar] [CrossRef]
- Driesch, H. The History and Theory of Vitalism; MacMillan: London, UK, 1914. [Google Scholar]
- Reinke, J. Die Schaffende Natur: Mit Bezugnahme auf Schopenhauer und Bergson; Quelle & Meyer: Leipzig, Germany, 1919. [Google Scholar]
- Reinke, J. Kritik der Abstammungslehre; Barth: Leipzig, Germany, 1920. [Google Scholar]
- Mayr, E. Toward a New Philosophy of Biology: Observations of an Evolutionist; Harvard University Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Needham, J. Organicism in Biology. J. Philos. Stud. 1928, 3, 29–40. [Google Scholar] [CrossRef]
- Monod, J. Le Hasard et la Necessite: Essai sur la Philosophie de la Biologie Moderne; Editions du Seuil: Paris, France, 1970. [Google Scholar]
- Schoepp-Cothenet, B.; van Lis, R.; Atteia, A.; Baymann, F.; Capowiez, L.; Ducluzeau, A.-L.; Duval, S.; Brink, F.T.; Russell, M.J.; Nitschke, W. On the universal core of bioenergetics. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 79–93. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. 1997, 154, 377–402. [Google Scholar] [CrossRef]
- Barge, L.M.; Branscomb, E.; Brucato, J.R.; Cardoso, S.S.S.; Cartwright, J.H.E.; Danielache, S.O.; Galante, D.; Kee, T.P.; Miguel, Y.; Mojzsis, S.; et al. Thermodynamics, Disequilibrium, Evolution: Far-From-Equilibrium Geological and Chemical Considerations for Origin-Of-Life Research. Orig. Life Evol. Biosph. 2017, 47, 39–56. [Google Scholar] [CrossRef]
- Chatterjee, S.; Yadav, S. The Coevolution of Biomolecules and Prebiotic Information Systems in the Origin of Life: A Visualization Model for Assembling the First Gene. Life 2022, 12, 834. [Google Scholar] [CrossRef]
- Deamer, D.W. Origin of Life. In What Everyone Needs to Know; Oxford University Press: Oxford, UK, 2020; ISBN 9780190098995. [Google Scholar]
- Todd, Z.R. Sources of Nitrogen-, Sulfur-, and Phosphorus-Containing Feedstocks for Prebiotic Chemistry in the Planetary Environment. Life 2022, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Neidhardt, F.C.; Ingraham, J.L.; Schaechter, M. Physiology of the Bacterial Cell: A Molecular Approach; Sinauer Associates: Sunderland, MA, USA, 1990. [Google Scholar]
- Nitschke, W.; McGlynn, S.E.; Milner-White, E.J.; Russell, M.J. On the antiquity of metalloenzymes and their substrates in bioenergetics. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Schoepp-Cothenet, B.; van Lis, R.; Philippot, P.; Magalon, A.; Russell, M.J.; Nitschke, W. The ineluctable requirement for the trans-iron elements molybdenum and/or tungsten in the origin of life. Sci. Rep. 2012, 2, 263. [Google Scholar] [CrossRef]
- Anbar, A.D. Elements and Evolution. Science 2008, 322, 1481–1483. [Google Scholar] [CrossRef]
- Brock, T.D.; Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Prentice-Hall: Bergen, NJ, USA, 2003; Chapter 2. [Google Scholar]
- Scott, D.; Amy, N.K. Molybdenum accumulation in chlD mutants of Escherichia coli. J. Bacteriol. 1989, 171, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.J.; Gray, H.B. Bioinorganic Chemistry. Curr. Opin. Chem. Biol. 1998, 2, 155–158. [Google Scholar] [CrossRef]
- Martin, R.B. Bioinorganic chemistry. In Encyclopedia of Molecular Cell Biology and Molecular Medicine; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Monosson, E. Evolution in a Toxic World; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Pyle, A.M. Ribozymes: A Distinct Class of Metalloenzymes. Science 1993, 261, 709–714. [Google Scholar] [CrossRef]
- Maret, W. The quintessence of metallomics: A harbinger of a different life science based on the periodic table of the bioelements. Metallomics 2022, 14, mfac051. [Google Scholar] [CrossRef] [PubMed]
