Sulforaphane Exposure Prevents Cadmium-Induced Toxicity and Mitochondrial Dysfunction in the Nematode Caenorhabditis elegans by Regulating the Insulin/Insulin-like Growth Factor Signaling (IIS) Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. C. elegans Strains
2.3. C. elegans Culture
2.4. Experimental Design
2.5. Survival
2.6. Lifespan Analysis
2.7. Lipofuscin Assay
2.8. Body Length
2.9. Mobility/Body-Bending Assay
2.10. Mitochondria-Associated Oxygen Consumption
2.11. Use of Fluorescent Probes
2.12. Quantification of Green Fluorescent Proteins (GFP)
2.13. Nuclear Localization of DAF-16
2.14. Image Analyses
2.15. Statistical Analysis and Calculation of the Mean Lethal Concentration (LC50)
3. Results
3.1. SFN Prevents CdCl2-Induced Decrease in Survival
3.2. SFN Prevents the Toxic Effects Induced by CdCl2
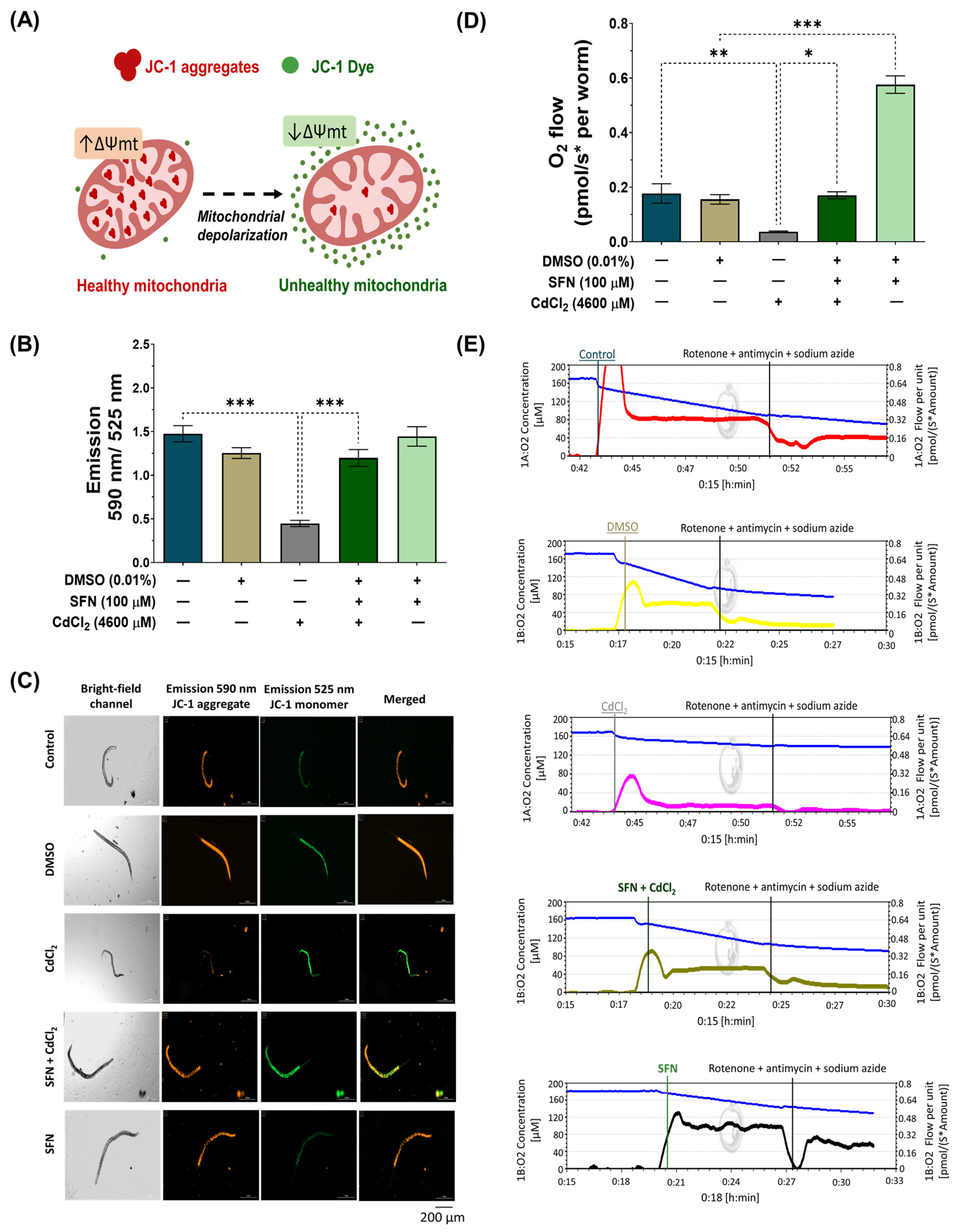
3.3. SFN Prevents CdCl2-Induced Mitochondrial Dysfunction
3.4. SFN Prevents the Decrease in Mitochondrial Mass Induced by CdCl2
3.5. SFN Prevents Oxidative Damage Induced by CdCl2
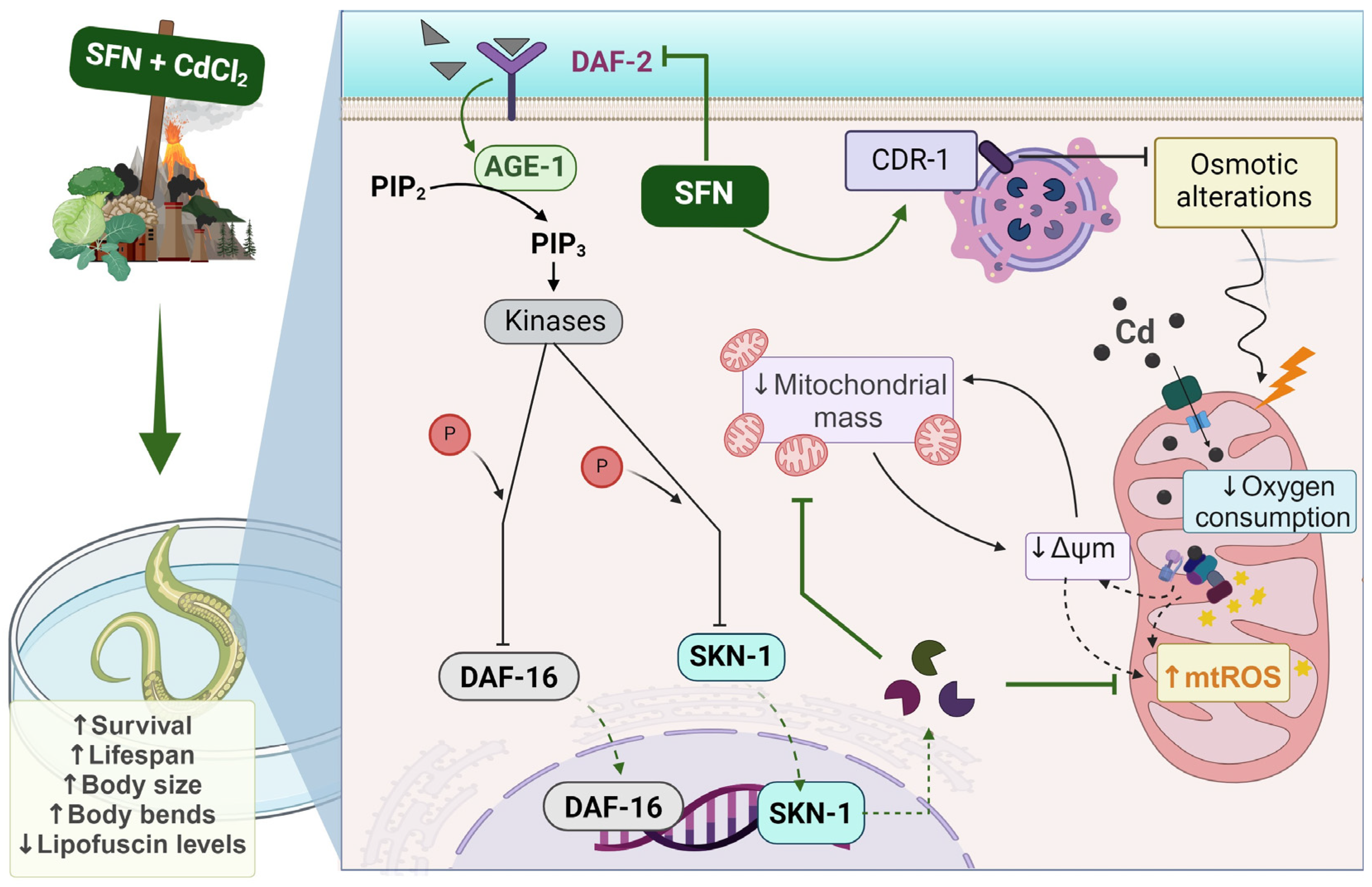
3.6. SFN-Mediated Protection against CdCl2 Toxicity Is Mediated by the IIS Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| age-1 | orthologous gene of phosphatidylinositol 3-kinase |
| C. elegans | Caenorhabditis elegans |
| Cd | cadmium |
| CdCl2 | cadmium chloride |
| cdr-1 o 2 | cadmium response genes |
| CI95 | confidence interval 95% |
| daf-16 | orthologous gene of forkhead box O (FoxO) |
| DAF-16 | protein of the orthologous gene of forkhead box O (FoxO) |
| DAF-18 | human tumor suppressor homologue/PTEN |
| daf-2 | homolog of tyrosine kinase receptor (IGFR) |
| DAF-2 | protein of gen homolog de tyrosine kinase receptor (IGFR) |
| DMSO | dimethyl sulfoxide |
| mtDNA | mitochondrial deoxyribonucleic acid |
| ER | Endoplasmic reticulum |
| ETS | electron transport system |
| FoxO | forkhead box O |
| FUdR | 5-fluoro-2′-desoxiuridina |
| GFP | green fluorescent protein |
| H2DCFDA | 6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate |
| IC95 | 95% confidence interval |
| IIS | insulin/insulin-like growth factor signaling pathway |
| IGF-1 | insulin-like growth factor-1 |
| K2HPO4 | potassium phosphate dibasic |
| KCl | potassium chloride |
| KH2PO4 | potassium phosphate monobasic |
| LC50 | Lethal concentration 50 |
| MgSO4•7H2O | magnesium sulfate heptahydrate |
| mtl-1 or 2 | Metallothionein-1 or 2 |
| Na2HPO4 | sodium hydrogen phosphate |
| NaCl | sodium chloride |
| NaClO | sodium hypochlorite |
| NaOH | sodium hydroxide |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| PDK-1 | 3-phosphoinositide-dependent protein kinase 1 |
| ROS | reactive oxygen species |
| SFN | sulforaphane |
| SGK-1 | glucocorticoid-regulated kinase 1 |
| skn1a | ortholog of Nrf1 |
| skn-1c | ortholog of nuclear factor erythroid 2–related factor 2 (Nrf2) |
| ΔΨm | mitochondrial membrane potential |
| Sirt1 | sirtuin 1 |
| NRF1 | nuclear respiratory factors 1 |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator 1 alpha |
| TFAM | mitochondrial transcription factor A |
| VDAC | voltage-dependent anion channel |
| DMT1 | divalent metal transporter 1 |
| MCU | mitochondrial calcium uniporter |
| PIP3 | phosphatidylinositol 3,4,5-triphosphate |
