Efficacy of Respiratory Syncytial Virus Vaccination to Prevent Lower Respiratory Tract Illness in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Concept
2.2. Research Strategy
2.3. Selection Criteria
2.4. Selection Criteria
2.5. Data Extraction
2.6. Quality Assessment (Risk of Bias)
2.7. Data Analysis
3. Results
3.1. Descriptive Analysis
3.2. Risk of Bias
3.3. Demographic Characteristics of the Included Studies
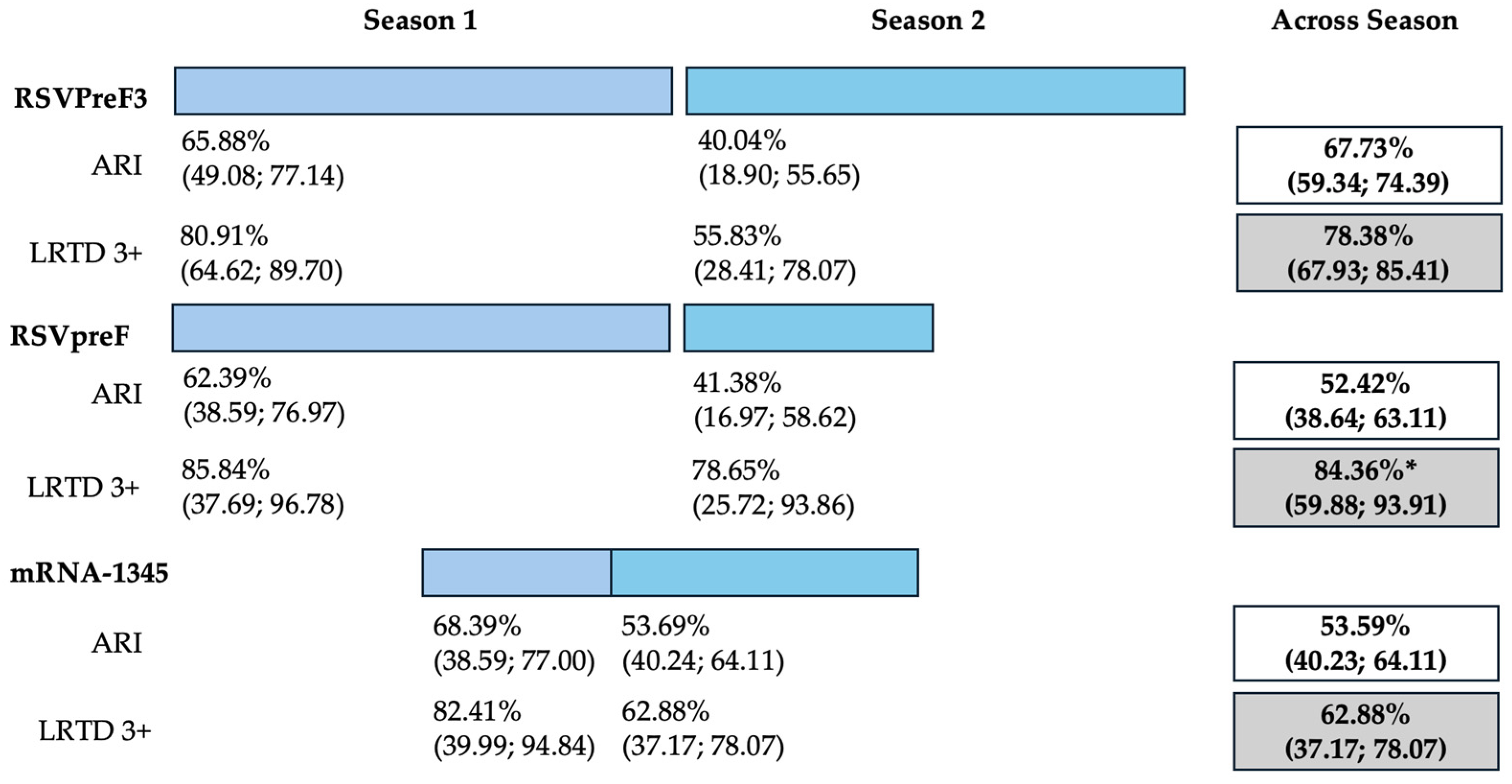
3.4. Effectiveness of RSV Vaccine
3.4.1. First Season
VE on ARI
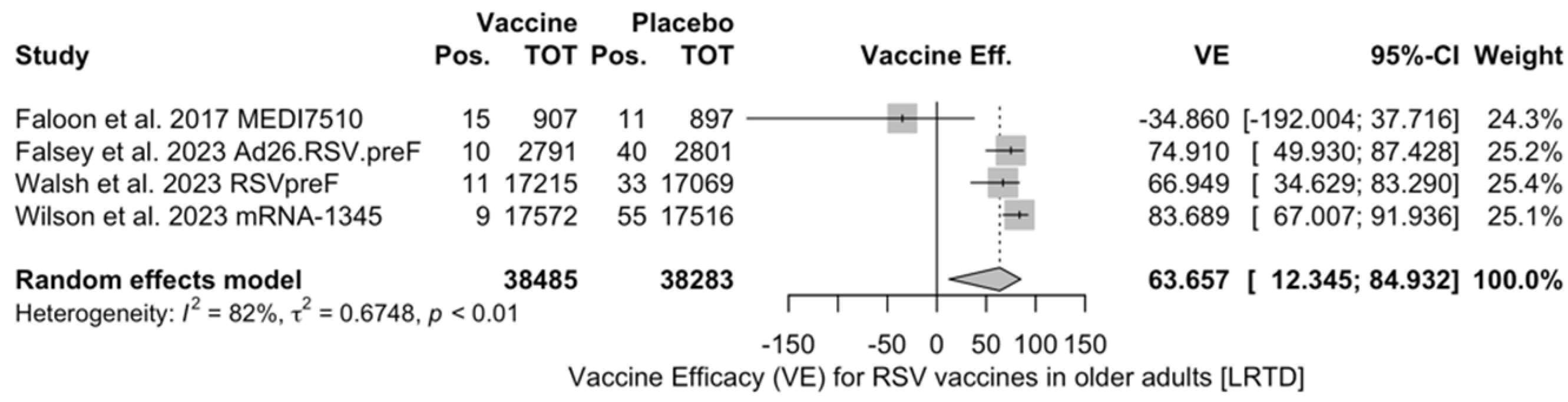
VE on LRTD with 2 Symptoms
VE on LRTD with 3 Symptoms or More
Comparison of Main Outcome by Age Groups
3.4.2. Follow-Up Studies
3.5. Sensitivity Analysis
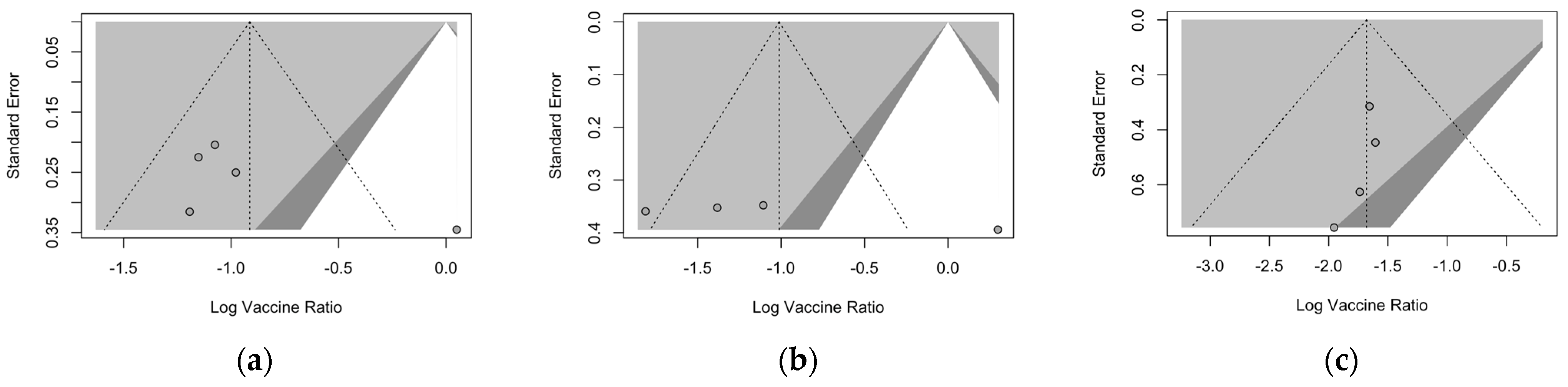
3.6. Publication Bias
4. Discussion
4.1. Summary of Main Findings and Their Generalizability
4.2. Limits and Implications for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Item | Definition |
|---|---|
| Population of interest | Adults aged 60 years or older having received at least one dose of any respiratory syncytial virus vaccine |
| Exposure | Exposed to RSV infection during the subsequent RSV season |
| Control/comparator | Adults aged 60 years or older not vaccinated against respiratory syncytial virus (placebo) |
| Outcome | Occurrence of lower respiratory tract disease and/or acute respiratory infection |
| Database | Keywords | No. of Entries |
|---|---|---|
| Pubmed | (“Vaccination”[MeSH Terms] OR (“vaccine”[Supplementary Concept] OR “vaccine”[All Fields] OR “Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR “vaccinable”[All Fields] OR “vaccinal”[All Fields] OR “vaccinate”[All Fields] OR “vaccinated”[All Fields] OR “vaccinates”[All Fields] OR “vaccinating”[All Fields] OR “vaccinations”[All Fields] OR “vaccination s”[All Fields] OR “vaccinator”[All Fields] OR “vaccinators”[All Fields] OR “vaccine s”[All Fields] OR “vaccined”[All Fields] OR “vaccines”[MeSH Terms] OR “vaccines”[All Fields] OR “vaccine”[All Fields] OR “vaccines”[All Fields] OR (“Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“immunization”[All Fields] AND “active”[All Fields]) OR “immunization active”[All Fields]) OR (“active immunisation”[All Fields] OR “Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“active”[All Fields] AND “immunization”[All Fields]) OR “active immunization”[All Fields]) OR (“active immunisations”[All Fields] OR “Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“active”[All Fields] AND “immunizations”[All Fields]) OR “active immunizations”[All Fields]) OR (“Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“immunizations”[All Fields] AND “active”[All Fields]) OR “immunizations active”[All Fields]))) AND (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “virus”[All Fields]) OR “respiratory syncytial virus”[All Fields] OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“syncytial”[All Fields] AND “virus”[All Fields] AND “respiratory”[All Fields]) OR “syncytial virus respiratory”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“syncytial”[All Fields] AND “viruses”[All Fields] AND “respiratory”[All Fields])) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“virus”[All Fields] AND “respiratory”[All Fields] AND “syncytial”[All Fields]) OR “virus respiratory syncytial”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“viruses”[All Fields] AND “respiratory”[All Fields] AND “syncytial”[All Fields]) OR “viruses respiratory syncytial”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“chimpanzee”[All Fields] AND “coryza”[All Fields] AND “agent”[All Fields]) OR “chimpanzee coryza agent”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“chimpanzee”[All Fields] AND “coryza”[All Fields] AND “agents”[All Fields])) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“coryza”[All Fields] AND “agent”[All Fields] AND “chimpanzee”[All Fields])) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“coryza”[All Fields] AND “agents”[All Fields] AND “chimpanzee”[All Fields])))) Filter: aged: 65+ years, 80 and older: 80+ years | 432 |
| EMBASE | (“pneumovirus’/exp” OR “pneumovirus” OR “pneumovirus infection” OR “human respiratory syncytial virus” OR “respiratory syncytial virus infection”) AND (“immunization” OR “vaccination”) Filter: aged: 65+ years, 80 and older: 80+ years | 344 |
| SCOPUS | (vaccination* OR immunization*) AND (“respiratory syncytial virus” OR “RSV” OR “bronchiolitis”) AND (adult* OR elderly OR elder*) Filter: adults | 721 |
| medRxiv | (respiratory syncytial virus OR RSV) AND (vaccine OR vaccination OR immunization) | 599 |
| Vaccine | Characteristics | Approved | Clinical Trial | Reference |
|---|---|---|---|---|
| RSVPreF3 | Subunit A stabilized prefusion F protein (120 μg); monovalent; Adjuvant: AS01 Single dose | FDA, EMA | NCT04886596 | [48,80,81] |
| RSVpreF | Bivalent (RSV A and B) stabilized prefusion F protein subunit (60 μg + 60 μg); Not adjuvated Single dose | FDA, EMA | NCT04886596 | [52,61] |
| Ad26.RSV.preF | Adenovirus serotype 26 vector-based RSV vaccine encoding prefusion F protein Single dose Monovalent | Ongoing | NCT03982199 | [82] |
| mRNA-1345 | Single mRNA sequence encoding for a stabilized prefusion F glycoprotein (50 µg) Single dose Monovalent | Ongoing | [51,85] | |
| MEDI7510 | RSV soluble fusion protein F antigen plus glucopyranosyl lipid A in stable emulsion (GLA-SE) adjuvantSingle dose (120 μg) Monovalent | Discontinued | NCT02508194 | [83] |
| Clinical Trial (Vaccine) | |||||
|---|---|---|---|---|---|
| NCT03982199 (Ad26.RSV.preF) [82] | NCT02508194 (MEDI7510) [83] | NCT04886596 (RSVPreF3) [48,80,81] | NCT05035212 (RSVpreF) [52,61] | NCT04886596 (mRNA-1345) [51,85] | |
| Signs/Symptoms | |||||
| Cough | Any | Any | New or increased | New or increased | Any |
| Shortness of breath | Any | Alternative with dyspnea | - | New or increased | Any |
| Dyspnea | Alternative with decreased oxygen saturation | Alternative with shortness of breath | New or increased | - | - |
