Coexistence of Intermetallic Complexions and Bulk Particles in Grain Boundaries in the ZEK100 Alloy
Abstract
:1. Introduction
2. Materials and Methods
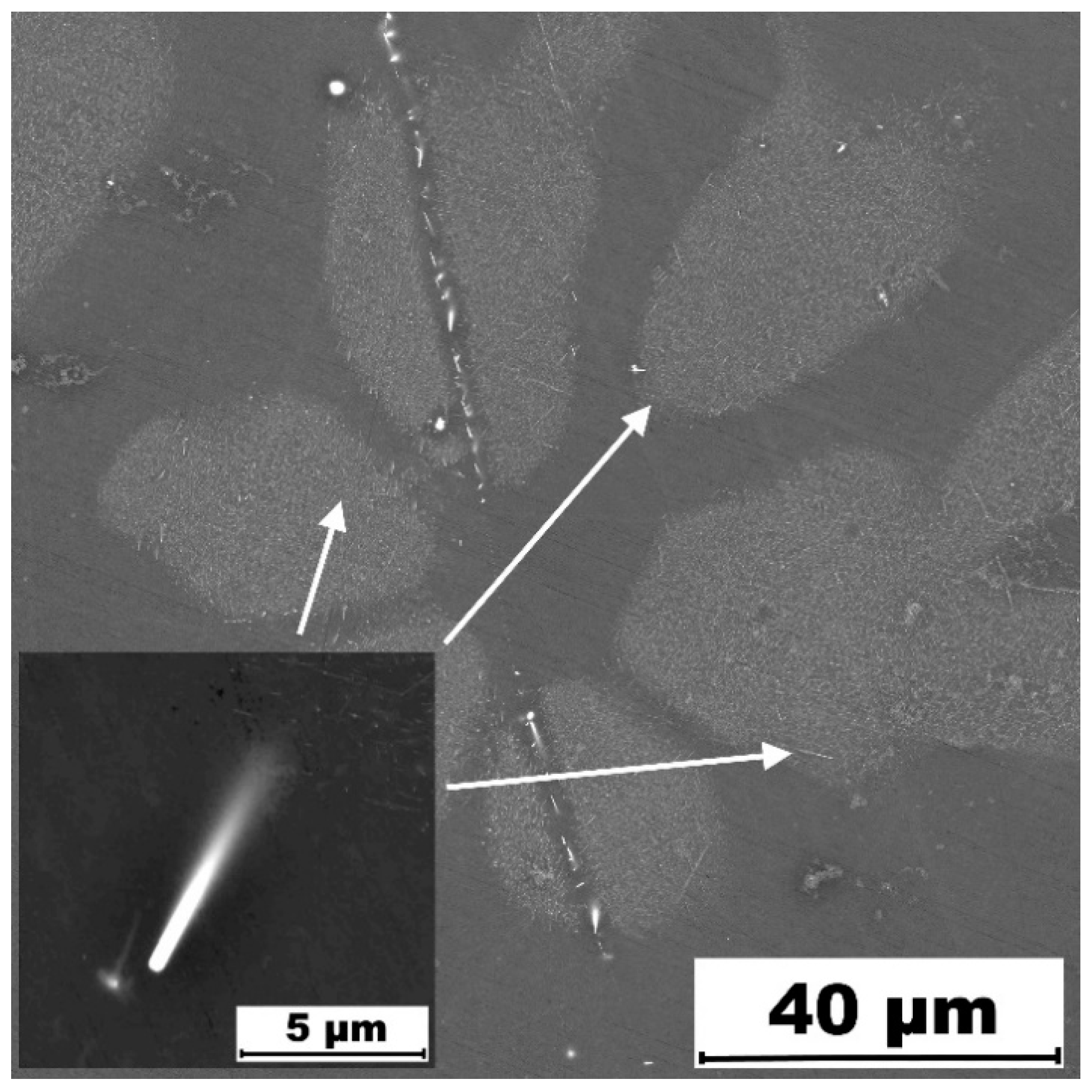
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryła, K.; Krystian, M.; Horky, J.; Mingler, B.; Mroczka, K.; Kurtyka, P.; Lityńska-Dobrzyńska, L. Improvement of Strength and Ductility of an EZ Magnesium Alloy by Applying Two Different ECAP Concepts to Processable Initial States. Mater. Sci. Eng. A 2018, 737, 318–327. [Google Scholar] [CrossRef]
- Bryła, K.; Morgiel, J.; Faryna, M.; Edalati, K.; Horita, Z. Effect of High-Pressure Torsion on Grain Refinement, Strength Enhancement and Uniform Ductility of EZ Magnesium Alloy. Mater. Lett. 2018, 212, 323–326. [Google Scholar] [CrossRef]
- Straumal, A.B.; Tsoy, K.V.; Mazilkin, I.A.; Nekrasov, A.N.; Bryła, K. Grain Boundary Wetting and Material Performance in an Industrial EZ33a Mg Cast Alloy. Arch. Metall. Mater. 2019, 64, 869–873. [Google Scholar] [CrossRef]
- Straumal, A.; Mazilkin, I.; Tzoy, K.; Straumal, B.; Bryła, K.; Baranchikov, A.; Eggeler, G. Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A. Metals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Trang, T.T.T.; Zhang, J.H.; Kim, J.H.; Zargaran, A.; Hwang, J.H.; Suh, B.C.; Kim, N.J. Designing a Magnesium Alloy with High Strength and High Formability. Nat. Commun. 2018, 9, 2522. [Google Scholar] [CrossRef] [Green Version]
- Oshida, Y. Magnesium Materials. From Mountain Bikes to Degradable Bone Grafts; De Gruyter Publishing: Berlin, Germany, 2021; p. 808. [Google Scholar] [CrossRef]
- Atrens, A.; Dietzel, W.; Srinivasan, P.B.; Winzer, N.; Kannan, M.B. Chapter 9: Stress Corrosion Cracking (SCC) of Magnesium Alloys. In Stress Corrosion Cracking: Theory and Practice; Woodhead Publishing: Cambridge, UK, 2011; pp. 341–380. [Google Scholar] [CrossRef]
- Martinez, D.C.; Dobkowska, A.; Marek, R.; Cwieka, H.; Jaroszewicz, J.; Plocinski, T.P.; Donik, C.; Helmholz, H.; Luthringer-Feyerabend, B.; Zeller-Plumhoff, B.; et al. In vitro and in vivo Degradation Behavior of Mg-0.45Zn-0.45Ca (ZX00) Screws for Orthopedic Applications. Bioact. Mat. 2023, 28, 132–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Kurukuri, S.; Worswick, M.J.; Li, D.Y.; Peng, Y.H.; Wu, P.D. The Strain Rate Sensitive and Anisotropic Behavior of Rare-Earth Magnesium Alloy ZEK100 Sheet. J. Magn. Alloys 2023, 11, 882–891. [Google Scholar] [CrossRef]
- Al-Samman, T.; Li, X. Sheet Texture Modification in Magnesium-Based Alloys by Selective Rare Earth Alloying. Mater. Sci. Eng. A 2011, 528, 3809–3822. [Google Scholar] [CrossRef]
- Patel, M.; Paudel, Y.; Mujahid, S.; Rhee, H.; El Kadiri, H. Self-Consistent Crystal Plasticity Modeling of Slip-Twin Interactions in Mg Alloys. Crystals 2023, 13, 653. [Google Scholar] [CrossRef]
- Ishiguro, Y.; Huang, X.; Tsukada, Y.; Koyama, T.; Chino, Y. Effect of Bending and Tension Deformation on the Texture Evolution and Stretch Formability of Mg–Zn–RE–Zr Alloy. Int. J. Miner. Metall. Mater. 2022, 29, 1334. [Google Scholar] [CrossRef]
- Griffits, D.; Davis, B.; Robson, J.D. The Influence of Strain Path on Rare Earth Recrystallization Textures in a Magnesium-Zinc-Rare Earth Alloy. Metal. Mater. Trans. A 2018, 49, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Habib, S.A.; Khan, A.S.; Gnäupel-Herold, T.; Lloyd, J.T.; Schoenfeld, S.E. Anisotropy, Tension-Compression Asymmetry and Texture Evolution of a Rare-Earth-Containing Magnesium Alloy Sheet, ZEK100, at Different Strain Rates and Temperatures: Experiments and Modeling. Int. J. Plast. 2017, 95, 163–190. [Google Scholar] [CrossRef]
