Preparation and Tribological Performance of Multi-Layer van der Waals Heterostructure WS2/h-BN
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Multi-Layer vdWH WS2/h-BN
- (1)
- Synthesis of PDDA@h-BN and SDBS@WS.
- (2)
- Synthesis of multi-layer vdWH WS2/h-BN.
2.3. Characterization
2.4. Tribological Tests
3. Results and Discussion
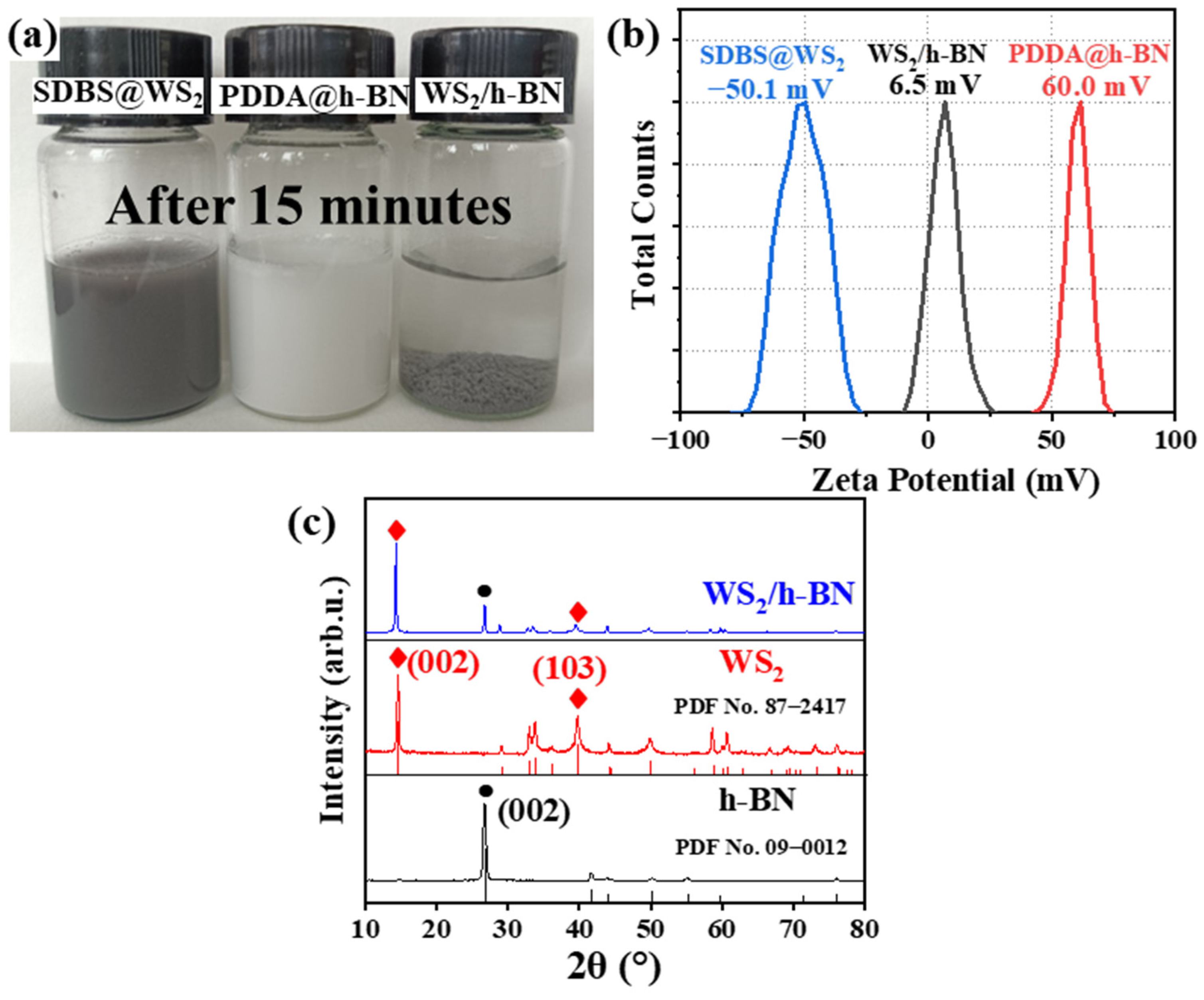
3.1. Subsection Characterization of PDDA@h-BN and SDBS@WS2
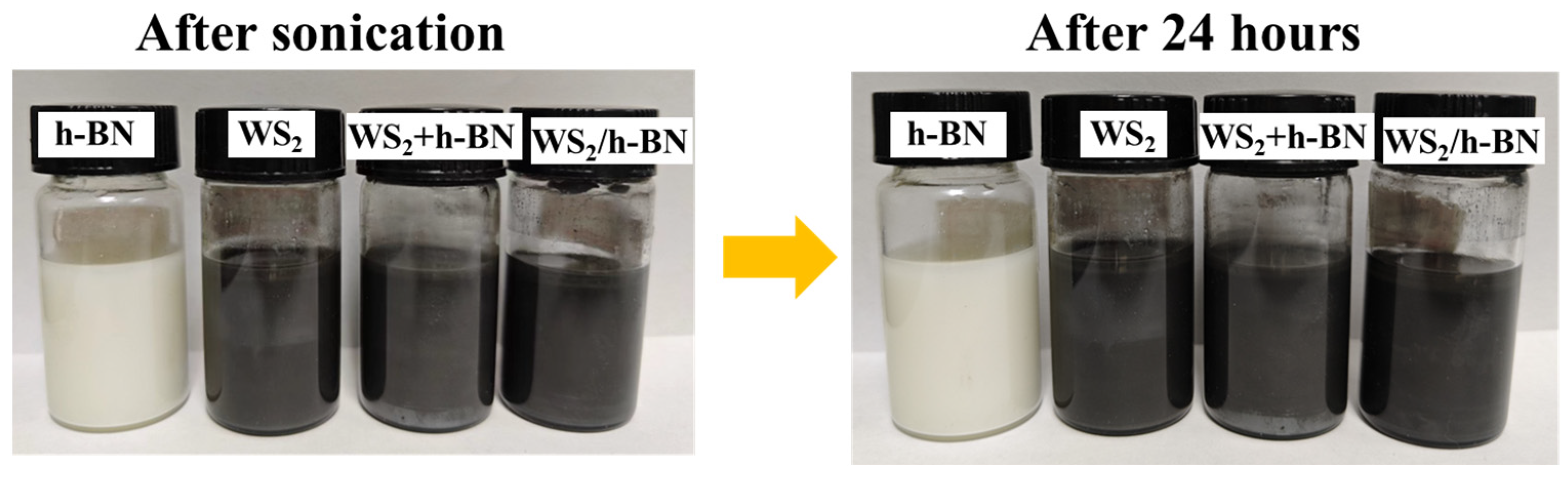