| Decreased oxygen saturation | Alternative with dyspnea | - | Any | - | Any |
| Oxygen requirement | - | - | Any | - | - |
| Wheezing | Any | Any | Any | New or increased | Any |
| Rales/Ronchi | Any | - | Alternative with crackles | - | Any |
| Crackles | - | - | Alternative with Rales/Ronchi | - | Any |
| Sputum production | Any | Any | New or increased | New or increased | Any |
| Tachypnea | Any | - | Any | Any | Any |
| Headache | Any | ||||
| Myalgias or Arthralgias | - | Any | Body aches | - | - |
| Malaise | Any | - | - | - | - |
| Fatigue | Any | Any | - | - | - |
| Fever (>37.8 °C) | Any | - | - | - | - |
| Feverishness | Any | Any | - | - | - |
| Pleuritic chest pain | - | - | - | - | Lasting ≥24 h |
| Radiologic evidence of pneumonia | - | - | - | - | Any |
| Case Definition | |||||
| Low Respiratory Tract Disease | Symptoms Shortness of breath Dyspnea or decreased oxygen saturation Cough Wheezing Rales and/or Rhonchi Sputum production Tachypnea Systemic symptoms Malaise Fatigue Fever 1 Feverishness 2 | Symptoms Dyspnea or shortness of breath Cough Wheezing Sputum production Systemic symptoms Myalgias or arthralgias Body aches Fatigue (tiredness) Headache Decreased appetite Feverishness | Symptoms Dyspnea (new or increased) Cough (new or increased) Sputum production (new or increased) Signs Wheezing (new or increased) Crackles and/or Rhonchi (new or increased) Tachypnea Decreased oxygen saturation Oxygen requirement | Shortness of breath (new or increased) Cough (new or increased) Wheezing (new or increased) Sputum production (new or increased) Tachypnea | Shortness of breath Cough Fever Wheezing Rales and/or Rhonchi Sputum production Tachypnea Hypoxemia Pleuritic chest pain ≥ 24 h Radiologic evidence of pneumonia |
| Study | Vaccinated (N.) | Placebo (N.) | Males | Age ≥70/75 years | White Ethnicity | |||
|---|---|---|---|---|---|---|---|---|
| Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | |||
| Ison et al. [48] | 12,470 | 12,503 | 5981 (47.96%) | 6074 (48.58%) | 5507 (44.16%) | 5521 (44.16%) | 9890 (79.31%) | 9936 (79.47%) |
| Papi et al. [81] | 12,466 | 12,494 | 5978 (47.95%) | 6067 (48.56%) | 5504 (44.15%) | 5519 (44.17%) | 9887 (79.31%) | 9932 (79.49%) |
| Feldman et al. [80] | 12,467 | 12,499 | 5979 (47.96%) | 6072 (48.58%) | 5504 (44.15%) | 5519 (44.16%) | 9987 (79.31%) | 9932 (79.46%) |
| Walsh et al. [52] | 17,215 | 17,069 | 8800 (51.12%) | 8601 (50.39%) | 6458 (37.51%) | 6389 (37.43%) | 13,475 (78.27%) | 13,360 (78.27%) |
| Wilson et al. [51] | 17,734 | 17,679 | 8974 (51.07%) | 8875 (50.67%) | 6453 (36.72%) | 6457 (36.86%) | 11,240 (63.97%) | 11,216 (64.03%) |
| Falsey et al. [82] | 2891 | 2891 | 1251 (43.27%) | 1197 (41.40%) | 765 (26.46%) | 759 (26.25%) | 2658 (91.94%) | 2690 (93.05%) |
| Falloon et al. [83] | 949 | 951 | 380 (41.90%) | 309 (34.45%) | 133 (14.66% | 135 (15.05%) | 771 (85.01%) | 773 (86.16%) |
| Study | Vaccinated (No.) | Placebo (No.) | COPD | Asthma | Congestive Hearth Disease | |||
|---|---|---|---|---|---|---|---|---|
| Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | |||
| Ison et al. [48] | 12,470 | 12,503 | 1131 (9.07%) | 1113 (8.90%) | 1193 (9.57%) | 1113 (8.90%) | 398 (3.19%) | 403 (3.22%) |
| Walsh et al. [52] | 17,215 | 17,069 | 960 (5.46%) | 978 (5.58%) | - | - | 53 (0.30 %) | 51 (0.29%) |
| Wilson et al. [51] | 17,734 | 17,679 | 1012 (5.88%) | 1080 (6.33%) | 1541 (8.95%) | 1508 (8.83%) | 293 (1.70%) | 307 (1.80%) |
| Falsey et al. [82] | 2891 | 2891 | 219 (7.58%) | 208 (7.19%) | 266 (9.20%) | 250 (8.65%) | 58 (2.01%) | 54 (1.87%) |
| Vaccine | Placebo (N.) | RSV A (n./N., %) | RSV B (n./N., %) | Ratio RSV A/B | ||||
|---|---|---|---|---|---|---|---|---|
| Season | ARI | LRTD 3+ | ARI | LRTD 3+ | ARI | LRTD 3+ | ||
| mRNA-1345 | 1st | 17,516 | 51 (0.29) | 10 (0.06) | 31 (0.18) | 7 (0.04) | 1.645 | 1.429 |
| FU | 18,045 | 106 (0.59) | 30 (0.17) | 80 (0.44) | 22 (0.12) | 1.325 | 1.364 | |
| RSVpreF | 1st | 17,069 | 12 (0.07) | 3 (0.02) | 45 (0.26) | 10 (0.06) | 0.267 | 0.300 |
| FU | 9992 | 62 (0.62) | 10 (0.10) | 25 (0.25) | 3 (0.03) | 2.480 | 3.333 | |
| RSVPreF3 | 1st | 12,494 | 32 (0.26) | 13 (0.10) | 61 (0.49) | 26 (0.21) | 0.525 | 0.500 |
| FU | 4991 | - | 34 (0.68) | - | 57 (1.14) | - | 0.596 | |
| Study | Vaccine | Day | GMI RSV A | GMI RSV B |
|---|---|---|---|---|
| Feldman et al. [80] | RSVPreF3 | 31 | 9.8 Range: 8.9–10.6 | 8.3 Range: 8.1–10.2 |
| Falsey et al. [82] | Ad26.RSV.preF | 15 | 12.1 | 9.4 |
| Papi et al. [81] | RSVPreF3 | 30 | 10.2 Range: 9.5–11 | 8.6 Range: 8.0–9.2 |
| Walsh et al. [52] (from Baber et al. [117]) | RSVpreF | 30 | 10.2 | 12.3 |
| Wilson et al. [51] (from Chen et al.) [118] | mRNA-1345 | 30 | Range: 12.1–16.6 | Range: 8.7–12.6 |
| Falloon et al. [83] | MEDI7510 | End of season | 4.6 Range: 4.3–4.9 | |
| Wilson et al. [85] | mRNA-1345 | 30 | 10.1 | 6.4 |




























References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV Disease in Infants and Young Children: Can We See a Brighter Future? Hum. Vaccines Immunother. 2022, 18, 2079322. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Provenzano, S.; Corrado, S.; Cerviere, M.P.; Parisi, S.; Marchesi, F.; Bottazzoli, M. Infodemiology of RSV in Italy (2017–2022): An Alternative Option for the Surveillance of Incident Cases in Pediatric Age? Children 2022, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Nair DNB, H.; Theodoratou, E.; Rudan, I.; Nokes, D.J.; Ngama HND, M.; Munywoki, P.K.; Dherani, M.; Nair, H.; James Nokes, D.; Gessner, B.D.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar]
- Luo, W.; Liu, Q.; Zhou, Y.; Ran, Y.; Liu, Z.; Hou, W.; Pei, S.; Lai, S. Spatiotemporal Variations of “Triple-Demic” Outbreaks of Respiratory Infections in the United States in the Post-COVID-19 Era. BMC Public Health 2023, 23, 2452. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.A.; Jain, B.; Raifman, J. Revamping Public Health Systems: Lessons Learned from the Tripledemic. Am. J. Prev. Med. 2023, 66, 185–188. [Google Scholar] [CrossRef]