| JC-1 | 5,5,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimi-dazoylcarbocyanine iodide |
| AKT1-2 | protein kinases B |
References
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–183. [Google Scholar]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-Remediation Approaches for Alleviation of Cadmium Contamination in Natural Resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef] [PubMed]
- Elinder, C.G.; Kjellstrom, T.; Hogstedt, C.; Andersson, K.; Spang, G. Cancer Mortality of Cadmium Workers. Occup. Environ. Med. 1985, 42, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Cadmium Sources and Toxicity. Toxics 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium Exposure in Living Organisms: A Short Review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- ATSDR ATSDR—Toxicological Profile. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15 (accessed on 19 April 2024).
- Bernhoft, R.A. Cadmium Toxicity and Treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed]
- Tägt, J.; Helte, E.; Donat-Vargas, C.; Larsson, S.C.; Michaëlsson, K.; Wolk, A.; Vahter, M.; Kippler, M.; Åkesson, A. Long-Term Cadmium Exposure and Fractures, Cardiovascular Disease, and Mortality in a Prospective Cohort of Women. Environ. Int. 2022, 161, 107114. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cruz, E.Y.; Amador-Martínez, I.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza Chaverri, J. Renal Damage Induced by Cadmium and Its Possible Therapy by Mitochondrial Transplantation. Chem. Biol. Interact. 2022, 361, 109961. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and Mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Pacini, A.; Gulisano, M.; Taddei, N.; Fiorillo, C.; Becatti, M. Cadmium-Induced Cytotoxicity: Effects on Mitochondrial Electron Transport Chain. Front. Cell Dev. Biol. 2020, 8, 604377. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Human Metabolism and Excretion of Cancer Chemoprotective Glucosinolates and Isothiocyanates of Cruciferous Vegetables. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 1091–1100. [Google Scholar] [PubMed]
- Zyla, K.; Plafker, S.M. Sulforaphane and Mitochondria. In Mitochondrial Physiology and Vegetal Molecules; de Oliveira, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 233–246. [Google Scholar]
- Arana-Hidalgo, D.; Silva-Palacios, A. Role of Sulforaphane in Endoplasmic Reticulum Homeostasis through Regulation of the Antioxidant Response. Life Sci. 2022, 299, 120554. [Google Scholar] [CrossRef]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Luo, Y.; Xie, Z. Sulforaphane Ameliorates Cadmium Induced Hepatotoxicity through the Up-Regulation of /Nrf2/ARE Pathway and the Inactivation of NF-ΚB. J. Funct. Foods 2021, 77, 104297. [Google Scholar] [CrossRef]
- Yang, S.-H.; Long, M.; Yu, L.-H.; Li, L.; Li, P.; Zhang, Y.; Guo, Y.; Gao, F.; Liu, M.-D.; He, J.-B. Sulforaphane Prevents Testicular Damage in Kunming Mice Exposed to Cadmium via Activation of Nrf2/ARE Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 1703. [Google Scholar] [CrossRef]
- Yang, S.-H.; Li, P.; Yu, L.-H.; Li, L.; Long, M.; Liu, M.-D.; He, J.-B. Sulforaphane Protect Against Cadmium-Induced Oxidative Damage in Mouse Leydigs Cells by Activating Nrf2/ARE Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 630. [Google Scholar] [CrossRef]
