A Warm Welcome to the Alps—The Northward Expansion of Trithemis annulata (Odonata, Libellulidae) in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Data
2.2. Northward Expansion, Range Size and Altitude
2.3. Species Distribution Modelling
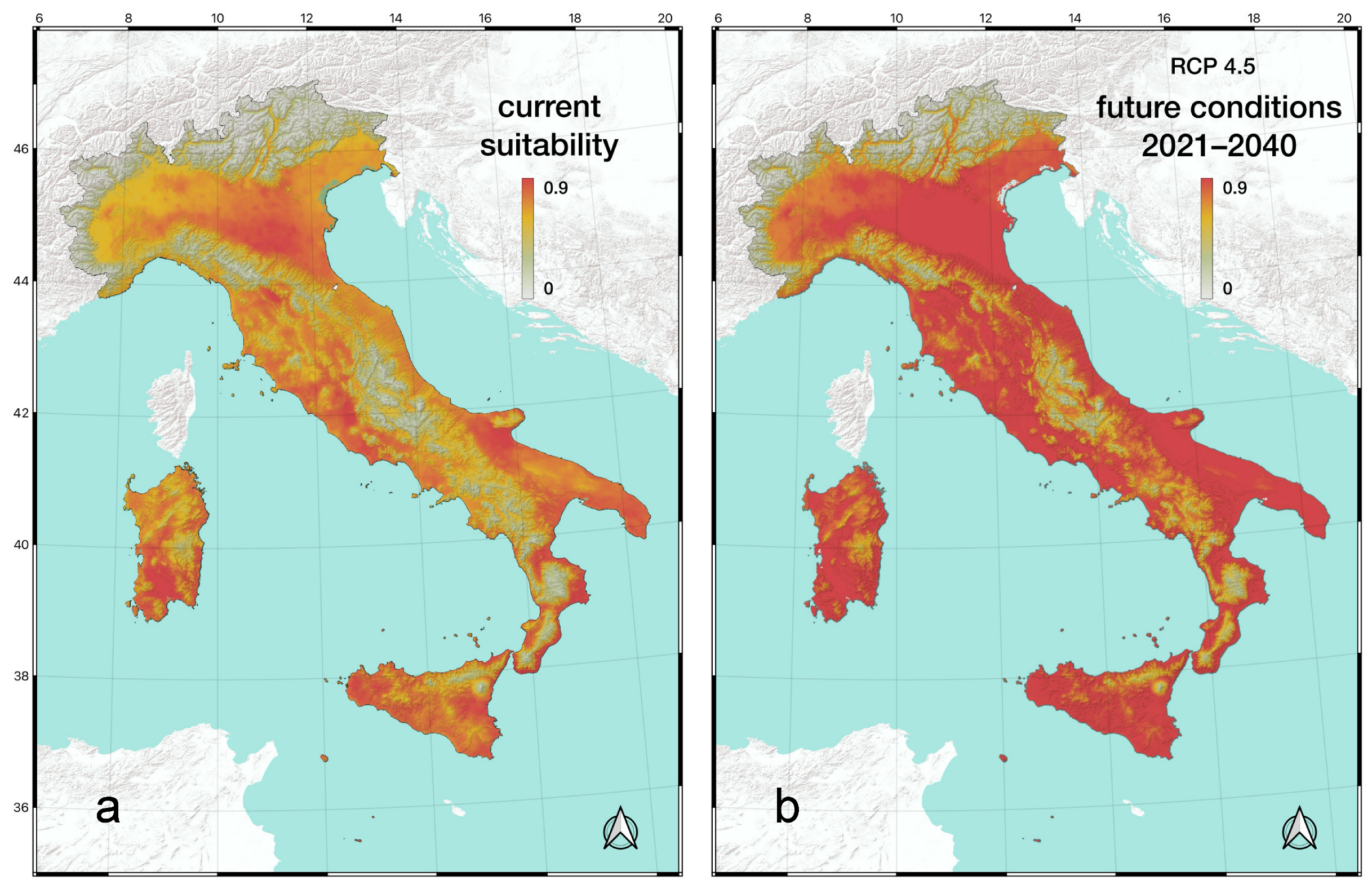
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. Proceedings of the IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Ossó, A.; Allan, R.P.; Hawkins, E.; Shaffrey, L.; Maraun, D. Emerging New Climate Extremes over Europe. Clim. Dyn. 2022, 58, 487–501. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ Warning to Humanity on Insect Extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Termaat, T.; van Strien, A.J.; van Grunsven, R.H.A.; De Knijf, G.; Bjelke, U.; Burbach, K.; Conze, K.; Goffart, P.; Hepper, D.; Kalkman, V.J.; et al. Distribution Trends of European Dragonflies under Climate Change. Divers. Distrib. 2019, 25, 936–950. [Google Scholar] [CrossRef]
- Engelhardt, E.K.; Biber, M.F.; Dolek, M.; Fartmann, T.; Hochkirch, A.; Leidinger, J.; Löffler, F.; Pinkert, S.; Poniatowski, D.; Voith, J.; et al. Consistent Signals of a Warming Climate in Occupancy Changes of Three Insect Taxa over 40 Years in Central Europe. Glob. Chang. Biol. 2022, 28, 3998–4012. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The Distributions of a Wide Range of Taxonomic Groups Are Expanding Polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Hassall, C.; Thompson, D.J.; French, G.C.; Harvey, I.F. Historical Changes in the Phenology of British Odonata Are Related to Climate. Glob. Chang. Biol. 2007, 13, 933–941. [Google Scholar] [CrossRef]
- Hassall, C. Odonata as Candidate Macroecological Barometers for Global Climate Change. Freshw. Sci. 2015, 34, 1040–1049. [Google Scholar] [CrossRef]
- Li, F.; Park, Y.-S. Habitat Availability and Environmental Preference Drive Species Range Shifts in Concordance with Climate Change. Divers. Distrib. 2020, 26, 1343–1356. [Google Scholar] [CrossRef]
- Shin, S.; Jung, K.S.; Kang, H.G.; Dang, J.-H.; Kang, D.; Han, J.E.; Kim, J.H. Northward Expansion Trends and Future Potential Distribution of a Dragonfly Ischnura senegalensis Rambur under Climate Change Using Citizen Science Data in South Korea. J. Ecol. Environ. 2021, 45, 33. [Google Scholar] [CrossRef]