- Becker, R.; Lloyd, J.T. A Reduced-Order Crystal Model for HCP Metals: Application to Mg. Mech. Mater. 2016, 98, 98–110. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Worswick, M.J. Experimental Fracture Characterisation of an Anisotropic Magnesium Alloy Sheet in Proportional and Non-proportional Loading Conditions. Int. J. Solids Struct. 2018, 144–145, 1–19. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Worswick, M.J. Fracture Characterization of Rolled Sheet Alloys in Shear Loading: Studies of Specimen Geometry, Anisotropy, and Rate Sensitivity. Exp. Mech. 2017, 57, 75–88. [Google Scholar] [CrossRef]
- Ray, A.K.; Wilkinson, D.S. The Effect of Microstructure on Damage and Fracture in AZ31B and ZEK100 Magnesium Alloys. Mater. Sci. Eng. A 2016, 658, 33–41. [Google Scholar] [CrossRef]
- Omer, K.; Butcher, C.; Worswick, M. Characterization of Heat Transfer Coefficient for Non-isothermal Elevated Temperature Forming of Metal Alloys. Int. J. Mater. Form. 2020, 13, 177–201. [Google Scholar] [CrossRef]
- Hadadzadeh, A.; Wells, M.A.; Javaid, A. Warm and Hot Deformation Behavior of As-Cast ZEK100 Magnesium Alloy. Exp. Mech. 2016, 56, 259–271. [Google Scholar] [CrossRef]
- Min, J.; Hector, L.G., Jr.; Lin, J.; Carter, J.T.; Sachdev, A.K. Spatio-temporal Characteristics of Propagative Plastic Instabilities in a Rare Earth Containing Magnesium Alloy. Int. J. Plast. 2014, 57, 52–76. [Google Scholar] [CrossRef]
- Li, Q.; Yea, W.; Gaoa, H.; Gao, L. Improving the Corrosion Resistance of ZEK100 Magnesium Alloy by Combining High-pressure Torsion Technology with Hydroxyapatite Coating. Mater. Des. 2019, 181, 107933. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Effects of Calcium and Rare-earth Elements on the Microstructure and Tension–Compression Yield Asymmetry of ZEK100 Alloy. Mater. Sci. Eng. A 2014, 618, 238–243. [Google Scholar] [CrossRef]
- Ertürk, S.; Brocks, W.; Bohlen, J.; Letzig, D.; Steglich, D. A Constitutive Law for the Thermo-mechanical Modelling of Magnesium Alloy Extrusion. Int. J. Mater. Form. 2012, 5, 325–339. [Google Scholar] [CrossRef]
- Dobroñ, P.; Chmelík, F.; Parfenenko, K.; Letzig, D.; Bohlen, J. On the Effect of the Extrusion Speed on Microstructure and Plastic Deformation of ZE10 and ZEK100 Magnesium Alloys—An Acoustic Emission Study. Acta Phys. Pol. 2012, 122, 593–596. [Google Scholar] [CrossRef]
- Javaid, A.; Czerwinski, F. Effect of Hot Rolling on Microstructure and Properties of the ZEK100 Alloy. J. Magn. Alloys 2019, 7, 27–37. [Google Scholar] [CrossRef]
- Boba, M.; Butcher, C.; Panahi, N.; Worswick, M.J.; Mishra, R.K.; Carter, J.T. Warm Forming Limits of Rare Earth-magnesium Alloy ZEK100 Sheet. Int. J. Mater. Form. 2017, 10, 181–191. [Google Scholar] [CrossRef]
- Roostaei, A.A.; Ling, Y.; Jahed, H.; Glinka, G. Applications of Neuber’s and Glinka’s Notch Plasticity Correction Rules to Asymmetric Magnesium Alloys Under Cyclic Load. Theor. Appl. Fract. Mech. 2020, 105, 102431. [Google Scholar] [CrossRef]
- Ling, Y.; Roostaei, A.A.; Glinka, G.; Jahed, H. Fatigue of ZEK100-F Magnesium Alloy: Characterisation and Modelling. Int. J. Fatigue 2019, 125, 179–186. [Google Scholar] [CrossRef]
