Wdr17 Regulates Cell Proliferation, Cell Cycle Progression and Apoptosis in Mouse Spermatocyte Cell Line
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bulk RNA-seq of Testes of Etv5 Knockout Mice
2.2. Single-Cell RNA Sequencing and Cell-Type Assignment
2.3. Cell Culture and Transfection
2.4. EdU Incorporation Assay
2.5. CCK-8 Assay
2.6. RNA Extraction and Quantitative PCR (qPCR)
2.7. Mitochondrial Membrane Potential and Apoptosis Detection
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
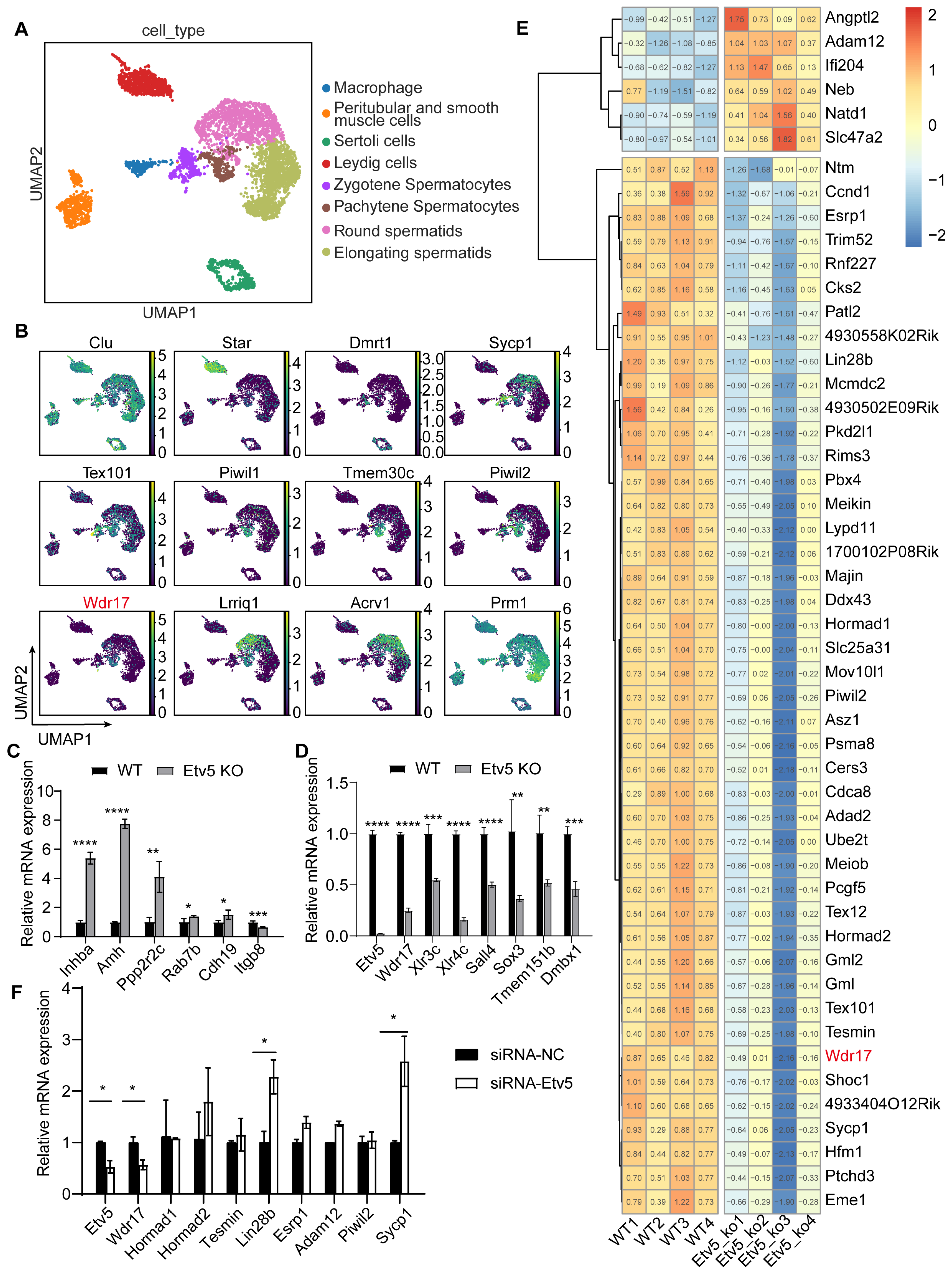
3.1. Transcriptomics Reveals Critical Role of Wdr17 in Mouse Spermatocyte Development
3.2. Wdr17 Affects the Proliferation of GC-2spd(ts) Cells
3.3. Wdr17 Knockdown Promotes Apoptosis in GC-2spd(ts) Cells
3.4. Wdr17 Overexpression Inhibits Apoptosis in GC-2spd(ts) Cells
3.5. Effects of Wdr17 on Cell Cycle Progression in GC-2spd(ts) Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef] [PubMed]
- White-Cooper, H.; Bausek, N. Evolution and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1465–1480. [Google Scholar] [CrossRef] [PubMed]
- Holstein, A.F.; Schulze, W.; Davidoff, M. Understanding spermatogenesis is a prerequisite for treatment. Reprod. Biol. Endocrinol. 2003, 1, 107. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, K.; Trauth, S.E.; Gribbins, K.M. Testicular histology and germ cell cytology during spermatogenesis in the Mississippi map turtle, Graptemys pseudogeographica kohnii, from Northeast Arkansas. Spermatogenesis 2014, 4, e992654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Li, G.; Zhang, M.; Xing, P.; Li, Z.; Chen, B.; Yang, H.; Wu, Z. Establishment of Etv5 gene knockout mice as a recipient model for spermatogonial stem cell transplantation. Biol. Open 2021, 10, bio056804. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ouyang, W.; Grigura, V.; Zhou, Q.; Carnes, K.; Lim, H.; Zhao, G.Q.; Arber, S.; Kurpios, N.; Murphy, T.L.; et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 2005, 436, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Bosch, E.; Ekici, A.B.; Winterpacht, A. Single-cell RNA sequencing of adult mouse testes. Sci. Data 2018, 5, 180192. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Bosch, E.; Ekici, A.B.; Winterpacht, A. Characterization of germ cell differentiation in the male mouse through single-cell RNA sequencing. Sci. Rep. 2018, 8, 6521. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, X.; Luo, X.; Wu, Z.; Yang, H. Modulation of cell cycle increases CRISPR-mediated homology-directed DNA repair. Cell Biosci. 2023, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Ma, Q.; Manske, G.L.; Shami, A.N.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.B.; et al. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Dev. Cell 2018, 46, 651–667.e610. [Google Scholar] [CrossRef] [PubMed]
- Murat, F.; Mbengue, N.; Winge, S.B.; Trefzer, T.; Leushkin, E.; Sepp, M.; Cardoso-Moreira, M.; Schmidt, J.; Schneider, C.; Mößinger, K.; et al. The molecular evolution of spermatogenesis across mammals. Nature 2023, 613, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Yan, Y.; Baron, M.; Wagner, F.; Barkley, D.; Chiodin, M.; Kim, S.Y.; Keefe, D.L.; Alukal, J.P.; Boeke, J.D.; et al. Widespread Transcriptional Scanning in the Testis Modulates Gene Evolution Rates. Cell 2020, 180, 248–262.e221. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Qiao, H. The Application of Single-Cell RNA Sequencing in Mammalian Meiosis Studies. Front. Cell Dev. Biol. 2021, 9, 673642. [Google Scholar] [CrossRef] [PubMed]
- Geisert, E.E.; Lu, L.; Freeman-Anderson, N.E.; Templeton, J.P.; Nassr, M.; Wang, X.; Gu, W.; Jiao, Y.; Williams, R.W. Gene expression in the mouse eye: An online resource for genetics using 103 strains of mice. Mol. Vis. 2009, 15, 1730–1763. [Google Scholar] [PubMed]
- Stöhr, H.; Mohr, N.; Fröhlich, S.; Mehdi, S.Q.; Bhattacharya, S.S.; Weber, B.H. Cloning and characterization of WDR17, a novel WD repeat-containing gene on chromosome 4q34. Biochim. Biophys. Acta 2002, 1579, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Ching, Y.H.; Chang, T.Y.; Hu, L.S.; Yong, Y.S.; Keak, P.Y.; Mustika, I.; Lin, M.D.; Liao, B.Y. Novel eye genes systematically discovered through an integrated analysis of mouse transcriptomes and phenome. Comput. Struct. Biotechnol. J. 2020, 18, 73–82. [Google Scholar] [CrossRef] [PubMed]





| Gene Symbol | Transcriptomic Data of Etv5 Knockout Testis | Single-Cell Transcriptomic Data [8,9] | ||||
|---|---|---|---|---|---|---|