- Olsen, K.; Svenning, J.-C.; Balslev, H. Niche Breadth Predicts Geographical Range Size and Northern Range Shift in European Dragonfly Species (Odonata). Diversity 2022, 14, 719. [Google Scholar] [CrossRef]
- Gil-Tapetado, D.; López-Collar, D.; Gómez, J.F.; Mañani-Pérez, J.; Cabrero-Sañudo, F.J.; Muñoz, J. Climate Change as a Driver of Insect Invasions: Dispersal Patterns of a Dragonfly Species Colonizing a New Region. PLoS ONE 2023, 18, e0291270. [Google Scholar] [CrossRef]
- Bota-Sierra, C.A.; García-Robledo, C.; Escobar, F.; Novelo-Gutiérrez, R.; Londoño, G.A. Environment, Taxonomy and Morphology Constrain Insect Thermal Physiology along Tropical Mountains. Funct. Ecol. 2022, 36, 1924–1935. [Google Scholar] [CrossRef]
- Taheri, S.; Naimi, B.; Rahbek, C.; Araújo, M.B. Improvements in Reports of Species Redistribution under Climate Change Are Required. Sci. Adv. 2021, 7, eabe1110. [Google Scholar] [CrossRef]
- Oertli, B. The Local Species Richness of Dragonflies in Mountain Waterbodies: An Indicator of Climate Warming? BIORISK–Biodivers. Ecosyst. Risk Assess. 2010, 5, 243–251. [Google Scholar] [CrossRef]
- Cancellario, T.; Miranda, R.; Baquero, E.; Fontaneto, D.; Martínez, A.; Mammola, S. Climate Change Will Redefine Taxonomic, Functional, and Phylogenetic Diversity of Odonata in Space and Time. NPJ Biodivers 2022, 1, 1. [Google Scholar] [CrossRef]
- Cadena, J.T.; Boudot, J.; Kalkman, V.J.; Marshall, L. Impacts of Climate Change on Dragonflies and Damselflies in West and Central Asia. Divers. Distrib. 2023, 29, 912–925. [Google Scholar] [CrossRef]
- Pélissié, M.; Johansson, F.; Hyseni, C. Pushed Northward by Climate Change: Range Shifts With a Chance of Co-Occurrence Reshuffling in the Forecast for Northern European Odonates. Environ. Entomol. 2022, 51, 910–921. [Google Scholar] [CrossRef]
- Jaeschke, A.; Bittner, T.; Reineking, B.; Beierkuhnlein, C. Can They Keep up with Climate Change?—Integrating Specific Dispersal Abilities of Protected Odonata in Species Distribution Modelling. Insect Conserv. Divers. 2013, 6, 93–103. [Google Scholar] [CrossRef]
- MacLean, S.A.; Beissinger, S.R. Species’ Traits as Predictors of Range Shifts under Contemporary Climate Change: A Review and Meta-Analysis. Glob. Chang. Biol. 2017, 23, 4094–4105. [Google Scholar] [CrossRef]