3.2. Characterization of Multi-Layer vdWH WS2/h-BN
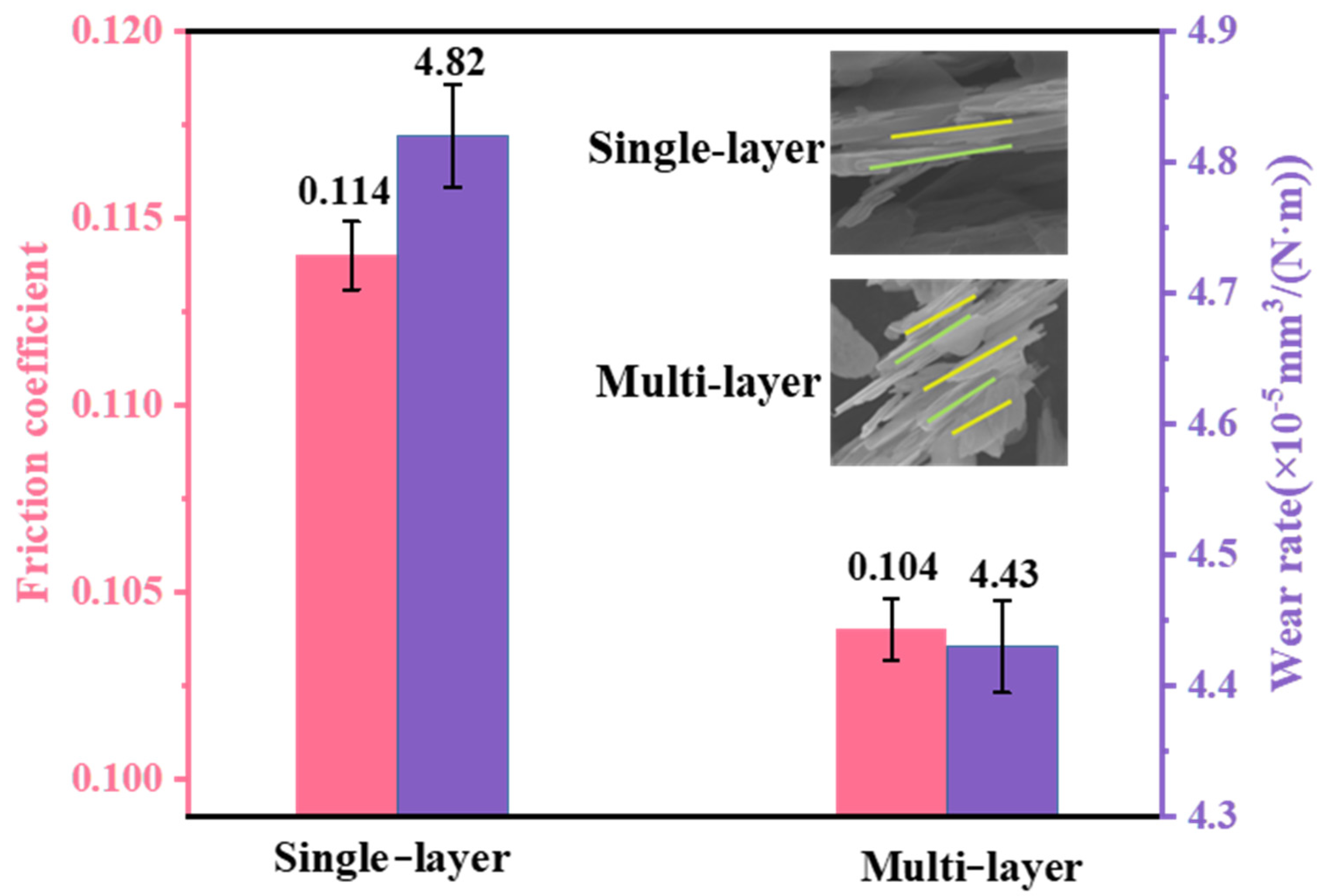
3.3. Tribological Property
3.4. Analysis of Lubrication Mechanism
4. Conclusions
- (1)
- SDBS and PDDA were used to modify WS2 and h-BN, which the negatively charged SDBS@WS2 and the positively charged PDDA@h-BN were successfully prepared. When the mass ratio of h-BN to PDDA was 1:1 and pH = 3, the zeta potential of PDDA@h-BN was 60.0 mV. Under the same conditions, the zeta potential of SDBS@WS2 was −50.1 mV.
- (2)
- The multi-layer (4 layers) vdWH WS2/h-BN synthesized by electrostatic interaction method had a sandwich-like structure, in which WS2 and h-BN were stacked alternately along the (002) crystal plane.
- (3)
- Compared with the base oil, the addition of multi-layer vdWH WS2/h-BN (1.0 wt%) reduced the friction coefficient (0.104) by 33.8% and the wear rate (4.43 × 10−5 mm3/(N·m)) by 16.8%. This arose from a natural lattice mismatch of 26.0% between the heterogeneous interfaces in multi-layer vdWH WS2/h-BN.