- Chen, G.; Ren, J.; Gao, H.; Cui, Y.; Chen, X. Pseudoelastic and Corrosion Behaviors of Mg ZEK100 Alloy under Cyclic Loading. Int. J. Fatigue 2017, 103, 466–477. [Google Scholar] [CrossRef]
- Mokdad, F.; Chen, D.L. Cyclic Deformation and Anelastic Behavior of ZEK100 Magnesium Alloy: Effect of Strain Ratio. Mater. Sci. Eng. A 2015, 640, 243–258. [Google Scholar] [CrossRef]
- Mokdad, F.; Chen, D.L. Strain-controlled Low Cycle Fatigue Properties of a Rare-earth Containing ZEK100 Magnesium Alloy. Mater. Des. 2015, 67, 436–447. [Google Scholar] [CrossRef]
- Min, J.; Lin, J. Anelastic Behavior and Phenomenological Modeling of Mg ZEK100-O Alloy Sheet under Cyclic Tensile Loading–Unloading. Mater. Sci. Eng. A 2013, 561, 174–182. [Google Scholar] [CrossRef]
- Noder, J.; Abedini, A.; Butcher, C. Evaluation of the VDA 238–100 Tight Radius Bend Test for Plane Strain Fracture Characterization of Automotive Sheet Metals. Exp. Mech. 2020, 60, 787–800. [Google Scholar] [CrossRef]
- Aslam, I.; Li, B.; McClelland, Z.; Horstemeyer, S.J.; Maa, Q.; Wang, P.T.; Horstemeyer, M.F. Three-point Bending Behavior of a ZEK100 Mg Alloy at Room Temperature. Mater. Sci. Eng. A 2014, 590, 168–173. [Google Scholar] [CrossRef]
- Min, J.; Lin, J.; Li, J. Forming Limits of Mg alloy ZEK100 Sheet in Preform Annealing Process. Mater. Des. 2014, 53, 947–953. [Google Scholar] [CrossRef]
- Min, J.; Hector, L.G., Jr.; Lin, J.; Carter, J.T. Analytical Method for Forming Limit Diagram Prediction with Application to a Magnesium ZEK100-O Alloy. J. Mater. Eng. Perform. 2013, 22, 3324–3336. [Google Scholar] [CrossRef] [Green Version]
- Antoniswamy, A.R.; Carpenter, A.J.; Carter, J.T.; Hector, L.G., Jr.; Taleff, E.M. Forming-Limit Diagrams for Magnesium AZ31B and ZEK100 Alloy Sheets at Elevated Temperatures. J. Mater. Eng. Perform. 2013, 22, 3389–3397. [Google Scholar] [CrossRef]
- Paudel, Y.R.; Indeck, J.; Hazeli, K.; Priddy, M.W.; Inal, K.; Rhee, H.; Barrett, C.D.; Whittington, W.R.; Limmer, K.R.; El Kadiri, H. Characterization and Modeling of {1012} Twin Banding in Magnesium. Acta Mater. 2020, 183, 438–451. [Google Scholar] [CrossRef]
- Bong, H.J.; Hu, X.; Sun, X.; Ren, Y. Temperature-dependent Constitutive Modeling of a Magnesium Alloy ZEK100 Sheet Using Crystal Plasticity Models Combined with in situ High-energy X-ray Diffraction Experiment. J. Magnes. Alloys 2022, 10, 2801–2816. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Jiang, Y.; Wu, P.; Wang, H. Multi-Island Genetic-Algorithm-Based Approach to Uniquely Calibrate Polycrystal Plasticity Models for Magnesium Alloys. JOM 2021, 73, 1395–1403. [Google Scholar] [CrossRef]
- Bong, H.J.; Hu, X.; Sun, X.; Ren, Y. Mechanism-based Constitutive Modeling of ZEK100 Magnesium Alloy with Crystal Plasticity and in-situ HEXRD Experiment. Int. J. Plast. 2019, 113, 35–51. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Worswick, M.J. Application of an Evolving Non-Associative Anisotropic-Asymmetric Plasticity Model for a Rare-Earth Magnesium Alloy. Metals 2018, 8, 1013. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Badreddine, H.; Saanouni, K. Thermodynamically-Consistent Constitutive Modeling of Hardening Asymmetry Including Isotropic Ductile Damage for Mg Alloys. Eur. J. Mech. A Solids 2019, 73, 169–180. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Nemcko, M.J.; Kurukuri, S.; Worswick, M.J. Constitutive Characterization of a Rare-Earth Magnesium Alloy Sheet (ZEK100-O) in Shear Loading: Studies of Anisotropy and Rate Sensitivity. Int. J. Mech. Sci. 2017, 128–129, 54–69. [Google Scholar] [CrossRef]