- Yang, S.-H.; Yu, L.-H.; Li, L.; Guo, Y.; Zhang, Y.; Long, M.; Li, P.; He, J.-B. Protective Mechanism of Sulforaphane on Cadmium-Induced Sertoli Cell Injury in Mice Testis via Nrf2/ARE Signaling Pathway. Molecules 2018, 23, 1774. [Google Scholar] [CrossRef] [PubMed]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Assessment of Sulforaphane-Induced Protective Mechanisms against Cadmium Toxicity in Human Mesenchymal Stem Cells. Environ. Sci. Pollut. Res. 2018, 25, 10080–10089. [Google Scholar] [CrossRef]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Sulforaphane Mitigates Cadmium-Induced Toxicity Pattern in Human Peripheral Blood Lymphocytes and Monocytes. Environ. Toxicol. Pharmacol. 2017, 55, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cruz, E.Y.; Eugenio-Pérez, D.; Ramírez-Magaña, K.J.; Pedraza-Chaverri, J. Effects of Vegetal Extracts and Metabolites against Oxidative Stress and Associated Diseases: Studies in Caenorhabditis elegans. ACS Omega 2023, 8, 8936–8959. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.K.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An Emerging Model in Biomedical and Environmental Toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef]
- Koga, M.; Zwaal, R.; Guan, K.-L.; Avery, L.; Ohshima, Y. A Caenorhabditis elegans MAP Kinase Kinase, MEK-1, Is Involved in Stress Responses. EMBO J. 2000, 19, 5148–5156. [Google Scholar] [CrossRef]
- Keshet, A.; Mertenskötter, A.; Winter, S.A.; Brinkmann, V.; Dölling, R.; Paul, R.J. PMK-1 P38 MAPK Promotes Cadmium Stress Resistance, the Expression of SKN-1/Nrf and DAF-16 Target Genes, and Protein Biosynthesis in Caenorhabditis elegans. Mol. Genet. Genom. 2017, 292, 1341–1361. [Google Scholar] [CrossRef] [PubMed]
- Lemire, B. Mitochondrial Genetics. In WormBook; The C. elegans Research Community; WormBook: Minneapolis, MN, USA, 2005; Available online: http://www.wormbook.org/chapters/www_mitogenetics/mitogenetics.pdf (accessed on 19 April 2024).
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T. Insulin/Insulin-Like Growth Factor Signaling in C. elegans. In WormBook; The C. elegans Research Community; WormBook: Minneapolis, MN, USA, 2013; pp. 1–43. Available online: https://www.ncbi.nlm.nih.gov/books/NBK179230/ (accessed on 19 April 2024). [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- The C. elegans Deletion Mutant Consortium. Large-Scale Screening for Targeted Knockouts in the Caenorhabditis elegans Genome. G3 Genes|Genomes|Genet. 2012, 2, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Senoo-Matsuda, N.; Yasuda, K.; Tsuda, M.; Ohkubo, T.; Yoshimura, S.; Nakazawa, H.; Hartman, P.S.; Ishii, N. A Defect in the Cytochrome b Large Subunit in Complex II Causes Both Superoxide Anion Overproduction and Abnormal Energy Metabolism in Caenorhabditis elegans. J. Biol. Chem. 2001, 276, 41553–41558. [Google Scholar] [CrossRef]
- Shukla, V.; Phulara, S.C.; Yadav, D.; Tiwari, S.; Kaur, S.; Gupta, M.M.; Nazir, A.; Pandey, R. Iridoid Compound 10-O-Trans-p-Coumaroylcatalpol Extends Longevity and Reduces Alpha Synuclein Aggregation in Caenorhabditis elegans. CNS Neurol. Disord. Drug Targets 2013, 11, 984–992. [Google Scholar] [CrossRef]