- (4)
- The multi-layer (4 layers) vdWH WS2/h-BN had better tribological property than single-layer vdWH WS2/h-BN. The friction coefficient (0.104) and wear rate (4.43 × 10−5 mm3/(N·m)) were 8.7% and 8.1% lower than those of single-layer vdWH WS2/h-BN, respectively. This was due to the fact that multi-layer vdWH WS2/h-BN provided more heterogeneous interfaces, which made its slip probability 15 times higher than that of single-layer vdWH WS2/h-BN.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tu, Y.; Zhang, L.; Zhang, X.; Kang, X. Improving the mechanical and tribological behavior of Cu-WS2 self-lubricating composite with the addition of WS2 nanosheet. Wear 2023, 530–531, 205013. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Klausen, L.H.; Li, Q.; Dong, M. Friction behaviors of two-dimensional materials at the nanoscale. Mater. Today Phys. 2022, 27, 100771. [Google Scholar] [CrossRef]
- Aldana, P.U.; Dassenoy, F.; Vacher, B.; Mogne, T.L.; Thiebaut, B.; Bouffet, A. Antispalling Effect of WS2 Nanoparticles on the Lubrication of Automotive Gearboxes. Tribol. Trans. 2016, 59, 178–188. [Google Scholar] [CrossRef]
- Kumari, S.; Chouhan, A.; Siva Kumar Konathala, L.N.; Sharma, O.P.; Ray, S.S.; Ray, A.; Khatri, O.P. Chemically functionalized 2D/2D hexagonal boron Nitride/Molybdenum disulfide heterostructure for enhancement of lubrication properties. Appl. Surf. Sci. 2022, 579, 152157–152168. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, 9439. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Mandelli, D.; Hod, O.; Urbakh, M.; Ma, M.; Zheng, Q. Robust microscale superlubricity in graphite/hexagonal boron nitride layered heterojunctions. Nat. Mater. 2018, 17, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, D.; Leven, I.; Hod, O.; Urbakh, M. Sliding friction of graphene/hexagonal-boron nitride heterojunctions: A route to robust superlubricity. Sci. Rep. 2017, 7, 10851. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Nicolini, P.; Du, L.; Yuan, J.; Wang, S.; Yu, H.; Tang, J.; Cheng, P.; Watanabe, K.; Taniguchi, T.; et al. UItra-low friction and edge-pinning effect in large-lattice-mismatch van der Waals heterostructures. Nat. Mater. 2022, 21, 47–53. [Google Scholar] [CrossRef]
- Tian, J.; Yin, X.; Li, J.; Qi, W.; Huang, P.; Chen, X.; Luo, J. Tribo-Induced Interfacial Material Transfer of an Atomic Force Microscopy Probe Assisting Superlubricity in a WS2/Graphene Heterojunction. ACS Appl. Mater. Interfaces 2019, 12, 4031–4040. [Google Scholar] [CrossRef]
- Wei, Z.; Li, B.; Xia, C.; Cui, Y.; He, J.; Xia, J.-B.; Li, J. Various Structures of 2D Transition-Metal Dichalcogenides and Their Applications. Small Methods 2018, 2, 1800094. [Google Scholar] [CrossRef]
- Dean, C.R.; Young, A.F.; Meric, I.; Lee, C.; Wang, L.; Sorgenfrei, S.; Watanabe, K.; Taniguchi, T.; Kim, P.; Shepard, K.L.; et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 2010, 5, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.-J.; Chen, Y.-Q.; Zhou, Z.-Y.; Sun, J.; Peng, X.-S.; Liu, X.-Z. Two-dimensional MoSe2/graphene heterostructure thin film with wafer-scale continuity via van der Waals epitaxy. Chem. Phys. Lett. 2020, 755, 137762. [Google Scholar] [CrossRef]
- Han, T.; Liu, H.; Wang, S.; Chen, S.; Yang, K. The Large-Scale Preparation and Optical Properties of MoS2/WS2 Vertical Hetero-Junction. Molecules 2020, 25, 1857. [Google Scholar] [CrossRef] [PubMed]