- Muhammad, W.; Mohammadi, M.; Kang, J.; Mishra, R.K.; Inal, K. An Elasto-Plastic Constitutive Model for Evolving Asymmetric/anisotropic Hardening Behavior of AZ31B and ZEK100 Magnesium Alloy Sheets Considering Monotonic and Reverse Loading Paths. Int. J. Plast. 2015, 70, 30–59. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saxena, K.K.; Malik, V.; Mohammed, K.A.; Prakash, C.; Buddhi, D.; Dixit, S. Significance of Alloying Elements on the Mechanical Characteristics of Mg-Based Materials for Biomedical Applications. Crystals 2022, 12, 1138. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, M.; Zhao, J.; Gao, L.; Li, M. In Vitro and in Vivo Degradation and Mechanical Properties of ZEK100 Magnesium Alloy Coated with Alginate, Chitosan and Mechano-growth Factor. Mater. Sci. Eng. C 2016, 63, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Grillo, C.A.; Alvarez, F.; Fernández Lorenzo de Mele, M.A. Degradation of Bioabsorbable Mg-based Alloys: Assessment of the Effects of Insoluble Corrosion Products and Joint Effects of Alloying Components on Mammalian Cells. Mater. Sci. Eng. C 2016, 58, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Reifenrath, J.; Marten, A.-K.; Angrisani, N.; Eifler, R.; Weizbauer, A. In vitro and in vivo Corrosion of the Novel Magnesium Alloy Mg–La–Nd–Zr: Influence of the Measurement Technique and in vivo Implant Location. Biomed. Mater. 2015, 10, 045021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, L.-L.; Gao, H.; Yuan, X.; Chen, X. Biodegradable Behaviour and Fatigue Life of ZEK100 Magnesium Alloy in Simulated Physiological Environment. Fatigue Fract. Eng. Mater. Struct. 2015, 38, 904–913. [Google Scholar] [CrossRef]
- Bondarenko, A.; Angrisani, N.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Reifenrath, J. Magnesium-based Bone Implants: Immunohistochemical Inalysis of Peri-implant Osteogenesis by Evaluation of Osteopontin and Osteocalcin Expression. J. Biomed. Mater. Res. Part A 2014, 102, 1449–1457. [Google Scholar] [CrossRef]
- Weizbauer, A.; Modrejewski, C.; Behrens, S.; Klein, H.; Helmecke, P.; Seitz, J.-M.; Windhagen, H.; Möhwald, K.; Reifenrath, J.; Waizy, H. Comparative in vitro Study and Biomechanical Testing of Two Different Magnesium Alloys. J. Biomater. Appl. 2014, 28, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term in vivo Degradation Behaviour and Biocompatibility of the Magnesium Alloy ZEK100 for use as a Biodegradable Bone Implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef]
- Reifenrath, J.; Angrisani, N.; Erdmann, N.; Lucas, A.; Waizy, H.; Seitz, J.M.; Bondarenko, A.; Meyer-Lindenberg, A. Degrading Magnesium Screws ZEK100: Biomechanical Testing, Degradation Analysis and Soft-tissue Biocompatibility in a Rabbit Model. Biomed. Mater. 2013, 8, 045012. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Biskup, C.; Schilling, T.; Haverich, A.; Bach, F.W.; Maier, H.J.; Hassel, T. Influence of Shot Peening on Surface Rougness and in vitro Load Cycles of Magnesium Alloys. Biomed. Tech. 2013, 58, 4060. [Google Scholar] [CrossRef]