- Chen, J.; Carey, J.R.; Ferris, H. Comparative Demography of Isogenic Populations of Caenorhabditis elegans. Exp. Gerontol. 2001, 36, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Dodd, W.; Choe, K. Isolation of a Hypomorphic Skn-1 Allele That Does Not Require a Balancer for Maintenance. G3 Genes|Genomes|Genet. 2016, 6, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Lehrbach, N.J.; Ruvkun, G. Proteasome Dysfunction Triggers Activation of SKN-1A/Nrf1 by the Aspartic Protease DDI-1. eLife 2016, 5, e17721. [Google Scholar] [CrossRef] [PubMed]
- Lehrbach, N.J.; Ruvkun, G. Endoplasmic Reticulum-Associated SKN-1A/Nrf1 Mediates a Cytoplasmic Unfolded Protein Response and Promotes Longevity. eLife 2019, 8, e44425. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, C.A.; Kern, C.C.; Butkeviciute, E.; McCarthy, E.; Dockrell, H.M.; Moreno-Altamirano, M.M.B.; Aguilar-López, B.A.; Bhosale, G.; Wang, H.; Gems, D.; et al. Mitochondrial Signature in Human Monocytes and Resistance to Infection in C. elegans During Fumarate-Induced Innate Immune Training. Front. Immunol. 2020, 11, 01715. [Google Scholar] [CrossRef] [PubMed]
- Angeli, S.; Foulger, A.; Chamoli, M.; Peiris, T.H.; Gerencser, A.; Shahmirzadi, A.A.; Andersen, J.; Lithgow, G. The Mitochondrial Permeability Transition Pore Activates the Mitochondrial Unfolded Protein Response and Promotes Aging. eLife 2021, 10, e63453. [Google Scholar] [CrossRef]
- Aghayeva, U.; Bhattacharya, A.; Hobert, O. A Panel of Fluorophore-Tagged Daf-16 Alleles. MicroPubl. Biol. 2020, 2020, 000210. [Google Scholar] [CrossRef]
- Ke, T.; Santamaría, A.; Tinkov, A.A.; Bornhorst, J.; Aschner, M. Generating Bacterial Foods in Toxicology Studies with Caenorhabditis elegans. Curr. Protoc. Toxicol. 2020, 84, e94. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook; The C. elegans Research Community; WormBook: Minneapolis, MN, USA, 2006; pp. 1–11. Available online: http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html (accessed on 19 April 2024). [CrossRef]
- Donkin, S.G.; Williams, P.L. Influence of Developmental Stage, Salts and Food Presence on Various End Points Using Caenorhabditis elegans for Aquatic Toxicity Testing. Environ. Toxicol. Chem. 1995, 14, 2139–2147. [Google Scholar] [CrossRef]
- Song, S.; Han, Y.; Zhang, Y.; Ma, H.; Zhang, L.; Huo, J.; Wang, P.; Liang, M.; Gao, M. Protective Role of Citric Acid against Oxidative Stress Induced by Heavy Metals in Caenorhabditis elegans. Environ. Sci. Pollut. Res. 2019, 26, 36820–36831. [Google Scholar] [CrossRef]
- Qi, Z.; Ji, H.; Le, M.; Li, H.; Wieland, A.; Bauer, S.; Liu, L.; Wink, M.; Herr, I. Sulforaphane Promotes C. elegans Longevity and Healthspan via DAF-16/DAF-2 Insulin/IGF-1 Signaling. Aging 2021, 13, 1649–1670. [Google Scholar] [CrossRef]
- Duran-Izquierdo, M.; Taboada-Alquerque, M.; Sierra-Marquez, L.; Alvarez-Ortega, N.; Stashenko, E.; Olivero-Verbel, J. Hydroalcoholic Extract of Haematoxylum Brasiletto Protects Caenorhabditis elegans from Cadmium-Induced Toxicity. BMC Complement. Med. Ther. 2022, 22, 184. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Daitoku, H.; Hirota, K.; Tamiya, H.; Yokoyama, A.; Kako, K.; Nagashima, Y.; Nakamura, A.; Shimada, T.; Watanabe, S.; et al. Asymmetric Arginine Dimethylation Determines Life Span in C. elegans by Regulating Forkhead Transcription Factor DAF-16. Cell Metab. 2011, 13, 505–516. [Google Scholar] [CrossRef]