| pvals_adj | log2FoldChange (knockout vs. wild-type) | Pachytene_Spermatocytes_pvals | Pachytene_Spermatocytes_Logfoldchanges | Pachytene_Spermatocytes_pvals_adj | Pachytene_Spermatocytes_Scores | |
| Neb | 0.035085187 | 1.115035706 | 7.94 × 10−60 | 4.2233295 | 5.80 × 10−57 | 16.313293 |
| Piwil2 | 0.001805853 | −1.210714668 | 2.27 × 10−57 | 4.5058355 | 1.40 × 10−54 | 15.964045 |
| Tesmin | 0.000433983 | −1.340550875 | 6.41 × 10−57 | 4.729641 | 3.83 × 10−54 | 15.899256 |
| Ntm | 0.007700911 | −1.033144584 | 4.68 × 10−55 | 3.2893615 | 2.50 × 10−52 | 15.628161 |
| Hormad1 | 0.00123195 | −1.394945387 | 2.88 × 10−43 | 3.8687394 | 8.59 × 10−41 | 13.791283 |
| Tex101 | 0.000340654 | −1.57479299 | 2.75 × 10−37 | 2.7778418 | 5.76 × 10−35 | 12.759866 |
| Slc47a2 | 4.60 × 10−5 | 1.064626668 | 1.28 × 10−31 | 3.233085 | 2.05 × 10−29 | 11.69966 |
| 1700102P08Rik | 0.004986544 | −1.091706396 | 1.06 × 10−29 | 3.0629256 | 1.50 × 10−27 | 11.318685 |
| Wdr17 | 0.013822108 | −1.000954672 | 8.94 × 10−29 | 4.1073084 | 1.24 × 10−26 | 11.1301985 |
| Hormad2 | 0.000508658 | −1.45355141 | 2.03 × 10−26 | 4.056227 | 2.46 × 10−24 | 10.6358595 |
| Pbx4 | 0.001542789 | −1.033426469 | 6.58 × 10−23 | 3.5383756 | 6.33 × 10−21 | 9.854076 |
| Adad2 | 0.000445121 | −1.351408826 | 8.64 × 10−23 | 5.3795614 | 8.16 × 10−21 | 9.826668 |
| Psma8 | 0.005144992 | −1.152660427 | 8.63 × 10−18 | 3.5972211 | 5.87 × 10−16 | 8.590912 |
| 4933404O12Rik | 0.001156883 | −1.167389415 | 3.43 × 10−17 | 4.6093717 | 2.20 × 10−15 | 8.430986 |
| Adam12 | 1.26 × 10−9 | 2.101374335 | 6.36 × 10−17 | 2.8372493 | 3.98 × 10−15 | 8.358305 |
| Sycp1 | 0.002365455 | −1.2813677 | 3.77 × 10−15 | 2.1614149 | 2.06 × 10−13 | 7.862474 |
| Lypd11 | 0.005179153 | −1.137202177 | 5.24 × 10−15 | 3.7817922 | 2.82 × 10−13 | 7.8209553 |
| Ptchd3 | 0.000741707 | −1.294829564 | 1.15 × 10−13 | 4.1466928 | 5.74 × 10−12 | 7.422454 |
| Ifi204 | 0.000501088 | 1.391344816 | 3.01 × 10−13 | 3.1548893 | 1.46 × 10−11 | 7.293736 |
| Mov10l1 | 0.000676886 | −1.469099226 | 5.96 × 10−12 | 3.0391064 | 2.54 × 10−10 | 6.8805127 |
| Esrp1 | 5.63 × 10−8 | −2.353795628 | 1.48 × 10−11 | 3.4791603 | 6.07 × 10−10 | 6.749511 |
| Lin28b | 4.77 × 10−5 | −1.589928027 | 1.48 × 10−10 | 3.0757754 | 5.43 × 10−9 | 6.4075217 |