- Weidling, M.; Besdo, S.; Schilling, T.; Bauer, M.; Hassel, T.; Haverich, A.; Wriggers, P. Finite Element Simulation of Myocardial Stabilising Structures and Development of New Designs. Biomed. Tech. 2013, 58, 4061. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.; Meyer-Lindenberg, A.; Goblet, F.; Bormann, D.; Stiller, W.; Seifert, H. Phantomuntersuchungen an einem Hochauflösenden CT zur Ex-vivo-Darstellung von Degradierbaren Magnesiumimplantaten und Simulierten Periimplantären Knochenschichten in Kaninchentibiae. Fortschr. Röntgenstr. 2012, 184, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Huehnerschulte, T.A.; Angrisani, N.; Rittershaus, D.; Bormann, D.; Windhagen, H.; Meyer-Lindenberg, A. In Vivo Corrosion of Two Novel Magnesium Alloys ZEK100 and AX30 and Their Mechanical Suitability as Biodegradable Implants. Materials 2011, 4, 1144–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huehnerschulte, T.A.; Reifenrath, J.; von Rechenberg, B.; Dziuba, D.; Seitz, J.M.; Bormann, D.; Windhagen, H.; Meyer-Lindenberg, A. In vivo Assessment of the Host Reactions to the Biodegradation of the Two Novel Magnesium Alloys ZEK100 and AX30 in an Animal Model. BioMed. Eng. OnLine 2012, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Seitz, J.-M.; Utermöhlen, D.; Wulf, E.; Klose, C.; Bach, F.-W. The Manufacture of Resorbable Suture Material from Magnesium—Drawing and Stranding of Thin Wires. Adv. Eng. Mater. 2011, 13, 1087–1095. [Google Scholar] [CrossRef]
- Seitz, J.-M.; Wulf, E.; Freytag, P.; Bormann, D.; Bach, F.-W. The Manufacture of Resorbable Suture Material from Magnesium. Adv. Eng. Mater. 2010, 12, 1099–1105. [Google Scholar] [CrossRef]
- Gao, H.; Ye, W.; Zhang, Z.; Gao, L. Ratcheting Behavior of ZEK100 Magnesium Alloy with Various Loading Conditions and Different Immersing Time. J. Mater. Res. 2017, 32, 2143–2152. [Google Scholar] [CrossRef]
- Zaghloul, B.; Kish, J.R. Corrosion Inhibition of Mg Alloy ZEK100 Sheet Metal by Dissolved Lithium Carbonate. J. Electrochem. Soc. 2021, 168, 081507. [Google Scholar] [CrossRef]
- Brady, M.P.; Rother, G.; Frith, M.G.; Ievlev, A.E.; Leonard, D.N.; Littrell, K.C.; Cakmak, E.; Meyer, H.M., III; Anovitz, L.M.; Davis, B. Temporal Evolution of Corrosion Film Nano-Porosity and Magnesium Alloy Hydrogen Penetration in NaCl Solution. J. Electrochem. Soc. 2020, 167, 131513. [Google Scholar] [CrossRef]
- Brady, M.P.; Leonard, D.N.; McNally, E.A.; Kish, J.R.; Meyer, H.M.; Cakmak, E.; Davis, B. Magnesium Alloy Effects on Plasma Electrolytic Oxidation Electro-Ceramic and Electro-Coat Formation and Corrosion Resistance. J. Electrochem. Soc. 2019, 166, C492–C508. [Google Scholar] [CrossRef]
- Binns, W.J.; Zargarzadah, F.; Dehnavi, V.; Chen, J.; Noël, J.J.; Shoesmith, D.W. Physical and Electrochemical Evidence for the Role of a Mg Hydride Species in Mg Alloy Corrosion. Corrosion 2019, 75, 58–68. [Google Scholar] [CrossRef]
- Brady, M.P.; Leonard, D.N.; Meyer, H.M., III; Thomson, J.K.; Unocic, K.A.; Elsentriecy, H.H.; Song, G.-L.; Kitchen, K.; Davis, B. Advanced Characterization Study of Commercial Conversion and Electrocoating Structures on Magnesium Alloys AZ31B and ZE10A. Surf. Coat. Technol. 2016, 294, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Dauphin-Ducharme, P.; Binns, W.J.; Snowden, M.E.; Shoesmith, D.W.; Mauzeroll, J. Determination of the Local Corrosion Rate of Magnesium Alloys Using a Shear Force Mounted Scanning Microcapillary Method. Faraday Discuss. 2015, 180, 331–345. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Binns, W.J.; Jakupi, P.; Shoesmith, D. The Influence of Microstructure on the Corrosion of Magnesium Alloy ZEK100. Corrosion 2015, 71, 242–254. [Google Scholar] [CrossRef]