- Kampkötter, A.; Gombitang Nkwonkam, C.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the Flavonoids Kaempferol and Fisetin on Thermotolerance, Oxidative Stress and FoxO Transcription Factor DAF-16 in the Model Organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A Living Model for Obesity and Aging Research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Branicky, R.; Wang, Y.; Khaki, A.; Liu, J.-L.; Kramer-Drauberg, M.; Hekimi, S. Stimulation of RAS-Dependent ROS Signaling Extends Longevity by Modulating a Developmental Program of Global Gene Expression. Sci. Adv. 2022, 8, eadc9851. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, B.; Zhang, S.; Fan, M.; Ji, X.; Zhang, S.; Wang, Z.; Qiao, K. Lentinan Protects Caenorhabditis elegans against Fluopyram-Induced Toxicity through DAF-16 and SKN-1 Pathways. Ecotoxicol. Environ. Saf. 2023, 265, 115510. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK Regulates Lifespan in Caenorhabditis elegans by Modulating Nuclear Translocation of Forkhead Transcription Factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Roh, J.-Y.; Park, Y.-J.; Choi, J. A Cadmium Toxicity Assay Using Stress Responsive Caenorhabditis elegans Mutant Strains. Environ. Toxicol. Pharmacol. 2009, 28, 409–413. [Google Scholar] [CrossRef]
- Różanowska, M.B. Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina. Antioxidants 2023, 12, 2111. [Google Scholar] [CrossRef]
- Gray, D.A.; Woulfe, J. Lipofuscin and Aging: A Matter of Toxic Waste. Sci. Aging Knowl. Environ. 2005, 2005, re1. [Google Scholar] [CrossRef] [PubMed]
- Maydata, A.G.; Espina, A.L.; Argilagos, M.E.R.; Núñez, E.M. Estrés Oxidativo, Alimentación y Suplementación Antioxidante En Patología Ocular: Historia Breve y Visión Futura. Rev. Cubana. Oftalmol. 2007, 20, 1–13. [Google Scholar]
- Dong, J.; Song, M.O.; Freedman, J.H. Identification and Characterization of a Family of Caenorhabditis elegans Genes That Is Homologous to the Cadmium-Responsive Gene Cdr-1. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2005, 1727, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Haas, K.L.; Freedman, J.H. Role of MTL-1, MTL-2, and CDR-1 in Mediating CadmiumSensitivity in Caenorhabditis elegans. Toxicol. Sci. 2012, 128, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Tian, S.; Teng, C.; Huang, L.; Liu, X.; Wang, J.; Zhang, Y.; Li, B.; Shan, Y. Sulforaphane Improves Lipid Metabolism by Enhancing Mitochondrial Function and Biogenesis In Vivo and In Vitro. Mol. Nutr. Food Res. 2019, 63, 1800795. [Google Scholar] [CrossRef]
- Höss, S.; Schlottmann, K.; Traunspurger, W. Toxicity of Ingested Cadmium to the Nematode Caenorhabditis elegans. Environ. Sci. Technol. 2011, 45, 10219–10225. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium Stress: An Oxidative Challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Ishii, N.; Fujii, M.; Hartman, P.S.; Tsuda, M.; Yasuda, K.; Senoo-Matsuda, N.; Yanase, S.; Ayusawa, D.; Suzuki, K. A Mutation in Succinate Dehydrogenase Cytochrome b Causes Oxidative Stress and Ageing in Nematodes. Nature 1998, 394, 694–697. [Google Scholar] [CrossRef]