- Nam, H.H.; Ison, M.G. Respiratory Syncytial Virus Infection in Adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Regassa, B.T.; Gebrewold, L.A.; Mekuria, W.T.; Kassa, N.A. Molecular Epidemiology of Respiratory Syncytial Virus in Children with Acute Respiratory Illnesses in Africa: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 04001. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Y.; Chmaisse, A.; Boutros, C.; Chamseddine, S.; Fayad, D.; Zaraket, H.; Dbaibo, G. The Burden of Respiratory Syncytial Virus (RSV) Infection in the Middle East and North Africa (MENA) Region across Age Groups: A Systematic Review. Vaccine 2021, 39, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Shaw, C.A.; Griffin, P.; Farinola, N.; Railkar, R.A.; Cao, X.; Liu, W.; Sachs, J.R.; Swenson, C.J.; Lee, H.; et al. A Phase 1, Randomized, Placebo-Controlled Study to Evaluate the Safety and Immunogenicity of an mRNA-Based RSV Prefusion F Protein Vaccine in Healthy Younger and Older Adults. Hum. Vaccines Immunother. 2021, 17, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Raybould, J.E.; Sastry, S.; de la Cruz, O. Respiratory Viruses in Transplant Recipients: More than Just a Cold. Clinical Syndromes and Infection Prevention Principles. Int. J. Infect. Dis. 2017, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Ciarlitto, C.; Guolo, S.; Brusco, C.; Cerone, G.; Antilici, L.; Schettini, L.; Piscitelli, A.L.; Chiara Vittucci, A.; Cutrera, R.; et al. Respiratory Syncytial Virus Bronchiolitis in Infancy: The Acute Hospitalization Cost. Front. Pediatr. 2021, 8, 594898. [Google Scholar] [CrossRef]
- Rha, B.; Curns, A.T.; Lively, J.Y.; Campbell, A.P.; Englund, J.A.; Boom, J.A.; Azimi, P.H.; Weinberg, G.A.; Staat, M.A.; Selvarangan, R.; et al. Respiratory Syncytial Virus-Associated Hospitalizations among Young Children: 2015–2016. Pediatrics 2020, 146, e20193611. [Google Scholar] [CrossRef]
- Leader, S.; Kohlhase, K. Respiratory Syncytial Virus-Coded Pediatric Hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–661. [Google Scholar]
- Leader, S.; Kohlhase, K.; Pearlman, M.H.; Williams, J.V.; Engle, W.A. Recent Trends in Severe Respiratory Syncytial Virus (RSV) among US Infants, 1997 to 2000. J. Pediatr. 2003, 143, S127–S132. [Google Scholar] [CrossRef]
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Bøås, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus–Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef]
- Kenmoe, S.; Nair, H. The Disease Burden of Respiratory Syncytial Virus in Older Adults. Curr. Opin. Infect. Dis. 2024, 37, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F. Respiratory Syncytial Virus, Influenza and SARS-CoV-2 in Homeless People from Urban Shelters: A Systematic Review and Meta-Analysis (2023). Epidemiologia 2024, 5, 41–79. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of Severe RSV Disease among Immunocompromised Children and Adults: A 10 Year Retrospective Study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Almeida, A.; Christaki, E.; Marques, T.M.; Tosatto, V.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Karagiannis, C.; Maikanti, P.; et al. Severity of RSV Infection in Southern European Elderly Patients during Two Consecutive Winter Seasons (2017–2018). J. Med. Virol. 2021, 93, 5152–5157. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Fernandes, J.; de Pouvourville, G.; Sosnowiez, K.; Elong, A.; Guilmet, C.; Omichessan, H.; Bureau, I.; Fagnani, F.; Emery, C.; et al. Respiratory Syncytial Virus-Related Hospital Stays in Adults in France from 2012 to 2021: A National Hospital Database Study. J. Clin. Virol. 2024, 171, 105635. [Google Scholar] [CrossRef]
- Polkowska-Kramek, A.; Begier, E.; Bruyndonckx, R.; Liang, C.; Beese, C.; Brestrich, G.; Tran, T.M.P.; Nuttens, C.; Casas, M.; Bayer, L.J.; et al. Estimated Incidence of Hospitalizations and Deaths Attributable to Respiratory Syncytial Virus Infections among Adults in Germany between 2015 and 2019. Infect. Dis. Ther. 2024, 13, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic Review on Respiratory Syncytial Virus Epidemiology in Adults and the Elderly in Latin America. Int. J. Infect. Dis. 2020, 90, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Nowalk, M.P.; D’Agostino, H.; Dauer, K.; Stiegler, M.; Zimmerman, R.K.; Balasubramani, G.K. Estimating the Burden of Adult Hospitalized RSV Infection Including Special Populations. Vaccine 2022, 40, 4121–4127. [Google Scholar] [CrossRef]
- Narejos Pérez, S.; Ramón Torrell, J.M.; Põder, A.; Leroux-Roels, I.; Pérez-Breva, L.; Steenackers, K.; Vandermeulen, C.; Meisalu, S.; McNally, D.; Bowen, J.S.; et al. Respiratory Syncytial Virus Disease Burden in Community-Dwelling and Long-Term Care Facility Older Adults in Europe and the United States: A Prospective Study. Open Forum Infect. Dis. 2023, 10, ofad111. [Google Scholar] [CrossRef]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory Syncytial Virus Disease Burden in Adults Aged 60 Years and Older in High-Income Countries: A Systematic Literature Review and Meta-Analysis. Influenza Other Respir. Viruses 2022, 17, e13031. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.P.; Tapia, L.I.; Catalán, P.; De la Maza, V.; Mejías, A. Intravenous Palivizumab in Respiratory Syncytial Virus Infection after Hematopoietic Stem Cell Transplant in Children. Pediatr. Blood Cancer 2017, 64, e26667. [Google Scholar] [CrossRef] [PubMed]