- Talini, G.; Gallori, E.; Maurel, M.-C. Natural and unnatural ribozymes: Back to the primordial RNA world. Res. Microbiol. 2009, 160, 457–465. [Google Scholar] [CrossRef]
- Farr, O.; Gaudu, N.; Danger, G.; Russell, M.J.; Ferry, D.; Nitschke, W.; Duval, S. Methanol on the rocks: Green rust transformation promotes the oxidation of methane. J. R. Soc. Interface 2023, 20, 20230386. [Google Scholar] [CrossRef]
- Rutten, M.G. Origin of life on Earth, its evolution and actualism. Evolution 1957, 11, 56–59. [Google Scholar] [CrossRef]
- Sillén, L.G. Regulation of O2, N2 and CO2 in the atmosphere; thoughts of a laboratory chemist. Tellus 1966, 18, 198. [Google Scholar] [CrossRef]
- Maden, B.H. No soup for starters? Autotrophy and the origins of metabolism. Trends Biochem. Sci. 1995, 20, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Yockey, H.P. Comments on “Let there be life: Thermodynamic reflections on biogenesis and evolution” by Avshalom C. Elitzur. J. Theor. Biol. 1995, 176, 349–355. [Google Scholar] [CrossRef]
- Branscomb, E.; Russell, M.J. Frankenstein or a submarine alkaline vent: Who is responsible for abiogenesis? Part 1: What is life—That it might create itself? BioEssays 2018, 40, e1700179. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cneos.jpl.nasa.gov/about/life_on_earth.html (accessed on 28 April 2024).
- Branscomb, E.; Russell, M.J. Why the Submarine Alkaline Vent is the Most Reasonable Explanation for the Emergence of Life. BioEssays 2018, 41, e1800208. [Google Scholar] [CrossRef] [PubMed]
- Branscomb, E.; Russell, M.J. On the beneficent thickness of water. Interface Focus 2019, 9, 20190061. [Google Scholar] [CrossRef]
- Branscomb, E. Boltzmann’s casino and the unbridgeable chasm in emergence of life research. arXiv 2023, arXiv:2312.00932. [Google Scholar]
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Guérin, B. Bioénergétique; EDP Sciences: Les Ulis, France, 2004. [Google Scholar]
- Russell, M.J. A self-sustaining serpentinization mega-engine feeds the fougerite nanoengines implicated in the emergence of guided metabolism. Front. Microbiol. 2023, 14, 1083. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Irwin, L.N. Life in the Universe; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Pascal, R.; Pross, A.; Sutherland, J.D. Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics. Open Biol. 2013, 3, 130156. [Google Scholar] [CrossRef]
- Davies, P.C.; Walker, S.I. The hidden simplicity of biology. Rep. Prog. Phys. 2016, 79, 102601. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.I. Origins of life: A problem for physics, a key issues review. Rep. Prog. Phys. 2017, 80, 092601. [Google Scholar] [CrossRef]
- Kompanichenko, V. Thermodynamic Jump from Prebiotic Microsystems to Primary Living Cells. Sci 2020, 2, 14. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Russell, M.J. On the origin of cells: An hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. 2003, 358, 27–85. [Google Scholar] [CrossRef]
- Martin, W.; Russell, M.J. On the origin of biochemistry at an alkaline hydrothermal vent. Philos. Philos. Trans. R. Soc. Lond. B 2007, 367, 1887–1925. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W.F. The Origin of Membrane Bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef]
- Lane, N.; Allen, J.F.; Martin, W. How did LUCA make a living? Chemiosmosis in the origin of life. BioEssays 2010, 32, 271–280. [Google Scholar] [CrossRef]