- Platts, P.J.; Mason, S.C.; Palmer, G.; Hill, J.K.; Oliver, T.H.; Powney, G.D.; Fox, R.; Thomas, C.D. Habitat Availability Explains Variation in Climate-Driven Range Shifts across Multiple Taxonomic Groups. Sci. Rep. 2019, 9, 15039. [Google Scholar] [CrossRef] [PubMed]
- Angert, A.L.; Crozier, L.G.; Rissler, L.J.; Gilman, S.E.; Tewksbury, J.J.; Chunco, A.J. Do Species’ Traits Predict Recent Shifts at Expanding Range Edges? Ecol. Lett. 2011, 14, 677–689. [Google Scholar] [CrossRef]
- Olsen, K.; Svenning, J.-C.; Balslev, H. Climate Change Is Driving Shifts in Dragonfly Species Richness across Europe via Differential Dynamics of Taxonomic and Biogeographic Groups. Diversity 2022, 14, 1066. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Migration of Terrestrial Arthropods in Relation to Habitat. Biol. Rev. 1962, 37, 171–211. [Google Scholar] [CrossRef]
- Grewe, Y.; Hof, C.; Dehling, D.M.; Brandl, R.; Brändle, M. Recent Range Shifts of European Dragonflies Provide Support for an Inverse Relationship between Habitat Predictability and Dispersal. Glob. Ecol. Biogeogr. 2013, 22, 403–409. [Google Scholar] [CrossRef]
- Hof, C.; Brändle, M.; Brandl, R. Lentic Odonates Have Larger and More Northern Ranges than Lotic Species. J. Biogeogr. 2006, 33, 63–70. [Google Scholar] [CrossRef]
- Solano, E.; Hardersen, S.; Audisio, P.; Amorosi, V.; Senczuk, G.; Antonini, G. Asymmetric Hybridization in Cordulegaster (Odonata: Cordulegastridae): Secondary Postglacial Contact and the Possible Role of Mechanical Constraints. Ecol. Evol. 2018, 8, 9657–9671. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, T.J.; Djernæs, M.; Nielsen, O.F.; Olsen, K. The Grasshopper Paradigm in Damselflies: Evidence for Phalanx-like Postglacial Recolonization of Europe from a Balkan Refugium in Platycnemis pennipes Pallas (Odonata: Zygoptera: Platycnemidae). Arthropod Syst. Phylogeny 2023, 81, 1019–1029. [Google Scholar] [CrossRef]
- Ott, J. The Expansion of Crocothemis erythraea (Brullé, 1832) in Germany—An Indicator of Climatic Changes. In Biology of Dragonflies; Scientific Publisher: Jodhpur, India, 2007. [Google Scholar]
- Damm, S.; Dijkstra, K.-D.B.; Hadrys, H. Red Drifters and Dark Residents: The Phylogeny and Ecology of a Plio-Pleistocene Dragonfly Radiation Reflects Africa’s Changing Environment (Odonata, Libellulidae, Trithemis). Mol. Phylogenetics Evol. 2010, 54, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, V.J.; Riservato, E.; Hardersen, S. Trithemis annulata (Palisot de Beauvois, 1807). In Atlas of the European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: The Netherlands, 2015; pp. 313–315. [Google Scholar]