- Permpalung, N.; Mahoney, M.V.; McCoy, C.; Atsawarungruangkit, A.; Gold, H.S.; Levine, J.D.; Wong, M.T.; LaSalvia, M.T.; Alonso, C.D. Clinical Characteristics and Treatment Outcomes among Respiratory Syncytial Virus (RSV)-Infected Hematologic Malignancy and Hematopoietic Stem Cell Transplant Recipients Receiving Palivizumab. Leuk. Lymphoma 2019, 60, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Cutland, C.L.; Downs, S.; Jones, S.; Van Niekerk, N.; Simoes, E.A.F.; Nunes, M.C. Burden of Respiratory Syncytial Virus Infection in South African Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Pregnant and Postpartum Women: A Longitudinal Cohort Study. Clin. Infect. Dis. 2018, 66, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, L.; Zhang, Y.; Zhang, X.; Zheng, M.; Kyaw, M.H. Burden of Respiratory Syncytial Virus Infections in China: Systematic Review and Meta-Analysis. J. Glob. Health 2015, 5, 020417. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.; Hall, C.B.; Katkin, J.P.; Shi, N.; Masaquel, A.S.; McLaurin, K.K.; Mahadevia, P.J. Healthcare Costs within a Year of Respiratory Syncytial Virus among Medicaid Infants. Pediatr. Pulmonol. 2010, 45, 772–781. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.K.; Farr, A.M.; Wade, S.W.; Diakun, D.R.; Stewart, D.L. Respiratory Syncytial Virus Hospitalization Outcomes and Costs of Full-Term and Preterm Infants. J. Perinatol. 2016, 36, 990–996. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Melgar, M.; Britton, A.; Roper, L.E.; Keipp Talbot, H.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Am. J. Transplant. 2023, 23, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Jones, J.M.; Roper, L.E.; Prill, M.M.; Ortega-Sanchez, I.R.; Moulia, D.L.; Wallace, M.; Godfrey, M.; Broder, K.R.; Tepper, N.K.; et al. Morbidity and Mortality Weekly Report Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus-Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 1115–1122. [Google Scholar]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar]
- Papazisis, G.; Topalidou, X.; Gioula, G.; González, P.A.; Bueno, S.M.; Kalergis, A.M. Respiratory Syncytial Virus Vaccines: Analysis of Pre-Marketing Clinical Trials for Immunogenicity in the Population over 50 Years of Age. Vaccines 2024, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A.; Roper, L.E.; Keipp Talbot, H.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Bastian, A.R.; Feldman, R.A.; Omoruyi, E.; de Paepe, E.; Hendriks, J.; van Zeeburg, H.; Godeaux, O.; Langedijk, J.P.M.; Schuitemaker, H.; et al. Phase 1 Safety and Immunogenicity Study of a Respiratory Syncytial Virus Vaccine With an Adenovirus 26 Vector Encoding Prefusion F (Ad26.RSV.preF) in Adults Aged ≥60 Years. J. Infect. Dis. 2020, 222, 979–988. [Google Scholar] [PubMed]
- Cicconi, P.; Jones, C.; Sarkar, E.; Silva-Reyes, L.; Klenerman, P.; de Lara, C.; Hutchings, C.; Moris, P.; Janssens, M.; Fissette, L.A.; et al. First-in-Human Randomized Study to Assess the Safety and Immunogenicity of an Investigational Respiratory Syncytial Virus (RSV) Vaccine Based on Chimpanzee-Adenovirus-155 Viral Vector-Expressing RSV Fusion, Nucleocapsid, and Antitermination Viral Proteins in Healthy Adults. Clin. Infect. Dis. 2020, 70, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Essink, B.; Harper, C.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Panozzo, C.A.; Wilson, E.; et al. Safety and Immunogenicity of an MRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults. J. Infect. Dis. 2024, jiae081. [Google Scholar] [CrossRef] [PubMed]
- Spearman, P.; Jin, H.; Knopp, K.; Xiao, P.; Gingerich, M.C.; Kidd, J.; Singh, K.; Tellier, M.; Radziewicz, H.; Wu, S.; et al. Intranasal Parainfluenza Virus Type 5 (PIV5)-Vectored RSV Vaccine Is Safe and Immunogenic in Healthy Adults in a Phase 1 Clinical Study. Sci. Adv. 2023, 9, eadj7611. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G.; Papi, A.; Athan, E.; Feldman, R.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; et al. Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons. Clin. Infect. Dis. 2024, ciae010. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Kabir, G.; Schultz, S.; Silbernagl, G.; Schmidt, D.; Jenkins, V.A.; Weidenthaler, H.; Stroukova, D.; Martin, B.K.; De Moerlooze, L. Reduced Respiratory Syncytial Virus Load, Symptoms, and Infections: A Human Challenge Trial of MVA-BN-RSV Vaccine. J. Infect. Dis. 2023, 228, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.A.; Phung, E.; Crank, M.C.; Morabito, K.M.; Villafana, T.; Dubovsky, F.; Falloon, J.; Esser, M.T.; Lin, B.C.; Chen, G.L.; et al. A Prefusion-Stabilized RSV F Subunit Vaccine Elicits B Cell Responses with Greater Breadth and Potency than a Postfusion F Vaccine. Sci. Transl. Med. 2022, 14, eade0424. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an MRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; Saeland, E.; Thoma, A.; van den Hoogen, W.; Tettero, L.; Drijver, J.; Vaneman, C.; van Polanen, Y.; Ritschel, T.; Bastian, A.R.; et al. RSV A2-Based Prefusion F Vaccine Candidates Induce RSV A and RSV B Cross Binding and Neutralizing Antibodies and Provide Protection against RSV A and RSV B Challenge in Preclinical Models. Vaccines 2023, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.; Cao, X.; Railkar, R.A.; Sachs, J.R.; Spellman, D.S.; Luk, J.; Shaw, C.A.; Cejas, P.J.; Citron, M.P.; Al-Ibrahim, M.; et al. Evaluation of a Stabilized RSV Pre-Fusion F MRNA Vaccine: Preclinical Studies and Phase 1 Clinical Testing in Healthy Adults. Vaccine 2023, 41, 6488–6501. [Google Scholar] [CrossRef] [PubMed]
- Samy, N.; Reichhardt, D.; Schmidt, D.; Chen, L.M.; Silbernagl, G.; Vidojkovic, S.; Meyer, T.P.; Jordan, E.; Adams, T.; Weidenthaler, H.; et al. Safety and Immunogenicity of Novel Modified Vaccinia Ankara-Vectored RSV Vaccine: A Randomized Phase I Clinical Trial. Vaccine 2020, 38, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.S.V.; Virta, M.; Williams, K.; Seppa, I.; Hartvickson, R.; Greenland, M.; Omoruyi, E.; Bastian, A.R.; Haazen, W.; Salisch, N.; et al. Phase 1/2a Safety and Immunogenicity of an Adenovirus 26 Vector Respiratory Syncytial Virus (RSV) Vaccine Encoding Prefusion F in Adults 18–50 Years and RSV-Seropositive Children 12–24 Months. J. Infect. Dis. 2023, 227, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Athan, E.; Baber, J.; Quan, K.; Scott, R.J.; Jaques, A.; Jiang, Q.; Li, W.; Cooper, D.; Cutler, M.W.; Kalinina, E.V.; et al. Safety and Immunogenicity of Bivalent RSVpreF Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Older Adults. Clin. Infect. Dis. 2023, ciad707. [Google Scholar] [CrossRef] [PubMed]