- Waizy, H.; Weizbauer, A.; Modrejewski, C.; Witte, F.; Windhagen, H.; Lucas, A.; Kieke, M.; Denken, B.; Behrens, P.; Meyer-Lindenberg, A.; et al. In vitro Corrosion of ZEK100 Plates in Hank’s Balanced Salt Solution. BioMed Eng. Online 2012, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, M.; Hassel, T.; Biskup, C.; Hartung, D.; Schilling, T.; Weidling, M.; Wriggers, P.; Wacker, F.; Bach, F.W.; Haverich, A. Geometric Adaption of Resorbable Myocardial Stabilizing Structures Based on the Magnesium Alloys LA63 and ZEK100 for the Support of Myocardial Grafts on the Left Ventricle. Biomed. Eng. 2012, 57, 22–25. [Google Scholar] [CrossRef]
- Krüger, R.; Seitz, J.-M.; Ewald, A.; Bach, F.-W.; Groll, J. Strong and Tough Magnesium Wire Reinforced Phosphate Cement Composites for Load-bearing Bone Replacement. J. Mech. Behav. Biomed. Mater. 2013, 20, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, D.L.; Bai, X.F.; Wang, P.Q.; Li, D.Y.; Jiang, X.Q. Microstructure and Mechanical Properties of Mg-to-Al Dissimilar Welded Joints with an Ag Interlayer using Ultrasonic Spot Welding. J. Magn. Alloys 2020, 8, 552–563. [Google Scholar] [CrossRef]
- Peng, H.; Chen, D.L.; Bai, X.F.; She, X.W.; Li, D.Y.; Jiang, X.Q. Ultrasonic Spot Welding of Magnesium-to-Aluminum Alloys with a Copper Interlayer: Microstructural Evolution and Tensile Properties. J. Manuf. Proc. 2019, 37, 91–100. [Google Scholar] [CrossRef]
- Peng, H.; Jiang, X.; Bai, X.; Li, D.; Chen, D. Microstructure and Mechanical Properties of Ultrasonic Spot Welded Mg/Al Alloy Dissimilar Joints. Metals 2018, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Macwan, A.; Chen, D.L. Ultrasonic Spot Welding of Rare-Earth Containing ZEK100 Magnesium Alloy to 5754 Aluminum Alloy. Mater. Sci. Eng. A 2016, 666, 139–148. [Google Scholar] [CrossRef]
- Macwan, A.; Chen, D.L. Ultrasonic Spot Welding of a Rare-Earth Containing ZEK100 Magnesium Alloy: Effect of Welding Energy. Metal. Mater Trans. A 2016, 47, 1686–1697. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Amirkhiz, B.S.; Worswick, M.J.; Gerlich, A.P. Microstructures and Properties of Mg alloy/DP600 Steel Dissimilar Refill Friction Stir Spot Welds. Sci. Technol. Weld. Join. 2015, 20, 494–501. [Google Scholar] [CrossRef]
- Rodriguez, R.I.; Jordon, J.B.; Rao, H.M.; Badarinarayan, H.; Yuan, W.; ElKadiri, H.; Allison, P.G. Microstructure, Texture, and Mechanical Properties of Friction Stir Spot Welded Rare-Earth Containing ZEK100 Magnesium Alloy Sheets. Mater. Sci. Eng. A 2014, 618, 637–644. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Z.; Li, Y.; Zhang, H.; Zhou, N. Laser Welding/Brazing of 5182 Aluminium Alloy to ZEK100 Magnesium Alloy Using a Nickel Interlayer. Sci. Technol. Weld. Join. 2018, 23, 543–550. [Google Scholar] [CrossRef]
- Yang, J.; Su, J.H.; Yu, Z.S.; Zhang, G.Z.; Lin, S.B.; Li, Y.L.; Zhou, N.Y. Influence of Ni Interlayer Width on Interfacial Reactions and Mechanical Properties in Laser Welding/Brazing of Al/Mg Lap Joint. Sci. Technol. Weld. Join. 2019, 25, 37–44. [Google Scholar] [CrossRef]
- Kuang, J.; Li, X.; Ye, X.; Tang, J.; Liu, H.; Wang, J.; Tang, G. Microstructure and Texture Evolution of Magnesium Alloys During Electropulse Treatment. Metall. Mater. Trans. A 2015, 46, 1789–1804. [Google Scholar] [CrossRef]