- Stefanov, T.; Vasilev, V. First Record of the Violet Dropwing Trithemis annulata (Palisot de Beauvois, 1807) (Odonata: Libellulidae) in Bulgaria. Acta Zool. Bulg. 2021, 73, 637–639. [Google Scholar]
- Koren, T.; Koller Šarić, K.; Kelava, L. The First Records of Trithemis annulata (Palisot de Beauvois, 1807) (Odonata: Libellulidae) in Croatia. Nat. Croat. Period. Musei Hist. Nat. Croat. 2022, 31, 293–302. [Google Scholar] [CrossRef]
- Puff, F.; Hilpold, A.; Schulze, C.H.; Guariento, E. Trithemis annulata (Insecta, Libellulidae) Reaches the Northernmost Italian Region Trentino-Alto Adige/Südtirol. Gredleriana 2023, 23, 107–114. [Google Scholar]
- Carchini, G.; Rota, E.; Utzeri, C. Lista Aggiornata Degli Odonati Italiani e Loro Distribuzione Regionale. Fragm. Entomol. 1985, 18, 91–103. [Google Scholar]
- Terzani, F. Trithemis annulata (Palisot de Beauvais, 1805) (Odonata: Libellulidae). In Bolletino della Società Entomologica Italiana; Segnalazioni Faunistiche Italiane (N. 163-197); Società Entomologica Italiana: Genova, Italy, 1991; Volume 123. [Google Scholar]
- Fabbri, R.A. Due Nuove Segnalazioni e Una Conferma per Le Specie Di Odonati Della Regione Emilia-Romagna (Insecta Odonata). Quad. Studi Nat. Romagna 2011, 34, 47–50. [Google Scholar]
- La Porta, G.; Dell’Otto, A.; Speziale, A.; Goretti, E.; Rebora, M.; Piersanti, S.; Gaino, E. Odonata Biodiversity in Some Protected Areas of Umbria, Central Italy. Odonatologica 2013, 42, 125–137. [Google Scholar]
- Gheza, G.; Ancarani, G.; Chiari, C.; Corazzato, C.; Galliani, C.; Minicò, A.; Sacchi, F.; Sand, M.L.; Piglia, A. Breeding of Trithemis annulata in Quarry Lakes in the Continental Area of Italy (Odonata: Libellulidae). Libellula 2019, 38, 137–155. [Google Scholar]
- Chiari, C.; Piglia, A.; Sacchi, F.; Sand, M. Presenza Di Trithemis annulata, Obelisco Violetto (Palisot De Beauvois, 1805) (Anisoptera: Libellulidae) in Provincia Di Brescia Nel 2018. «NATURA BRESCIANA» Ann. Mus. Civ. Sc. Nat. 2020, 43, 145–148. [Google Scholar]
- NASA JPL NASA Shuttle Radar Topography Mission Global 1 Arc Second 2013; NASA: Washington, DC, USA, 2013.