- Melov, S.; Ravenscroft, J.; Malik, S.; Gill, M.S.; Walker, D.W.; Clayton, P.E.; Wallace, D.C.; Malfroy, B.; Doctrow, S.R.; Lithgow, G.J. Extension of Life-Span with Superoxide Dismutase/Catalase Mimetics. Science 2000, 289, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 Transcription Factor Can Protect against Oxidative Stress and Increase Lifespan in C. elegans by Distinct Mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, Stress Responses, and Aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of Mitophagy and Mitochondrial Biogenesis during Ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef]
- Le, Q.G.; Ishiwata-Kimata, Y.; Kohno, K.; Kimata, Y. Cadmium Impairs Protein Folding in the Endoplasmic Reticulum and Induces the Unfolded Protein Response. FEMS Yeast Res. 2016, 16, fow049. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. Biomed. Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-X.; Sen, I.; Janssens, G.E.; Zhou, X.; Fonslow, B.R.; Edgar, D.; Stroustrup, N.; Swoboda, P.; Yates, J.R.; Ruvkun, G.; et al. DAF-16/FOXO and HLH-30/TFEB Function as Combinatorial Transcription Factors to Promote Stress Resistance and Longevity. Nat. Commun. 2018, 9, 4400. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Tapia, E.; Pedraza-Chaverri, J. Modulation of Mitochondrial Functions by the Indirect Antioxidant Sulforaphane: A Seemingly Contradictory Dual Role and an Integrative Hypothesis. Free Radic. Biol. Med. 2013, 65, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Jardim, F.R.; de Almeida, F.J.S.; Luckachaki, M.D.; de Oliveira, M.R. Effects of Sulforaphane on Brain Mitochondria: Mechanistic View and Future Directions. J. Zhejiang Univ. Sci. B 2020, 21, 263–279. [Google Scholar] [CrossRef]
- Thévenod, F.; Fels, J.; Lee, W.-K.; Zarbock, R. Channels, Transporters and Receptors for Cadmium and Cadmium Complexes in Eukaryotic Cells: Myths and Facts. BioMetals 2019, 32, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. The VDAC Channel: Molecular Basis for Selectivity. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol Rescues Cadmium-Induced Mitochondrial Injury by Enhancing Transcriptional Regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a Pathway in TCMK-1 Cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, C.; Sun, Y.-C.; Zhang, Q.; Lv, M.-W.; Guo, K.; Li, J.-L. Cadmium Exposure Triggers Mitochondrial Dysfunction and Oxidative Stress in Chicken (Gallus Gallus) Kidney via Mitochondrial UPR Inhibition and Nrf2-Mediated Antioxidant Defense Activation. Sci. Total Environ. 2019, 689, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kasai, S.; Yamazaki, H.; Tatara, Y.; Mimura, J.; Engler, M.J.; Tanji, K.; Nikaido, Y.; Inoue, T.; Suganuma, H.; et al. Sulforaphane Increase Mitochondrial Biogenesis-Related Gene Expression in the Hippocampus and Suppresses Age-Related Cognitive Decline in Mice. Int. J. Mol. Sci. 2022, 23, 8433. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Pi, H.; Xu, S.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; Li, R.; et al. Melatonin Improves Mitochondrial Function by Promoting MT1/SIRT1/PGC-1 Alpha-Dependent Mitochondrial Biogenesis in Cadmium-Induced Hepatotoxicity In Vitro. Toxicol. Sci. 2014, 142, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 00435. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Imai, H. Hydrogen Peroxide Produced by Superoxide Dismutase SOD-2 Activates Sperm in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 14804–14813. [Google Scholar] [CrossRef]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Molecular Inhibitory Mechanisms of Antioxidant Enzymes in Rat Liver and Kidney by Cadmium. Toxicology 2002, 179, 37–50. [Google Scholar] [CrossRef]