| 4930558K02Rik | 3.67 × 10−5 | −1.121742116 | 8.09 × 10−10 | 2.089895 | 2.75 × 10−8 | 6.1432333 |
| Patl2 | 0.002401623 | −1.818866565 | 2.50 × 10−9 | 3.3682396 | 8.02 × 10−8 | 5.961757 |
| Mcmdc2 | 0.00023271 | −1.46087782 | 2.40 × 10−8 | 3.0477593 | 6.87 × 10−7 | 5.5803294 |
| Gml | 0.000163156 | −1.650853404 | 3.19 × 10−8 | 2.3788683 | 9.05 × 10−7 | 5.5304885 |
| Asz1 | 0.006742716 | −1.180976405 | 4.03 × 10−8 | 3.0195432 | 1.13 × 10−6 | 5.4895577 |
| Cers3 | 0.006782458 | −1.103098215 | 4.26 × 10−8 | 3.4379182 | 1.19 × 10−6 | 5.479924 |
| Cks2 | 0.000508101 | −1.034688964 | 1.00 × 10−7 | 2.1928904 | 2.66 × 10−6 | 5.3263083 |
| Natd1 | 6.71 × 10−6 | 1.07067814 | 5.05 × 10−7 | 2.3129487 | 1.21 × 10−5 | 5.024413 |
| Shoc1 | 0.002652382 | −1.264923666 | 3.73 × 10−6 | 2.813658 | 7.96 × 10−5 | 4.6260476 |
| Ube2t | 0.002919971 | −1.200702292 | 4.96 × 10−6 | 2.5175054 | 0.000104762 | 4.566415 |
| Rims3 | 0.000578389 | −1.02391401 | 5.28 × 10−6 | 4.227404 | 0.000110944 | 4.5532537 |
| Rnf227 | 3.63 × 10−5 | −1.479084615 | 5.48 × 10−6 | 3.7630215 | 0.00011478 | 4.545429 |
| Gml2 | 0.00281273 | −1.445208949 | 7.47 × 10−5 | 2.2389789 | 0.001264598 | 3.9608386 |
| Pkd2l1 | 0.001499767 | −1.038933229 | 0.000105601 | 3.7833967 | 0.001722635 | 3.8773482 |
| Ccnd1 | 0.000782232 | −1.027401117 | 0.000191913 | 2.944979 | 0.00295234 | 3.7294312 |
| Pcgf5 | 0.00025788 | −1.40635133 | 0.00019446 | 2.1380026 | 0.002984545 | 3.726107 |
| Meikin | 0.004490066 | −1.012027443 | 0.000229154 | 3.6511478 | 0.00345783 | 3.6844974 |
| Cdca8 | 0.0060417 | −1.039677017 | 0.000235647 | 2.5353625 | 0.003550373 | 3.677374 |
| Majin | 0.001037518 | −1.444400641 | 0.000259084 | 3.5810795 | 0.003879766 | 3.6531093 |
| Slc25a31 | 0.002702153 | −1.216286706 | 0.000301156 | 2.2676058 | 0.004455657 | 3.6143038 |
| Angptl2 | 0.001980206 | 1.00202547 | 0.000315776 | 2.965626 | 0.004647562 | 3.602002 |
| Ddx43 | 0.002753675 | −1.111080912 | 0.000390876 | 3.4711144 | 0.005626907 | 3.5461683 |
| Meiob | 0.0005354 | −1.553742147 | 0.000578764 | 2.8036695 | 0.008004477 | 3.4413757 |
| Trim52 | 1.01 × 10−5 | −1.221516545 | 0.000843951 | 2.2543037 | 0.011140829 | 3.3379626 |
| Hfm1 | 0.003331661 | −1.183825782 | 0.000905049 | 3.0592759 | 0.011781762 | 3.3184922 |