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS Monthly High-Resolution Gridded Multivariate Climate Dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; Elith, J.; Dudík, M.; Ferrier, S.; Huettmann, F.; et al. Effects of Sample Size on the Performance of Species Distribution Models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Collins, S.D.; McIntyre, N.E. Modeling the Distribution of Odonates: A Review. Freshw. Sci. 2015, 34, 1144–1158. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping Species Distributions with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef]
- Elith, J.; Burgman, M. Predictions and Their Validation: Rare Plants in the Central Highlands, Victoria, Australia. In Predicting Species Occurrences: Issues of Accuracy and Scale; Island Press: Covelo, CA, USA, 2002; pp. 303–314. [Google Scholar]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Team: Boston, MA, USA, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Volodin, E.M.; Yurova, A.Y. Summer Temperature Standard Deviation, Skewness and Strong Positive Temperature Anomalies in the Present Day Climate and under Global Warming Conditions. Clim. Dyn. 2013, 40, 1387–1398. [Google Scholar] [CrossRef]
- Corso, A.; Janni, O.; Pavesi, M.; Viganò, M. Update to the Status of Pantala flavescens (Fabricius,1798) and Trithemis kirbyi Selys,1891 for Italy and Central Mediterranean Basin (Odonata Libellulidae). Biodivers. J. 2017, 8, 33–38. [Google Scholar]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Thomas, C.D. A Northward Shift of Range Margins in British Odonata. Glob. Chang. Biol. 2005, 11, 502–506. [Google Scholar] [CrossRef]
- Nagano, K.; Hiraiwa, M.K.; Ishiwaka, N.; Seko, Y.; Hashimoto, K.; Uchida, T.; Sánchez-Bayo, F.; Hayasaka, D. Global Warming Intensifies the Interference Competition by a Poleward-Expanding Invader on a Native Dragonfly Species. R. Soc. Open Sci. 2023, 10, 230449. [Google Scholar] [CrossRef]
- Bonet Betoret, C. Expansión De Trithemis annulata En Europa En Los Años 80 Y 90 (Odonata). Boletín Soc. Entomológica Aragonesa 2000, 27, 85–86. [Google Scholar]
- Bush, A.; Hermoso, V.; Linke, S.; Nipperess, D.; Turak, E.; Hughes, L. Freshwater Conservation Planning under Climate Change: Demonstrating Proactive Approaches for Australian Odonata. J. Appl. Ecol. 2014, 51, 1273–1281. [Google Scholar] [CrossRef]
- Simaika, J.P.; Samways, M.J. Predicted Range Shifts of Dragonflies over a Wide Elevation Gradient in the Southern Hemisphere. Freshw. Sci. 2015, 34, 1133–1143. [Google Scholar] [CrossRef]
- Devictor, V.; van Swaay, C.; Brereton, T.; Brotons, L.; Chamberlain, D.; Heliölä, J.; Herrando, S.; Julliard, R.; Kuussaari, M.; Lindström, Å.; et al. Differences in the Climatic Debts of Birds and Butterflies at a Continental Scale. Nat. Clim. Chang. 2012, 2, 121. [Google Scholar] [CrossRef]
- Cerini, F. Long-Term Shifts in the Communities of Odonata: Effect of Chance or Climate Change? North West. J. Zool. 2020, 16, 6. [Google Scholar]
- Harabiš, F.; Dolný, A. The Effect of Ecological Determinants on the Dispersal Abilities of Central European Dragonflies (Odonata). Odonatologica 2011, 40, 17–26. [Google Scholar]
- Ghirardi, N.; Bresciani, M.; Pinardi, M.; Nizzoli, D.; Viaroli, P. Pit Lakes from Gravel and Sand Quarrying in the Po River Basin: An Opportunity for Riverscape Rehabilitation and Ecosystem Services Improvement. Ecol. Eng. 2023, 196, 107103. [Google Scholar] [CrossRef]