- Díez-Domingo, J.; Sáez-Llorens, X.; Rodriguez-Weber, M.A.; Epalza, C.; Chatterjee, A.; Chiu, C.H.; Lin, C.Y.; Berry, A.A.; Martinón-Torres, F.; Baquero-Artigao, F.; et al. Safety and Immunogenicity of a ChAd155-Vectored Respiratory Syncytial Virus (RSV) Vaccine in Healthy RSV-Seropositive Children 12-23 Months of Age. J. Infect. Dis. 2023, 227, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Green, C.A.; Scarselli, E.; Voysey, M.; Capone, S.; Vitelli, A.; Nicosia, A.; Cortese, R.; Thompson, A.J.; Sande, C.S.; De Lara, C.; et al. Safety and Immunogenicity of Novel Respiratory Syncytial Virus (RSV) Vaccines Based on the RSV Viral Proteins F, N and M2-1 Encoded by Simian Adenovirus (PanAd3-RSV) and MVA (MVA-RSV); Protocol for an Open-Label, Dose-Escalation, Single-Centre, Phase 1 Clinical Trial in Healthy Adults. BMJ Open 2015, 5, e008748. [Google Scholar] [CrossRef] [PubMed]
- Malkin, E.; Yogev, R.; Abughali, N.; Sliman, J.; Wang, C.K.; Zuo, F.; Yang, C.F.; Eickhoff, M.; Esser, M.T.; Tang, R.S.; et al. Safety and Immunogenicity of a Live Attenuated RSV Vaccine in Healthy RSV-Seronegative Children 5 to 24 Months of Age. PLoS ONE 2013, 8, e77104. [Google Scholar] [CrossRef]
- Walsh, E.; Falsey, A.; Patton, M.; Stacey, H.; Eiras, D.P.; Jiang, Q.; Woodside, J.; Mikati, T.; Kalinina, E.; Cooper, D.; et al. Efficacy of a Bivalent RSVpreF Vaccine in Older Adults Beyond a First RSV Season. In Proceedings of the 8th ReSViNET Conference, Mumbai, India, 13–16 February 2024; Respiratory Syncytial Virus Foundation: Mumbai, India, 2024; pp. 99–100. [Google Scholar]
- Karron, R.A.; Luongo, C.; Woods, S.; Oliva, J.; Collins, P.L.; Buchholz, U.J.; Council-Dibitetto, C.; Gatto, M.; Ghasri, T.; Gormley, A.; et al. Evaluation of the Live-Attenuated Intranasal Respiratory Syncytial Virus (RSV) Vaccine RSV/6120/ΔNS2/1030s in RSV-Seronegative Young Children. J. Infect. Dis. 2024, 229, 346–354. [Google Scholar] [CrossRef]
- Cunningham, C.K.; Karron, R.A.; Muresan, P.; Kelly, M.S.; McFarland, E.J.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; et al. Evaluation of Recombinant Live-Attenuated Respiratory Syncytial Virus (RSV) Vaccines RSV/ΔNS2/Δ1313/I1314L and RSV/276 in RSV-Seronegative Children. J. Infect. Dis. 2022, 226, 2069–2078. [Google Scholar] [CrossRef]
- Graham, B.S.; Modjarrad, K.; McLellan, J.S. Novel Antigens for RSV Vaccines. Curr. Opin. Immunol. 2015, 35, 30–38. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Tang, S.F.; Shi, Y. Efficacy and Safety of Respiratory Syncytial Virus Vaccination during Pregnancy to Prevent Lower Respiratory Tract Illness in Newborns and Infants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 2023, 11, 1260740. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Mintzker, Y.; Blum, D.; Adler, L. Replacing PICO in Non-Interventional Studies. BMJ Evid.-Based Med. 2023, 28, 284. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Von Hippel, P.T. The Heterogeneity Statistic I2 Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Krumpal, I. Determinants of Social Desirability Bias in Sensitive Surveys: A Literature Review. Qual. Quant. 2013, 47, 2025–2047. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). OHAT Risk of Bias Rating Tool for Human and Animal Studies; National Toxicology Program: Research Triangle Park, NC, USA, 2015. [Google Scholar]
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing Risk of Bias in Human Environmental Epidemiology Studies Using Three Tools: Different Conclusions from Different Tools. Syst. Rev. 2020, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A Re-Evaluation of Random-Effects Meta-Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3900051070. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.G.; Antonelli-Incalzi, R.; Steenackers, K.; Lee, D.G.; Papi, A.; Ison, M.G.; Fissette, L.; David, M.P.; Marechal, C.; Van Der Wielen, M.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine Is Efficacious in Older Adults with Underlying Medical Conditions. Clin. Infect. Dis. 2024, 78, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Bart, S.; Ervin, J.; Bastian, A.R.; Menten, J.; De Paepe, E.; Vandenberghe, S.; Chan, E.K.H.; et al. Efficacy and Safety of an Ad26.RSV.PreF–RSV PreF Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Falloon, J.; Yu, J.; Esser, M.T.; Villafana, T.; Yu, L.; Dubovsky, F.; Takas, T.; Levin, M.J.; Falsey, A.R. An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. J. Infect. Dis. 2017, 216, 1362–1370. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; Office of Health Assessment and Translation (OHAT), Division of the National Toxicology Program, National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2019. [Google Scholar]
- Wilson, E.; Goswami, J.; Doreski, P.A.; Marc, G.P.; Jimenez, G.; Priddy, F.; Lin, N.; Le Cam, N.; Karen Slobod, K.; Stoszek, S.K.; et al. Efficacy and Safety of MRNA-1345, an RSV Vaccine, in Older Adults: Results through ≥6 Months of Follow-up and Evaluation of Correlate of Protection Against RSV. In Proceedings of the 8th ReSViNET Conference, Mumbai, India, 13–16 February 2024; Respiratory Syncytial Virus Society: Mumbai, India, 2024; pp. 87–88. [Google Scholar]
- Falloon, J.; Talbot, H.K.; Curtis, C.; Ervin, J.; Krieger, D.; Dubovsky, F.; Takas, T.; Yu, J.; Yu, L.; Lambert, S.L. Dose Selection for an Adjuvanted Respiratory Syncytial Virus F Protein Vaccine for Older Adults Based on Humoral and Cellular Immune Responses. Clin. Vaccine Immunol. 2017, 24, e00157-17. [Google Scholar] [CrossRef]
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D.; et al. A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. Pfizer Announces Positive Top-Line Data for Full Season Two Efficacy of ABRYSVO® for RSV in Older Adults. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two (accessed on 28 March 2024).