- Cahn, J.W. Critical Point Wetting. J. Chem. Phys. 2008, 66, 3667. [Google Scholar] [CrossRef]
- Ebner, C.; Saam, W.F. New Phase-Transition Phenomena in Thin Argon Films. Phys. Rev. Lett. 1977, 38, 1486. [Google Scholar] [CrossRef]
- Straumal, B.B.; Polyakov, S.A.; Bischoff, E.; Gust, W.; Mittemeijer, E.J. Faceting of Σ3 and Σ9 grain boundaries in copper. Interface Sci. 2001, 9, 287–292. [Google Scholar] [CrossRef]
- Maksimova, E.L.; Shvindlerman, L.S.; Straumal, B.B. Transformation of Σ17 special tilt boundaries to general boundaries in tin. Acta Metall. 1988, 36, 1573–1583. [Google Scholar] [CrossRef]
- Laporte, D.; Watson, E.B. Experimental and Theoretical Constraints on Melt Distribution in Crustal Sources: The Effect of Crystalline Anisotropy on Melt Interconnectivity. Chem. Geol. 1995, 124, 161–184. [Google Scholar] [CrossRef]
- Noskovich, O.I.; Rabkin, E.I.; Semenov, V.N.; Straumal, B.B.; Shvindlerman, L.S. Wetting and premelting phase transitions in 38°[100] tilt grain boundaries in (Fe–12at.%Si) Zn alloy in the vicinity of the A2–B2 bulk ordering in Fe–12at.%Si alloy. Acta Metall. Mater. 1991, 39, 3091–3098. [Google Scholar] [CrossRef]
- Chang, L.-S.; Rabkin, E.; Straumal, B.B.; Hoffmann, S.; Baretzky, B.; Gust, W. Grain boundary segregation in the Cu–Bi system. Defect Diff. Forum 1998, 156, 135–146. [Google Scholar] [CrossRef]
- Ross, D.; Bonn, D.; Meunier, J. Observation of Short-Range Critical Wetting. Nature 1999, 400, 737–739. [Google Scholar] [CrossRef]
- Straumal, B.B.; Gust, W.; Watanabe, T. Tie lines of the grain boundary wetting phase transition in the Zn-rich part of the Zn–Sn phase diagram. Mater. Sci. Forum 1999, 294–296, 411–414. [Google Scholar] [CrossRef]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid Phase Sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Rabkin, E.I.; Shvindlerman, L.S.; Straumal, B.B. Grain Boundaries: Phase Transitions and Critical Phenomena. Int. J. Mod. Phys. B 1991, 5, 2989–3028. [Google Scholar] [CrossRef]
- Straumal, B.; Rabkin, E.; Lojkowski, W.; Gust, W.; Shvindlerman, L.S. Pressure influence on the grain boundary wetting phase transition in Fe–Si alloys. Acta Mater. 1997, 45, 1931–1940. [Google Scholar] [CrossRef]
- Straumal, A.B.; Mazilkin, I.A.; Tsoi, K.V.; Baretzky, B.; Straumal, B.B. “Wetting” Phase Transitions by the Second Solid Phase for Linear Defects (Grain Boundary Triple Junctions). JETP Lett. 2020, 112, 257–261. [Google Scholar] [CrossRef]
- Straumal, A.B.; Bokstein, B.S.; Petelin, A.L.; Straumal, B.B.; Baretzky, B.; Rodin, A.O.; Nekrasov, A.N. Apparently Complete Grain Boundary Wetting in Cu–In Alloys. J. Mater. Sci. 2012, 47, 8336–8343. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Pouraliakbar, H.; Hong, S.I.; Pavese, M. Grain Boundary Transition Associated Intergranular Failure Analysis at TMAZ/SZ Interface of Dissimilar AA7475-AA2198 Joints by Friction Stir Welding. Mater. Lett. 2020, 280, 128557. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Pouraliakbar, H.; Saboori, A.; Hong, S.I.; Pavese, M. Comparative Insight into the Interfacial Phase Evolutions during Solution Treatment of Dissimilar Friction Stir Welded AA2198-AA7475 and AA2198-AA6013 Aluminum Sheets. Materials 2021, 14, 1290. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kogtenkova, O.; Zięba, P. Wetting Transition of Grain Boundary Triple Junctions. Acta Mater. 2008, 56, 925–933. [Google Scholar] [CrossRef]