- Altwegg, R.; Collingham, Y.C.; Erni, B.; Huntley, B. Density-Dependent Dispersal and the Speed of Range Expansions. Divers. Distrib. 2013, 19, 60–68. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Boudot, J.-P.; Futahashi, R.; Abbott, J.C.; Bota-Sierra, C.A.; Guralnick, R.; Bybee, S.M.; Ware, J.; Belitz, M.W. Diversity of Palaearctic Dragonflies and Damselflies (Odonata). Diversity 2022, 14, 966. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Boudot, J.-P.; Bernard, R.; De Knijf, G.; Suhling, F.; Termaat, T. Diversity and Conservation of European Dragonflies and Damselflies (Odonata). Hydrobiologia 2018, 811, 269–282. [Google Scholar] [CrossRef]
- Weihrauch, F.; Burbach, K.; Hölken, U.; Netz, H.J.; Stettmer, C. Neue Nachweise von Orthetrum albistylum aus Bayern (Odonata: Libellulidae). Libellula Suppl. 2003, 4, 61–72. [Google Scholar]
- Fekete, J.; De Knijf, G.; Dinis, M.; Padisák, J.; Boda, P.; Mizsei, E.; Várbíró, G. Winners and Losers: Cordulegaster Species under the Pressure of Climate Change. Insects 2023, 14, 348. [Google Scholar] [CrossRef]
- Rödder, D.; Schmitt, T.; Gros, P.; Ulrich, W.; Habel, J.C. Climate Change Drives Mountain Butterflies towards the Summits. Sci. Rep. 2021, 11, 14382. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, R.; González-Megías, A.; Jay-Robert, P.; Marquéz-Ferrando, R. Climate Change and Elevational Range Shifts: Evidence from Dung Beetles in Two European Mountain Ranges. Glob. Ecol. Biogeogr. 2014, 23, 646–657. [Google Scholar] [CrossRef]
- Konvicka, M.; Maradova, M.; Benes, J.; Fric, Z.; Kepka, P. Uphill Shifts in Distribution of Butterflies in the Czech Republic: Effects of Changing Climate Detected on a Regional Scale. Glob. Ecol. Biogeogr. 2003, 12, 403–410. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Rodríguez, P.; Córdoba-Aguilar, A. Can Dragonfly and Damselfly Communities Be Used as Bioindicators of Land Use Intensification? Ecol. Indic. 2019, 107, 105553. [Google Scholar] [CrossRef]
- Riservato, E.; Festi, A.; Fabbri, R.; Grieco, C.; Hardersen, S.; La Porta, G.; Landi, F.; Siesa, M.E.; Utzeri, C. Odonata: Atlante delle libellule Italiane: Preliminare; Ed. Belvedere: Latina, Italy, 2014; ISBN 978-88-89504-38-3. [Google Scholar]
- Boano, G.; Sindaco, R.; Riservato, E.; Fasano, S.; Barbero, R. Atlante Degli Odonati Del Piemonte e Della Valle d’Aosta; Memorie dell’Associazione Naturalistca Piemontese; Associazione Naturalistica Piemontese: Carmagnola, Italy, 2007; Volume VI. [Google Scholar]
- Vary, P.P.; Sansault, E. Première Observation de Trithemis annulata En Région Centre-Val de Loire (Odonata: Libellulidae). Martinia 2019, 56. [Google Scholar]
- Vinko, D.; Salamun, A. First Record of Violet Dropwing Trithemis annulata (Palisot de Beauvois, 1807) (Odonata: Libellulidae) in Slovenia. Nat. Slov. 2021, 23, 25–37. [Google Scholar] [CrossRef]
- Essl, F.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kühn, I.; Lenzner, B.; Pauchard, A.; Pyšek, P.; et al. A Conceptual Framework for Range-Expanding Species That Track Human-Induced Environmental Change. BioScience 2019, 69, 908–919. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Porta, G.; Hardersen, S. A Warm Welcome to the Alps—The Northward Expansion of Trithemis annulata (Odonata, Libellulidae) in Italy. Insects 2024, 15, 340. https://doi.org/10.3390/insects15050340
La Porta G, Hardersen S. A Warm Welcome to the Alps—The Northward Expansion of Trithemis annulata (Odonata, Libellulidae) in Italy. Insects. 2024; 15(5):340. https://doi.org/10.3390/insects15050340
Chicago/Turabian StyleLa Porta, Gianandrea, and Sönke Hardersen. 2024. "A Warm Welcome to the Alps—The Northward Expansion of Trithemis annulata (Odonata, Libellulidae) in Italy" Insects 15, no. 5: 340. https://doi.org/10.3390/insects15050340