- Ohnishi, T. NADH-quinone oxidoreductase, the most complex complex. J. Bioenerg. Biomembr. 1995, 25, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, S.; Schoepp-Cothenet, B.; Ceccaldi, P.; Guigliarelli, B.; Magalon, A. The prokaryotic Mo/W-bisPGD enzymes family: A catalytic workhorse in bioenergetics. Biochim. Biophys. Acta-Bioenerg. 2013, 1827, 1048–1085. [Google Scholar] [CrossRef]
- Nitschke, W.; Schoepp-Cothenet, B.; Duval, S.; Zuchan, K.; Farr, O.; Baymann, F.; Panico, F.; Minguzzi, A.; Branscomb, E.; Russell, M.J. Aqueous electrochemistry: The toolbox for life’s emergence from redox disequilibria. Electrochem. Sci. Adv. 2022, 3, e2100192. [Google Scholar] [CrossRef]
- Ducluzeau, A.L.; van Lis, R.; Duval, S.; Schoepp-Cothenet, B.; Russell, M.J.; Nitschke, W. Was nitric oxide the first deep electron sink? Trends Biochem. Sci. 2009, 34, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Baymann, F.; Lebrun, E.; Brugna, M.; Schoepp-Cothenet, B.; Giudici-Orticoni, M.; Nitschke, W. The redox protein construction kit: Pre-last universal common ancestor evolution of energy-conserving enzymes. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2003, 358, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Grein, F.; Ramos, A.R.; Venceslau, S.S.; Pereira, I.A. Unifying concepts in anaerobic respiration: Insights from dissimilatory sulfur metabolism. Biochim. Et Biophys. Acta-Bioenerg. 2013, 1827, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Refojo, P.N.; Sousa, F.L.; Teixeira, M.; Pereira, M.M. The alternative complex III: A different architecture using known building modules. Biochim. Et Biophys. Acta-Bioenerg. 2010, 1797, 1869–1876. [Google Scholar] [CrossRef]
- Karavaeva, V.; Sousa, F.L. Modular structure of Complex II: An evolutionary perspective. Biochim. Biophys. Acta-Bioenerg. 2023, 1864, 148916. [Google Scholar]
- Zuchan, K.; Baymann, F.; Baffert, C.; Brugna, M.; Nitschke, W. The dyad of the Y-junction- and a flavin module unites diverse redox enzymes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2021, 1862, 148401. [Google Scholar] [CrossRef]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 1977, 41, 100–180. [Google Scholar] [CrossRef]
- de Gomez-Puyou, M.T.; de Jesus Garcia, J.; Gomez-Puyou, A. Synthesis of pyrophosphate and ATP by soluble mitochondrial F1. Biochemistry 1993, 32, 2213–2218. [Google Scholar] [CrossRef]
- Chi, A.; Kemp, R.G. The primordial high energy compound: ATP or inorganic pyrophosphate? J. Biol. Chem. 2000, 275, 35677–35679. [Google Scholar] [CrossRef]
- Kirchhoff, H. Molecular crowding and order in photosynthetic membranes. Trends Plant Sci. 2008, 13, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Cartron, M.L.; Mullin, N.; Qian, P.; Leggett, G.J.; Hunter, C.N.; Hobbs, J.K. Direct Imaging of Protein Organization in an Intact Bacterial Organelle Using High-Resolution Atomic Force Microscopy. ACS Nano 2017, 11, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Huang, F.; Mason, D.; Zhao, G.-Y.; Johnson, G.N.; Mullineaux, C.W.; Liu, L.-N. Dissecting the Native Architecture and Dynamics of Cyanobacterial Photosynthetic Machinery. Mol. Plant 2017, 10, 1434–1448. [Google Scholar] [CrossRef]
- Mullineaux, C.W.; Liu, L.-N. Membrane Dynamics in Phototrophic Bacteria. Annu. Rev. Microbiol. 2020, 74, 633–654. [Google Scholar] [CrossRef]
- Salton, M.; Freer, J. Composition of the membranes isolated from several gram-positive bacteria. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1965, 107, 531–538. [Google Scholar] [CrossRef]
- Luisi, P.L.; Walde, P.; Oberholzer, T. Lipid vesicles as possible intermediates in the origin of life. Curr. Opin. Colloid Interface Sci. 1999, 4, 33–39. [Google Scholar] [CrossRef]