- Newairy, A.A.; El-Sharaky, A.S.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. The Hepatoprotective Effects of Selenium against Cadmium Toxicity in Rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.K.; Mishra, P.C. Mechanism of Action of Sulforaphane as a Superoxide Radical Anion and Hydrogen Peroxide Scavenger by Double Hydrogen Transfer: A Model for Iron Superoxide Dismutase. J. Phys. Chem. B 2015, 119, 7825–7836. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Talalay, P. Antioxidant Functions of Sulforaphane: A Potent Inducer of Phase II Detoxication Enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry Extract Ameliorates Oxidative Stress in Caenorhabditis elegans via the SKN-1/Nrf2 Pathway. J. Funct. Foods 2020, 70, 103977. [Google Scholar] [CrossRef]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 Target Genes That Control C. elegans Life-Span and Metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Song, Y.; Xie, J.; Xie, J.; Chen, Y.; Li, P.; Liu, D.; Hu, X.; Yu, Q. Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans. Foods 2022, 11, 1590. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.T.G.; Rodrigues, L.B.L.; Salgueiro, W.G.; de Castro Dal Forno, A.H.; Rodrigues, C.F.; Sacramento, M.; Franco, J.; Alves, D.; de Paula Oliveira, R.; Pinton, S.; et al. Organoselenotriazoles Attenuate Oxidative Damage Induced by Mitochondrial Dysfunction in Mev-1 Caenorhabditis elegans Mutants. J. Trace Elem. Med. Biol. 2019, 53, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xing, X. Pre-Treatment with Mild UV Irradiation Suppresses Reproductive Toxicity Induced by Subsequent Cadmium Exposure in Nematodes. Ecotoxicol. Environ. Saf. 2010, 73, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, P.; Yang, Y.; Shen, L. Formation of a Combined Ca/Cd Toxicity on Lifespan of Nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2010, 73, 1221–1230. [Google Scholar] [CrossRef]
- Ji, H.; Qi, Z.; Schrapel, D.; Le, M.; Luo, Y.; Yan, B.; Gladkich, J.; Schaefer, M.; Liu, L.; Herr, I. Sulforaphane Targets TRA-1/GLI Upstream of DAF-16/FOXO to Promote C. elegans Longevity and Healthspan. Front. Cell Dev. Biol. 2021, 9, 784999. [Google Scholar] [CrossRef]
- Wang, S.; Lin, D.; Cao, J.; Wang, L. APPA Increases Lifespan and Stress Resistance via Lipid Metabolism and Insulin/IGF-1 Signal Pathway in Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 24, 13682. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lai, C.; Tsai, M.; Wu, J.; Ho, C. Molecular Mechanisms for Anti-aging by Natural Dietary Compounds. Mol. Nutr. Food Res. 2012, 56, 88–115. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.-G.; Chen, T.-Y.; Fahey, J.W.; Talalay, P. Keap1-Nrf2 Signaling: A Target for Cancer Prevention by Sulforaphane. Top. Curr. Chem. 2013, 329, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Paek, J.; Lo, J.Y.; Narasimhan, S.D.; Nguyen, T.N.; Glover-Cutter, K.; Robida-Stubbs, S.; Suzuki, T.; Yamamoto, M.; Blackwell, T.K.; Curran, S.P. Mitochondrial SKN-1/Nrf Mediates a Conserved Starvation Response. Cell Metab. 2012, 16, 526–537. [Google Scholar] [CrossRef]