| Eme1 | 0.00421195 | −1.007867675 | 0.00189948 | 3.7456686 | 0.022814588 | 3.105515 |
| 4930502E09Rik | 0.034908872 | −1.256370566 | 0.002200087 | 4.013551 | 0.025873651 | 3.0618024 |
| Tex12 | 0.000777606 | −1.504324304 | 0.002631263 | 3.172405 | 0.030188345 | 3.007823 |
| Genes | Primer Sequence (5′ to 3′) | mRNA Accession No. |
|---|---|---|
| Xiap | F: CGAGCTGGGTTTCTTTATACCG R: GCAATTTGGGGATATTCTCCTGT | NM_001301639.1 |
| Smac | F: ATGACAGCGGTTGGCCTTT R: TCCTGTACCTGTGACTTCACC | NM_023232.3 |
| Omi | F: TAGGACCCCGGATCTCTGG R: GACCCCAACCCCACAACAG | NM_019752.3 |
| Apaf1 | F: AGTAATGGGTCCTAAGCATGTTG R: GCGATTGGGAAAATCACGTAAAA | NM_001042558.1 |
| Bad | F: AAGTCCGATCCCGGAATCC R: GCTCACTCGGCTCAAACTCT | NM_007522.3 |
| Esrp1 | F: CAGTTTAACCAGTCAGTGAGCAATG R: TCAGGCAGTAACACATTCTTCTTGG | NM_001290383.1 |
| Adam12 | F: AAGTGTGGAAATGGCTATGTGGAAG R: GGTAGCGTTACAGCAGCGATTC | NM_007400.3 |
| Hormad2 | F: AGGACGATGGCACTACTGAGATAG R: TTCGCTGACCTTCTTCTTCTTTCTG | NM_001417965.1 |
| Hormad1 | F: AAGTGGATGCTTGGATGCTATGATG R: TTGTCTGAGGATCTCCTGGATTGG | NM_001289532.1 |
| Tesmin | F: GGTGAGGAAGCAGAGCAGGAG R: GGACTTGAACTCGATGTGGAGAATC | NM_001039657.2 |
| Lin28b | F: GGCCTTGAGTCAATACGGGT R: ATCCTGCCGTCTCCACCTAT | NM_001031772.2 |
| β-actin | F: GGCTGTATTCCCCTCCATCG R: CCAGTTGGTAACAATGCCATGT | NM_007393.5 |
| Ccnd1 | F: ATTTCCAACCCACCCTCCAT R: GGGGTCCTTGTTTAGCCAGAG | NM_001379248.1 |
| Ccne1 | F: TGTTACAGATGGCGCTTGCT R: GCCAGGACACAATGGTCAGA | NM_007633.2 |
| Ccna2 | F: GTCAACCCCGAAAAACTGGC R: TTAAGAGGAGCAACCCGTCG | NM_009828.3 |
| Cdk2 | F: CGGAGTGGTGTACAAAGCCA R: TCGGATGGCAGTACTGGGTA | NM_016756.4 |
| Cdk4 | F: CCTGCCGGTTGAGACCATTA R: TCAGGTCCCGGTGAACAATG | NM_001355005.1 |
| Cdk6 | F: TCCTGCTCCAGTCCAGCTAT R: CCACGTCTGAACTTCCACGA | NM_009873.3 |
| P53 | F: CCATGGCCCCTGTCATCTTT R: TGAGGGGAGGAGAGTACGTG | NM_001127233.1 |
| P21 | F: GCAAAGTGTGCCGTTGTCTC R: CGTCTCCGTGACGAAGTCAA | NM_001111099.2 |
| Dmbx1 | F: GTTCCCACGGAGAAGGCAAGG R: TCCGACAGGCTCAGTTGAAGTTC | NM_001025567.1 |
| Tmem151b | F: GAGGAGGACGAGGACGAGGAG R: GCCGATGGACGATGAGGACAG | NM_001013749.3 |
| Sox3 | F: ACAACTCCGAGATCAGCAAGCG R: TCCTTCTTGAGCAGCGTCTTGG | NM_009237.3 |
| Sall4 | F: CCAGGACGACGCAGCAGAAG R: CCAACACAGAGAAGCCCAGAGAG | NM_175303.5 |
| Cdh19 | F: TGGCTATCATGCTCGCATCCTATAC R: CAGACAAGGCTCCAGGCTGAC | NM_001081386.2 |