- Deamer, D. The Role of Lipid Membranes in Life’s Origin. Life 2017, 7, 5. [Google Scholar] [CrossRef]
- Purvis, G.; Šiller, L.; Crosskey, A.; Vincent, J.; Wills, C.; Sheriff, J.; Xavier, C.; Telling, J. Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents. Commun. Earth Environ. 2024, 5, 30. [Google Scholar] [CrossRef]
- Bayro, M.J.; Daviso, E.; Belenky, M.; Griffin, R.G.; Herzfeld, J. An amyloid organelle, solid-state NMR evidence for cross-β as-sembly of gas vesicles. J. Biol. Chem. 2012, 287, 3479–3484. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Aussignargues, C.; Zarzycki, J.; Cai, F.; Sutter, M. Bacterial microcompartments. Nat. Rev. Microbiol. 2018, 16, 277–290. [Google Scholar] [CrossRef]
- Holmes, A.O.M.; Kalli, A.C.; Goldman, A. The Function of Membrane Integral Pyrophosphatases from Whole Organism to Single Molecule. Front. Mol. Biosci. 2019, 6, 132. [Google Scholar] [CrossRef]
- Russell, M.J.; Daniel, R.M.; Hall, A.J.; Sherringham, J.A. A hydrothermally precipitated catalytic iron sulphide membrane as a first step toward life. J. Mol. Evol. 1994, 39, 231–243. [Google Scholar] [CrossRef]
- Nitschke, W.; Russell, M.J. Hydrothermal Focusing of Chemical and Chemiosmotic Energy, Supported by Delivery of Catalytic Fe, Ni, Mo/W, Co, S and Se, Forced Life to Emerge. J. Mol. Evol. 2009, 69, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Why are cells powered by proton gradients. Nat. Educ. 2010, 3, 2. [Google Scholar]
- Russell, M.J. On Irish bacteriometallogenesis and its wider connotations. In Irish-Type Deposits around the World; Andrew, C.J., Hitzman, M.W., Stanley, G., Eds.; Irish Association for Economic Geology: Dublin, Ireland, 2023; pp. 45–94. [Google Scholar]
- Jackson, J.B. Natural pH Gradients in Hydrothermal Alkali Vents Were Unlikely to Have Played a Role in the Origin of Life. J. Mol. Evol. 2016, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, W.; Mühlenhoff, U.; Liebl, U. Evolution. In Photosynthesis: A Comprehensive Treatise; Raghavendra, A.S., Ed.; Cambridge University Press: Cambridge, MA, USA, 2000; Chapter 22. [Google Scholar]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Frenkel-Pinter, M.; Samanta, M.; Ashkenasy, G.; Leman, L.J. Prebiotic Peptides: Molecular Hubs in the Origin of Life. Chem. Rev. 2020, 120, 4707–4765. [Google Scholar] [CrossRef] [PubMed]
- Adamski, P.; Eleveld, M.; Sood, A.; Kun, Á.; Szilágyi, A.; Czárán, T.; Szathmáry, E.; Otto, S. From self-replication to replicator systems en route to de novo life. Nat. Rev. Chem. 2020, 4, 386–403. [Google Scholar] [CrossRef]
- Kauffman, S.A. Approaches to the origin of life on Earth. Life 2011, 1, 34–48. [Google Scholar] [CrossRef]
- Ashkenasy, G.; Jagasia, R.; Yadav, M.; Ghadiri, M.R. Design of a directed molecular network. Proc. Natl. Acad. Sci. USA 2004, 101, 10872–10877. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitschke, W.; Farr, O.; Gaudu, N.; Truong, C.; Guyot, F.; Russell, M.J.; Duval, S. The Winding Road from Origin to Emergence (of Life). Life 2024, 14, 607. https://doi.org/10.3390/life14050607
Nitschke W, Farr O, Gaudu N, Truong C, Guyot F, Russell MJ, Duval S. The Winding Road from Origin to Emergence (of Life). Life. 2024; 14(5):607. https://doi.org/10.3390/life14050607
Chicago/Turabian StyleNitschke, Wolfgang, Orion Farr, Nil Gaudu, Chloé Truong, François Guyot, Michael J. Russell, and Simon Duval. 2024. "The Winding Road from Origin to Emergence (of Life)" Life 14, no. 5: 607. https://doi.org/10.3390/life14050607