- Dong, J.; Boyd, W.A.; Freedman, J.H. Molecular Characterization of Two Homologs of the Caenorhabditis elegans Cadmium-Responsive Gene Cdr-1: Cdr-4 and Cdr-6. J. Mol. Biol. 2008, 376, 621–633. [Google Scholar] [CrossRef]
- Garcia-Neto, W.; Cabrera-Orefice, A.; Uribe-Carvajal, S.; Kowaltowski, A.J.; Alberto Luévano-Martínez, L. High Osmolarity Environments Activate the Mitochondrial Alternative Oxidase in Debaryomyces Hansenii. PLoS ONE 2017, 12, e0169621. [Google Scholar] [CrossRef] [PubMed]
- Lewiston, N.; Newman, A.; Robin, E.; Holtzman, D. Shark Heart Mitochondria: Effects of External Osmolality on Respiration. Science 1979, 206, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Grauso, M.; Lan, A.; Andriamihaja, M.; Bouillaud, F.; Blachier, F. Hyperosmolar Environment and Intestinal Epithelial Cells: Impact on Mitochondrial Oxygen Consumption, Proliferation, and Barrier Function in Vitro. Sci. Rep. 2019, 9, 11360. [Google Scholar] [CrossRef]








| Evaluation | Probe | Concentration (μM) | Exposure Time (Hours) |
|---|---|---|---|
| Intracellular ROS | 2′,7′-dichlorodihydrofluoresceine diacetate (H2DCFDA) | 50 | 4 |
| Mitochondrial ROS | MitoSOXTM Red | 5 | 24 |
| Mitochondrial mass | MitoTracker® Green | 5 | 24 |
| Mitochondrial membrane potential (Δψm) | 5,5,6,6-Tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) | 5 | 3 |
| Treatment | Mean Lifespan (Days ± SEM) | Max Lifespan (Days) | P (log-Rank) | Number of Experiments (n) | Total Number of Nematodes | |
|---|---|---|---|---|---|---|
| vs. Control | vs. CdCl2 | |||||
| Control | 19 ± 0.45 | 25 | 5 | 109 | ||
| DMSO | 18 ± 0.50 | 25 | ns | 5 | 105 | |
| CdCl2 | 8 ± 0.56 | 20 | <0.0001 | 5 | 104 | |
| SFN+CdCl2 | 14 ± 0.68 | 25 | <0.0001 | <0.0001 | 5 | 103 |
| SFN | 20 ± 0.58 | 30 | 0.0002 | 5 | 102 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cruz, E.Y.; Aparicio-Trejo, O.E.; Eugenio-Pérez, D.; Juárez-Peredo, E.; Zurita-León, M.; Valdés, V.J.; Pedraza-Chaverri, J. Sulforaphane Exposure Prevents Cadmium-Induced Toxicity and Mitochondrial Dysfunction in the Nematode Caenorhabditis elegans by Regulating the Insulin/Insulin-like Growth Factor Signaling (IIS) Pathway. Antioxidants 2024, 13, 584. https://doi.org/10.3390/antiox13050584
Hernández-Cruz EY, Aparicio-Trejo OE, Eugenio-Pérez D, Juárez-Peredo E, Zurita-León M, Valdés VJ, Pedraza-Chaverri J. Sulforaphane Exposure Prevents Cadmium-Induced Toxicity and Mitochondrial Dysfunction in the Nematode Caenorhabditis elegans by Regulating the Insulin/Insulin-like Growth Factor Signaling (IIS) Pathway. Antioxidants. 2024; 13(5):584. https://doi.org/10.3390/antiox13050584
Chicago/Turabian StyleHernández-Cruz, Estefani Yaquelin, Omar Emiliano Aparicio-Trejo, Dianelena Eugenio-Pérez, Elí Juárez-Peredo, Mariana Zurita-León, Víctor Julián Valdés, and José Pedraza-Chaverri. 2024. "Sulforaphane Exposure Prevents Cadmium-Induced Toxicity and Mitochondrial Dysfunction in the Nematode Caenorhabditis elegans by Regulating the Insulin/Insulin-like Growth Factor Signaling (IIS) Pathway" Antioxidants 13, no. 5: 584. https://doi.org/10.3390/antiox13050584