- Macknojia, A.; Ayyagari, A.; Zambrano, D.; Rosenkranz, A.; Shevchenko, E.V.; Berman, D. Macroscale Superlubricity Induced by MXene/MoS2 Nanocomposites on Rough Steel Surfaces under High Contact Stresses. ACS Nano 2023, 17, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Liang, P.; Shu, H.; Zhang, L.; Wang, D.; Chao, D.; Liu, Z.; Du, X.; Wan, H.; Wang, H. Interface Synergistic Effect from Layered Metal Sulfides of MoS2/SnS2 van der Waals Heterojunction with Enhanced Li-Ion Storage Performance. J. Phys. Chem. C 2018, 122, 24600–24608. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhou, F.; Qu, J.; Song, J. 2D van der Waals Heterojunction Nanophotonic Devices: From Fabrication to Performance. Adv. Funct. Mater. 2021, 31, 2104260. [Google Scholar] [CrossRef]
- Han, T.; Liu, H.; Chen, S.; Chen, Y.; Wang, S.; Li, Z. Fabrication and Characterization of MoS2/h-BN and WS2/h-BN Heterostructures. Micromachines 2020, 11, 1114–1130. [Google Scholar] [CrossRef]
- Purbayanto, M.A.K.; Chandel, M.; Birowska, M.; Rosenkranz, A.; Jastrzębska, A.M. Optically Active MXenes in Van der Waals Heterostructures. Adv. Mater. 2023, 35, e2301850. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhang, X.; Wang, C.; Zhao, X.; Li, J.; Jin, H. Convenient Synthesis of WS2/MoS2 Heterostructures with Enhanced Photocatalytic Performance. J. Phys. Chem. C 2019, 123, 27363–27368. [Google Scholar] [CrossRef]
- Ren, K.; Yu, G.; Zhang, Z.; Wu, W.; Tian, P.; Chhattal, M.; Gong, Z.; Li, Y.; Zhang, J. Self-organized transfer film-induced ultralow friction of Graphene/MoWS4 heterostructure nanocomposite. Appl. Surf. Sci. 2022, 572, 151443. [Google Scholar] [CrossRef]
- Ni, X.; Li, Z.; Wang, Y. Adsorption Characteristics of Anionic Surfactant Sodium Dodecylbenzene Sulfonate on the Surface of Montmorillonite Minerals. Front. Chem. 2018, 6, 390–400. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhao, J.; Song, J.; Su, C.; Wang, Z. Surface modified TiO2 floating photocatalyst with PDDA for efficient adsorption and photocatalytic inactivation of Microcystis aeruginosa. Water Res. 2018, 131, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired modification of h-BN for high thermal conductive composite films with aligned structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5709. [Google Scholar] [CrossRef]
- Sui, Y.; Li, P.; Dai, X.; Zhang, C. Green self-assembly of h-BN@PDA@MoS2 nanosheets by polydopamine as fire hazard suppression materials. React. Funct. Polym. 2021, 165, 105965–105977. [Google Scholar] [CrossRef]
- Lv, F.; Lu, X.; Song, J.; Zhu, M.; Wang, S.; Xu, Y.; Chang, X. Enhanced Aramid/Al2O3 interfacial properties by PDDA modification for the preparation of composite insulating paper. Res. Chem. Intermed. 2022, 48, 4815–4835. [Google Scholar] [CrossRef]
- Vaziri, H.S.; Shokuhfar, A.; Afghahi, S.S.S. Synthesis of WS2/CNT hybrid nanoparticles for fabrication of hybrid aluminum matrix nanocomposite. Mater. Res. Express 2020, 7, 025034–025048. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Haghighi, A.H.; Sheydaei, M. Poly(ethylene trisulfide)/graphene oxide nanocomposites. J. Therm. Anal. Calorim. 2016, 128, 427–442. [Google Scholar] [CrossRef]