- Falloon, J.; Ji, F.; Curtis, C.; Bart, S.; Sheldon, E.; Krieger, D.; Dubovsky, F.; Lambert, S.; Takas, T.; Villafana, T.; et al. A Phase 1a, First-in-Human, Randomized Study of a Respiratory Syncytial Virus F Protein Vaccine with and without a Toll-like Receptor-4 Agonist and Stable Emulsion Adjuvant. Vaccine 2016, 34, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Baral, R.; Higgins, D.; Khan, S.; Kochar, S.; Li, Y.; Ortiz, J.R.; Cherian, T.; Feikin, D.; Jit, M.; et al. Value Profile for Respiratory Syncytial Virus Vaccines and Monoclonal Antibodies. Vaccine 2023, 41, S7–S40. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E.; Scott, D.A.; Gurtman, A.; Zareba, A.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Jiang, Q.; et al. Phase 1/2 Randomized Study of the Immunogenicity, Safety, and Tolerability of a Respiratory Syncytial Virus Prefusion F Vaccine in Adults with Concomitant Inactivated Influenza Vaccine. J. Infect. Dis. 2022, 225, 2056–2066. [Google Scholar] [CrossRef]
- Comeaux, C.A.; Bart, S.; Bastian, A.R.; Klyashtornyy, V.; De Paepe, E.; Omoruyi, E.; Van Der Fits, L.; Van Heesbeen, R.; Heijnen, E.; Callendret, B.; et al. Safety, Immunogenicity, and Regimen Selection of Ad26.RSV.PreF-Based Vaccine Combinations: A Randomized, Double-Blind, Placebo-Controlled, Phase 1/2a Study. J. Infect. Dis. 2024, 229, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; De Paepe, E.; Devincenzo, J.; Gymnopoulou, E.; Menten, J.; Murray, B.; Rosemary Bastian, A.; Vandebosch, A.; Haazen, W.; Noulin, N.; et al. Prevention of Respiratory Syncytial Virus Infection in Healthy Adults by a Single Immunization of Ad26.RSV.PreF in a Human Challenge Study. J. Infect. Dis. 2022, 226, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.D.; et al. Arterial Events, Venous Thromboembolism, Thrombocytopenia, and Bleeding after Vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population Based Cohort Study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Cizmeci, D.; Yuan, D.; Mehta, N.; Tolboom, J.; De Paepe, E.; van Heesbeen, R.; Sadoff, J.; Comeaux, C.A.; Heijnen, E.; et al. Vaccine-Induced Antibody Fc-Effector Functions in Humans Immunized with a Combination Ad26.RSV.PreF/RSV PreF Protein Vaccine. J. Virol. 2023, 97, e00771-23. [Google Scholar] [CrossRef] [PubMed]
- Widagdo, W.; Bastian, A.R.; Jastorff, A.M.; Scheys, I.; De Paepe, E.; Comeaux, C.A.; Ligtenberg, N.; Callendret, B.; Heijnen, E. Concomitant Administration of Ad26.RSV.PreF/RSV PreF Protein Vaccine and High-Dose Influenza Vaccine in Adults 65 Years and Older: A Noninferiority Trial. J. Infect. Dis. 2023, jiad594. [Google Scholar] [CrossRef]
- Chu, H.Y.; Chin, J.; Pollard, J.; Zerr, D.M.; Englund, J.A. Clinical Outcomes in Outpatient Respiratory Syncytial Virus Infection in Immunocompromised Children. Influenza Other Respir. Viruses 2016, 10, 205–210. [Google Scholar] [CrossRef]
- Yoon, J.G.; Noh, J.Y.; Choi, W.S.; Park, J.J.; Suh, Y.B.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Clinical Characteristics and Disease Burden of Respiratory Syncytial Virus Infection among Hospitalized Adults. Sci. Rep. 2020, 10, 12106. [Google Scholar] [CrossRef]
- Reeves, R.M.; van Wijhe, M.; Lehtonen, T.; Stona, L.; Teirlinck, A.C.; Vazquez Fernandez, L.; Li, Y.; Osei-Yeboah, R.; Fischer, T.K.; Heikkinen, T.; et al. A Systematic Review of European Clinical Practice Guidelines for Respiratory Syncytial Virus Prophylaxis. J. Infect. Dis. 2022, 226, S110–S116. [Google Scholar] [CrossRef]
- Loubet, P.; Lenzi, N.; Valette, M.; Foulongne, V.; Krivine, A.; Houhou, N.; Lagathu, G.; Rogez, S.; Alain, S.; Duval, X.; et al. Clinical Characteristics and Outcome of Respiratory Syncytial Virus Infection among Adults Hospitalized with Influenza-like Illness in France. Clin. Microbiol. Infect. 2017, 23, 253–259. [Google Scholar] [CrossRef]
- Widmer, K.; Zhu, Y.; Williams, J.V.; Griffin, M.R.; Edwards, K.M.; Talbot, H.K. Rates of Hospitalizations for Respiratory Syncytial Virus, Human Metapneumovirus, and Influenza Virus in Older Adults. J. Infect. Dis. 2012, 206, 56–62. [Google Scholar] [CrossRef]
- Malosh, R.E.; Martin, E.T.; Callear, A.P.; Petrie, J.G.; Lauring, A.S.; Lamerato, L.; Fry, A.M.; Ferdinands, J.; Flannery, B.; Monto, A.S. Respiratory Syncytial Virus Hospitalization in Middle-Aged and Older Adults. J. Clin. Virol. 2017, 96, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Korsten, K.; Adriaenssens, N.; Coenen, S.; Butler, C.; Ravanfar, B.; Rutter, H.; Allen, J.; Falsey, A.; Pirçon, J.Y.; Gruselle, O.; et al. Burden of Respiratory Syncytial Virus Infection in Community-Dwelling Older Adults in Europe (RESCEU): An International Prospective Cohort Study. Eur. Respir. J. 2021, 57, 2002688. [Google Scholar] [CrossRef]
- Ellis, S.E.; Coffey, C.S.; Mitchel, E.F.; Dittus, R.S.; Griffin, M.R. Influenza-and Respiratory Syncytial Virus-Associated Morbidity and Mortality in the Nursing Home Population. J. Am. Geriatr. Soc. 2003, 51, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Atamna, A.; Babich, T.; Froimovici, D.; Yahav, D.; Sorek, N.; Ben-Zvi, H.; Leibovici, L.; Bishara, J.; Avni, T. Morbidity and Mortality of Respiratory Syncytial Virus Infection in Hospitalized Adults: Comparison with Seasonal Influenza. Int. J. Infect. Dis. 2021, 103, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Surie, D.; Yuengling, K.A.; Decuir, J.; Zhu, Y.; Gaglani, M.; Ginde, A.A.; Keipp Talbot, H.; Casey, J.D.; Mohr, N.M.; Ghamande, S.; et al. Disease Severity of Respiratory Syncytial Virus Compared with COVID-19 and Influenza among Hospitalized Adults Aged ≥60 Years—IVY Network, 20 U.S. States, February 2022–May 2023. Morb. Mortal. Wkly. Rev. 2023, 72, 1083–1088. [Google Scholar] [CrossRef]