- Cantwell, P.R.; Tang, M.; Dillon, S.J.; Luo, J.; Rohrer, G.S.; Harmer, M.P. Grain Boundary Complexions. Acta Mater. 2014, 62, 1–48. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Schütz, G.; Straumal, A.B.; Baretzky, B. Observation of Pseudopartial Grain Boundary Wetting in the NdFeB-Based Alloy. J. Mater. Eng. Perform. 2016, 25, 3303–3309. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Gusak, A.M.; Bulatov, M.F.; Straumal, A.B.; Baretzky, B. Grain Boundary Phenomena in NdFeB-Based Hard Magnetic Alloys. Rev. Adv. Mater. Sci. 2014, 38, 17–28. [Google Scholar]
- Straumal, B.B.; Rodin, A.O.; Shotanov, A.E.; Straumal, A.B.; Kogtenkova, O.A.; Baretzky, B. Pseudopartial Grain Boundary Wetting: Key to the Thin Intergranular Layers. In Defect and Diffusion Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2013; Volume 333, pp. 175–192. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Sauvage, X.; Valiev, R.Z.; Straumal, A.B.; Gusak, A.M. Pseudopartial Wetting of Grain Boundaries in Severely Deformed Al-Zn Alloys. Russ. J. Non-Ferr. Met. 2015, 56, 44–51. [Google Scholar] [CrossRef]
- Langenohl, L.; Brink, T.; Richter, G.; Dehm, G.; Liebscher, C.H. Atomic-resolution observations of silver segregation in a [111] tilt grain boundary in copper. Phys. Rev. B 2023, 107, 134112. [Google Scholar] [CrossRef]
- Bishara, H.; Langenohl, L.; Zhou, X.; Gault, B.; Best, J.P.; Dehm, G. Decoupling the Electrical Resistivity Contribution of Grain Boundaries in Dilute Fe-alloyed Cu Thin Films. Scr. Mater. 2023, 230, 115393. [Google Scholar] [CrossRef]
- Brink, T.; Langenohl, L.; Bishara, H.; Dehm, G. Universality of Grain Boundary Phases in fcc metals: Case Study on High-Angle [111] Symmetric Tilt Grain Boundaries. Phys. Rev. B 2023, 107, 054103. [Google Scholar] [CrossRef]





| Concentration of Elements, wt.% | Matrix | Matrix + (Zr) | GB Phase 1 | GB Phase 2 |
|---|---|---|---|---|
| Mg | 97.71 ± 0.02 | 98.43 ± 0.02 | 73.31 ± 0.13 | 79.59 ± 0.123 |
| Zn | 1.12 ± 0.08 | 0.98 ± 0.09 | 10.38 ± 0.26 | 3.96 ± 0.49 |
| Zr | 0.01 ± 0.07 | 0.14 ± 0.07 | 0.34 ± 0.2 | 13.17 ± 0.7 |
| Ce | 0.12 ± 0.1 | 0.1 ± 0.11 | 6.873 ± 0.29 | 0.36 ± 0.66 |
| Nd | 0.02 ± 0.11 | 0.08 ± 0.11 | 0.91 ± 0.5 | 0.18 ± 0.8 |
| Y | 0.03 ± 0.07 | 0.06 ± 0.02 | 0.19 ± 0.19 | 2.32 ± 0.37 |
| La | 0.15 ± 0.1 | 0.19 ± 0.1 | 7.76 ± 0.29 | 0.42 ± 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straumal, B.; Tsoy, K.; Druzhinin, A.; Orlov, V.; Khrapova, N.; Davdian, G.; Gerstein, G.; Straumal, A. Coexistence of Intermetallic Complexions and Bulk Particles in Grain Boundaries in the ZEK100 Alloy. Metals 2023, 13, 1407. https://doi.org/10.3390/met13081407
Straumal B, Tsoy K, Druzhinin A, Orlov V, Khrapova N, Davdian G, Gerstein G, Straumal A. Coexistence of Intermetallic Complexions and Bulk Particles in Grain Boundaries in the ZEK100 Alloy. Metals. 2023; 13(8):1407. https://doi.org/10.3390/met13081407
Chicago/Turabian StyleStraumal, Boris, Kristina Tsoy, Aleksandr Druzhinin, Valery Orlov, Natalya Khrapova, Gregory Davdian, Gregory Gerstein, and Alexander Straumal. 2023. "Coexistence of Intermetallic Complexions and Bulk Particles in Grain Boundaries in the ZEK100 Alloy" Metals 13, no. 8: 1407. https://doi.org/10.3390/met13081407