- Cho, E.-C.; Chang-Jian, C.-W.; Zheng, J.-H.; Huang, J.-H.; Lee, K.-C.; Ho, B.-C.; Hsiao, Y.-S. Microwave-assisted synthesis of TiO2/WS2 heterojunctions with enhanced photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2018, 91, 489–498. [Google Scholar] [CrossRef]
- Shu, D.; Li, Y.; Liu, A.; Zhang, H.; Zhou, Y. Facile route to preparation of positively charged GO using poly (diallyldimethylammoniumchloride). Fuller. Nanotub. Carbon Nanostruct. 2019, 28, 394–401. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Wang, J.; Ye, X.; Lei, W.; Xue, M.; Tang, H.; Li, C. Synthesis of ultrathin WS2 nanosheets and their tribological properties as lubricant additives. Nanoscale Res. Lett. 2016, 11, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, X.; Zhao, Z.; Yue, Z.; Feng, P.; Ma, X.; Li, H.; Ye, X.; Zhu, M. Heterostructured rGO/MoS2 nanocomposites toward enhancing lubrication function of industrial gear oils. Carbon 2022, 191, 84–97. [Google Scholar] [CrossRef]
- Feng, P.; Ren, Y.; Li, Y.; He, J.; Zhao, Z.; Ma, X.; Fan, X.; Zhu, M. Synergistic lubrication of few-layer Ti3C2Tx/MoS2 heterojunction as a lubricant additive. Friction 2022, 10, 2018–2032. [Google Scholar] [CrossRef]
- Neves, G.O.; Araya, N.; Salvaro, D.B.; Lamim, T.d.S.; Giacomelli, R.O.; Binder, C.; Klein, A.N.; de Mello, J.D.B. Tribologically induced nanostructural evolution of carbon materials: A new perspective. Friction 2023, 12, 144–163. [Google Scholar] [CrossRef]
- Shao, X.; Wang, L.; Yang, Y.; Yang, T.; Deng, G.; He, Y.; Dong, L.; Wang, H.; Yang, J. Influence of preload on the tribological performance of MoS2/GO composite lubricating coating. Tribol. Int. 2023, 181, 108306. [Google Scholar] [CrossRef]
- Kong, S.; Wang, J.; Hu, W.; Li, J. Effects of Thickness and Particle Size on Tribological Properties of Graphene as Lubricant Additive. Tribol. Lett. 2020, 68, 112–122. [Google Scholar] [CrossRef]
- Hod, O.; Meyer, E.; Zheng, Q.; Urbakh, M. Structural superlubricity and ultralow friction across the length scales. Nature 2018, 563, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Grey, F.; Zheng, Q. The high-speed sliding friction of graphene and novel routes to persistent superlubricity. Sci. Rep. 2014, 4, 4875–4882. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Ding, Q.; Yang, L.; Liu, B.; Bai, L. Enhancement on lubrication performances of water lubricants by multilayer graphene. Tribol. Lett. 2023, 72, 5–25. [Google Scholar] [CrossRef]
- Liang, H.; Chen, X.; Bu, Y.; Xu, M.; Zheng, G.; Gao, K.; Hua, X.; Fu, Y.; Zhang, J. Macroscopic superlubricity of potassium hydroxide solution achieved by incorporating in-situ released graphene from friction pairs. Friction 2022, 11, 567–579. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Sun, Y.; Shang, F.; Zhang, J.; Yao, J.; Yan, Z.; Shen, H. Preparation and Tribological Performance of Multi-Layer van der Waals Heterostructure WS2/h-BN. Lubricants 2024, 12, 163. https://doi.org/10.3390/lubricants12050163
Fang Y, Sun Y, Shang F, Zhang J, Yao J, Yan Z, Shen H. Preparation and Tribological Performance of Multi-Layer van der Waals Heterostructure WS2/h-BN. Lubricants. 2024; 12(5):163. https://doi.org/10.3390/lubricants12050163
Chicago/Turabian StyleFang, Yunqi, Yang Sun, Fengqin Shang, Jing Zhang, Jiayu Yao, Zihan Yan, and Hangyan Shen. 2024. "Preparation and Tribological Performance of Multi-Layer van der Waals Heterostructure WS2/h-BN" Lubricants 12, no. 5: 163. https://doi.org/10.3390/lubricants12050163