- Celante, H.; Oubaya, N.; Fourati, S.; Beaune, S.; Khellaf, M.; Casalino, E.; Ricard, J.D.; Vieillard-Baron, A.; Heming, N.; Mekontso Dessap, A.; et al. Prognosis of Hospitalised Adult Patients with Respiratory Syncytial Virus Infection: A Multicentre Retrospective Cohort Study. Clin. Microbiol. Infect. 2023, 29, e1–e943. [Google Scholar] [CrossRef]
- Blanco, I.; Diego, I.; Bueno, P.; Casas-Maldonado, F.; Miravitlles, M. Geographic Distribution of COPD Prevalence in the World Displayed by Geographic Information System Maps. Eur. Respir. J. 2019, 54, 1900610. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Ferraro, P.; Gualerzi, G.; Ranzieri, S.; Henry, B.M.; Said, Y.B.; Pyatigorskaya, N.V.; Nevolina, E.; Wu, J.; Bragazzi, N.L.; et al. Point-of-Care Diagnostic Tests for Detecting SARS-CoV-2 Antibodies: A Systematic Review and Meta-Analysis of Real-World Data. J. Clin. Med. 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Zaniboni, A.; Satta, E.; Ranzieri, S.; Marchesi, F. Potential Use of Exhaled Breath Condensate for Diagnosis OfSARS-CoV-2 Infections: A Systematic Review AndMeta-Analysis. Diagnostics 2022, 12, 2245. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Vila, J.; Hansen, G.; Manissero, D.; Pareja, J.; Rao, S.N.; Visseaux, B. Systematic Review on the Association between Respiratory Virus Real-Time PCR Cycle Threshold Values and Clinical Presentation or Outcomes. J. Antimicrob. Chemother. 2021, 76, III33–III49. [Google Scholar] [CrossRef] [PubMed]
- Wishaupt, J.O.; van der Ploeg, T.; Smeets, L.C.; de Groot, R.; Versteegh, F.G.A.; Hartwig, N.G. Pitfalls in Interpretation of CT-Values of RT-PCR in Children with Acute Respiratory Tract Infections. J. Clin. Virol. 2017, 90, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Onwuchekwa, C.; Moreo, L.M.; Menon, S.; Machado, B.; Curcio, D.; Kalina, W.; Atwell, J.E.; Gessner, B.D.; Siapka, M.; Agarwal, N.; et al. Underascertainment of Respiratory Syncytial Virus Infection in Adults Due to Diagnostic Testing Limitations: A Systematic Literature Review and Meta-Analysis. J. Infect. Dis. 2023, 228, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Boytchev, H. Maternal RSV Vaccine: Further Analysis Is Urged on Preterm Births. BMJ 2023, 381, 1021. [Google Scholar] [CrossRef] [PubMed]
- Boytchev, H. Concerns over Informed Consent for Pregnant Women in Pfizer’s RSV Vaccine Trial. BMJ 2023, 383, 2620. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J. Maternal RSV Vaccine—Weighing Benefits and Risk. N. Engl. J. Med. 2024, 390, 1050–1051. [Google Scholar] [CrossRef]
- Baber, J.; Arya, M.; Moodley, Y.; Jaques, A.; Jiang, Q.; Swanson, K.A.; Cooper, D.; Maddur, M.S.; Loschko, J.; Gurtman, A.; et al. A Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine with and without Adjuvant in Healthy Older Adults. J. Infect. Dis. 2022, 226, 2054–2063. [Google Scholar] [CrossRef]
- Chen, G.L.; Mithani, R.; Kapoor, A.; Lu, S.; El Asmar, L.; Panozzo, C.A.; Shaw, C.A.; Stoszek, S.K.; August, A. 234. Safety and Immunogenicity of MRNA-1345, an MRNA-Based RSV Vaccine in Younger and Older Adult Cohorts: Results from a Phase 1, Randomized Clinical Trial. Open Forum Infect. Dis. 2022, 9, S164–S165. [Google Scholar] [CrossRef]



| Study | Vaccine | Study Period (from … to) | Countries | RCT | Season | Vaccinated (N.) | Placebo (N.) | ROB Assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | |||||||||
| Studies reporting on the first season after the delivery of RSV vaccine | ||||||||||||||
| Ison et al. [48] FP | RSVPreF3 | Subunit, PreF, Adj, MV (A) | 25/05/2021 30/09/2023 | 17 | Phase 3 NCT04886596 | 1 + 2 | 12,470 | 12,503 |  |  |  |  |  |  |
| Feldman et al. [80] FP | RSVPreF3 | Subunit, PreF, Adj, MV (A) | 25/05/2021 31/01/2022 | 17 | Phase 3 NCT04886596 | 1 | 12,466 | 12,494 |  |  |  |  |  |  |
| Papi et al. [81] FP | RSVPreF3 | Subunit, PreF, Adj, MV (A) | 25/05/2021 31/01/2022 | 17 | Phase 3 NCT04886596 | 1 | 12,467 | 12,499 |  |  |  |  |  |  |
| Walsh et al. [52] FP | RSVpreF | Subunit, PreF, BV (A + B) | 31/08/2021 14/07/2022 | 7 | Phase 3 NCT05035212 | 1 | 17,215 | 17,069 |  |  |  |  |  |  |
| Wilson et al. [51] FP | mRNA-1345 | mRNA; PreF; MV (A) | 17/11/2021 31/10/2022 | 22 | Phase 2/3 NCT05127434 | 1 | 17,734 | 17,679 |  |  |  |  |  |  |
| Falsey et al. [82] FP | Ad26.RSV.preF | Vector-based PreF; MV (A) | 05/08/2019 20/03/2020 | 1 | Phase 2b NCT03982199 | 1 | 2891 | 2891 |  |  |  |  |  |  |
| Falloon et al. [83] FP | MEDI7510 | Protein-based PostF; MV (A) | 30/09/2015 09/09/2016 | 7 | Phase 2 NCT02508194 | 1 | 949 | 951 |  |  |  |  |  |  |
| Follow-up studies | ||||||||||||||
| Ison et al. [48] FP | RSVPreF3 | Subunit, PreF, Adj, MV (A) | 25/05/2021 30/09/2023 | 17 | Phase 3 NCT04886596 | Full season 2 | 4991 | 10,031 |  |  |  |  |  |  |
| Walsh et al. [61] AR | RSVpreF | Subunit, PreF, BV (A + B) | 31/08/2021 undefined | 7 | Phase 3 NCT05035212 | Mid-season 2 | 10,027 | 9992 |  |  |  |  |  |  |