| Rab7b | F: TGGGACACAGGTGGTCAGGAG R: AATGCCAGGATACAGCCATCGG | NM_001311096.1 |
| Itgb8 | F: GGGAGTGTGAAGGTGGCAGATG R: AGTGCTGTGCTGAGGCTGATG | NM_177290.4 |
| Ppp2r2c | F: GGACGACCTACGCATCAACCTC R: TGCTGCTGCTGTAGACGAAGAG | NM_001360003.1 |
| Amh | F: ACTCGCTTGGTTCGTGCTCTG R: GGGTGACAGCAGCAGTAATAGGG | NM_007445.3 |
| Inhba | F: CTCGCTCTCCTTCCACTCAACAG R: AGCCACACTCCTCCACAATCATG | NM_008380.2 |
| Xlr3c | F: ATCGCTGAAGAGCTGAGACG R: GTGGAGGAGCAGCAGTCTTT | NM_011727.2 |
| Rb | F: TAACCTTGAACCTGCTTGTCCTCTC R: GGCTGCTTGTGTCTCTGTATTTGC | NM_009029.3 |
| E2f | F: GTGGCTGCTGACTCACTCCTG R: TCTCTAATGCCCTCACCCTCCTC | NM_001291105.1 |
| Casp9 | F: TGAAGAACGACCTGACTGCCAAG R: ATGAGAGAGGATGACCACCACAAAG | NM_001277932.1 |
| Casp3 | F: GACTGGAAAGCCGAAACTCTTCATC R: AGTCCCACTGTCTGTCTCAATGC | NM_001284409.1 |
| Casp7 | F: ACTCCACGGTTCCAGGTTATTACTC R: AGGTCCTTGCCATGCTCATTCAG | NM_007611.3 |
| Wdr17 | F: TCCAGGTGGCAGTGACAACTTG R: TCGTTAGTTCTTGGGCTTCTGAGG | NM_001372372.1 |
| Piwil2 | F: TAAAACTCACCCCTCTGGTGC R: ACAGGATCCATTGAGAGGCT | NM_001364321.1 |
| Sycp1 | F: TGAGGGGAAGCTCACGGTT R: CGAACAGTGTGAAGGGCTTTTG | NM_011516.2 |
| Etv5 | F: AAGAATCGGCCAGCCATGAA R: TCCGGGAAGGCCATAGAGAA | NM_001358428.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Jin, T.; Ji, X.; Zhang, Q.; Zhang, X.; Wu, Z.; Li, Z.; Yang, H. Wdr17 Regulates Cell Proliferation, Cell Cycle Progression and Apoptosis in Mouse Spermatocyte Cell Line. Animals 2024, 14, 1418. https://doi.org/10.3390/ani14101418
Zhao X, Jin T, Ji X, Zhang Q, Zhang X, Wu Z, Li Z, Yang H. Wdr17 Regulates Cell Proliferation, Cell Cycle Progression and Apoptosis in Mouse Spermatocyte Cell Line. Animals. 2024; 14(10):1418. https://doi.org/10.3390/ani14101418
Chicago/Turabian StyleZhao, Xin, Taili Jin, Xi Ji, Qiuyan Zhang, Xianyu Zhang, Zhenfang Wu, Zicong Li, and Huaqiang Yang. 2024. "Wdr17 Regulates Cell Proliferation, Cell Cycle Progression and Apoptosis in Mouse Spermatocyte Cell Line" Animals 14, no. 10: 1418. https://doi.org/10.3390/ani14101418
APA StyleZhao, X., Jin, T., Ji, X., Zhang, Q., Zhang, X., Wu, Z., Li, Z., & Yang, H. (2024). Wdr17 Regulates Cell Proliferation, Cell Cycle Progression and Apoptosis in Mouse Spermatocyte Cell Line. Animals, 14(10), 1418. https://doi.org/10.3390/ani14101418