| Wilson et al. [85] AR | mRNA-1345 | mRNA; PreF; MV (A) | 17/11/2021 undefined | 22 | Phase 3 NCT04886596 | 12 months after delivery | 18,112 | 18,045 |  |  |  |  |  |  |
 = definitively low;
= definitively low;  = probably low;
= probably low;  = probably high.
= probably high.| Trial (Vaccine) | RSV Confirmation | Endpoint | Characteristics |
|---|---|---|---|
| NCT03982199 (Ad26.RSV.preF) | RT-qPCR | 1 | VE against first episode of LRTD defined as 3 or more symptoms of LRTD |
| 2 | VE against first episode of LRTD defined as 2 or more symptoms of LRTD | ||
| 3 | VE against first episode of LRTD defined as 1 or more symptoms of LRTD + systemic symptoms | ||
| NCT02508194 (MEDI7510) | RT-qPCR | 1 | VE against first episode of ARI plus ≥ 1 symptoms from any 2 or 3 locations |
| 2 | VE against first episode of LRTD defined as 2 or more symptoms of LRTD | ||
| NCT04886596 (RSVPreF3) | RT-qPCR | VE against first episode of LRTD defined as at least 1 sign + 1 further sign or symptom of LRTD OR VE against first episode of LRTD defined as at least 3 signs OR symptoms of LRTD | |
| NCT05035212 (RSVpreF) | RT-qPCR OR NAAT | 1 | VE against first episode of LRTD defined as at least 2 signs/symptoms of LRTD |
| 2 | VE against first episode of LRTD defined as at least 3 signs/symptoms of LRTD | ||
| NCT04886596 (mRNA-1345) | RT-qPCR | 1 | VE against first episode of LRTD defined as at least 2 signs/symptoms of LRTD |
| 2 | VE against first episode of LRTD defined as at least 3 signs/symptoms of LRTD |
| Variable | Studies | No. of Subjects (/Total, %) | No. of Placebo (/Total, %) | RR (95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 (95% CI) | τ2 (95% CI) | Q (p-Value) | |||||
| Demographic data | |||||||
| Age > 70/75 years | [48,51,52,80,81,82,83] | 19,316/51,055 (37.83%) | 19,216/50,876 (37.77%) | 1000 (0.984; 1.015) | 0.0% (0.0; 79.2) | 0.001 (0.001; 0.002) | 0.18 (0.996) |
| White ethnicity | [48,51,52,80,81,82,83] | 38,034/51,055 (74.50%) | 37,975/50,876 (74.64%) | 0.996 (0.990; 1.003) | 0.0% (0.0; 79.2) | 0.001 (0.001; 0.002) | 2.09 (0.300) |
| Male Gender | [48,51,52,80,81,82,83] | 25,386/51,055 (49.72%) | 25,056/50,876 (49.25%) | 1.024 (0.987; 1.061) | 71.2% (27.1; 88.6) | 0.001 (0.000; 0.056) | 13.89 (0.008) |
| Comorbidities | |||||||
| COPD | [48,51,52,82] | 3322/49,979 (6.65%) | 3379/50,148 (6.74%) | 0.986 (0.942; 1.033) | 0.0% (0.0; 84.7) | 0.001 (0.000; 0.024) | 0.38 (0.945) |
| Congestive heart disease | [48,51,52,82] | 802/49,979 (1.60%) | 815/50,148 (1.63%) | 0.986 (0.896; 1.086) | 0.0% (0.0; 84.7) | 0.001 (0.000; 0.015) | 0.38 (0.945) |
| Asthma | [48,52,82] | 3000/32,576 (9.21%) | 2871/32,463 (8.84%) | 1.049 (0.999; 1.101) | 0.0% (0.0; 89.6) | 0.001 (0.000; 0.014) | 0.52 (0.772) |
| Outcome | Studies | No. of Subjects (/Total, %) | No. of Placebo (/Total, %) | VE (95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 (95% CI) | τ2 (95% CI) | Q (p-Value) | |||||
| 1st season, overall | |||||||
| ARI | [48,51,52,82,83] | 110/50,954 (0.22%) | 293/50,781 (0.58%) | 59.88% (41.17; 72.64) | 61.0% (0.0; 85.2) | 0.120 (0.000; 2.127) | 10.15 (0.038) |
| LRTD (2 symptoms) | [51,52,82,83] | 45/38,485 (0.12%) | 139/38,283 (0.36%) | 63.66% (12.35; 84.93) | 82.5% (0.0; 93.2) | 0.675 (0.122; 11.471) | 17.14 (<0.001) |
| LRTD (3+ symptoms) | [48,51,52,82] | 23/50,047 (0.05%) | 124/49,884 (0.25%)) | 81.38% (70.94; 88.06) | 0.0% (0.0; 84.7) | 0.000 (0.001; 0.012) | 0.17 (0.982) |
| 1st season, RSV A | |||||||
| ARI | [51,52,83] | 32/35,694 (0.09%) | 79/35,482 (0.22%) | 51.17% (−16.28; 84.23) | 82.2% (45.3; 94.2) | 0.604 (0.062; 26.472) | 11.25 (0.004) |
| LRTD (3+ symptoms) | [51,52,81] | 4/47,253 (0.01%) | 26/47,079 (0.06%) | 83.76% (52.95; 94.40) | 0.0% (0.0; 89.6) | 0.000 (0.000; 13.311) | 0.60 (0.741) |
| 1st season, RSV B | |||||||
| ARI | [51,52,83] | 38/35,694 (0.11%) | 87/35,482 (0.25%) | 51.56% (−50.11; 85.37)) | 85.4% (57.1; 95.0) | 0.866 (0.129; 40.735) | 13.69 (0.001) |
| LRTD (3+ symptoms) | [51,52,81] | 8/47,253 (0.02%) | 43/47,079 (0.09%) | 80.72% (58.79; 90.98) | 0.0% (0.0; 89.6) | 0.000 (0.000; 10.341) | 0.64 (0.727) |
| 1st season, only adults aged ≥70/75 years | |||||||
| ARI | [51,52,82] | 18/13,627 (0.13%) | 69/13,546 (0.51%) | 72.31% (48.58; 85.09) | 22.1% (0.0; 91.9) | 0.079 (0.000; 10.349) | 2.57 (0.277) |
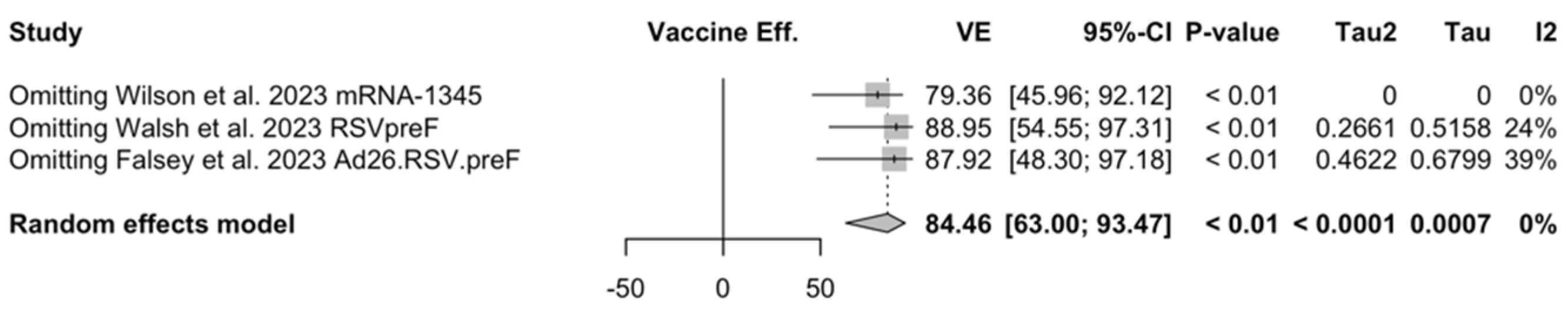
| LRTD (2 symptoms) | [51,52,82] | 6/13,627 (0.04%) | 46 13,546 (0.34%) | 84.46% (63.00; 93.47) | 0.0% (0.0; 89.6) | 0.000 (0.000; 29.114) | 1.79 (0.409) |
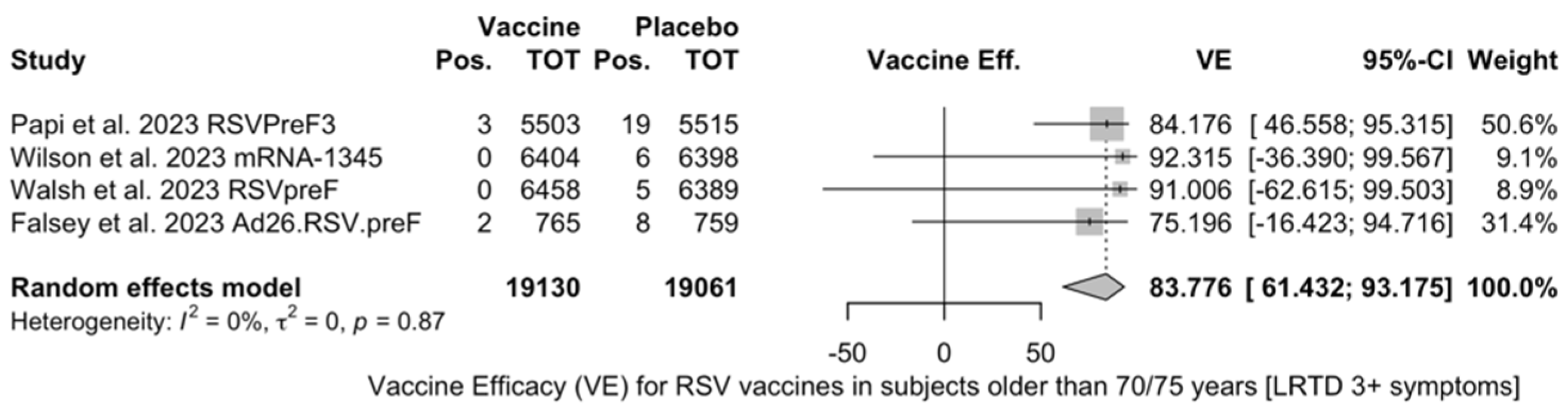
| LRTD (3+ symptoms) | [51,52,81,82] | 5/19,130 (0.03%) | 38/19,061 (0.20%) | 83.78%) (61.43; 93.18) | 0.0% (0.0; 84.7) | 0.000 (0.000; 2.732) | 0.71 (0.871) |
| Follow-up studies | |||||||
| ARI | [48,61,85] | 190/33,130 (0.57%) | 451/38,068 (1.18%) | 46.64% (35.94; 55.55) | 1.8% (0.0; 89.9) | 0.003 (0.000; 0.788) | 2.04 (0.361) |
| LRTD (3+ symptoms) | [48,61,85] | 42/33,130 (0.13%) | 156/38,068 (0.41%) | 61.15% (45.29; 72.40) | 0.0% (0.0; 89.6) | 0.003 (0.000; 5.411) | 1.19 (0.553) |
| Follow-up studies, RSV A | |||||||
| LRTD (3+ symptoms) | [48,61,85] | 13/33,130 (0.03%) | 53/38,068 (0.14%) | 70.55% (45.51; 84.08) | 0.0% (0.0; 89.6) | 0.000 (0.000; 17.178) | 1.18 (0.554) |
| Follow-up studies, RSV B | |||||||
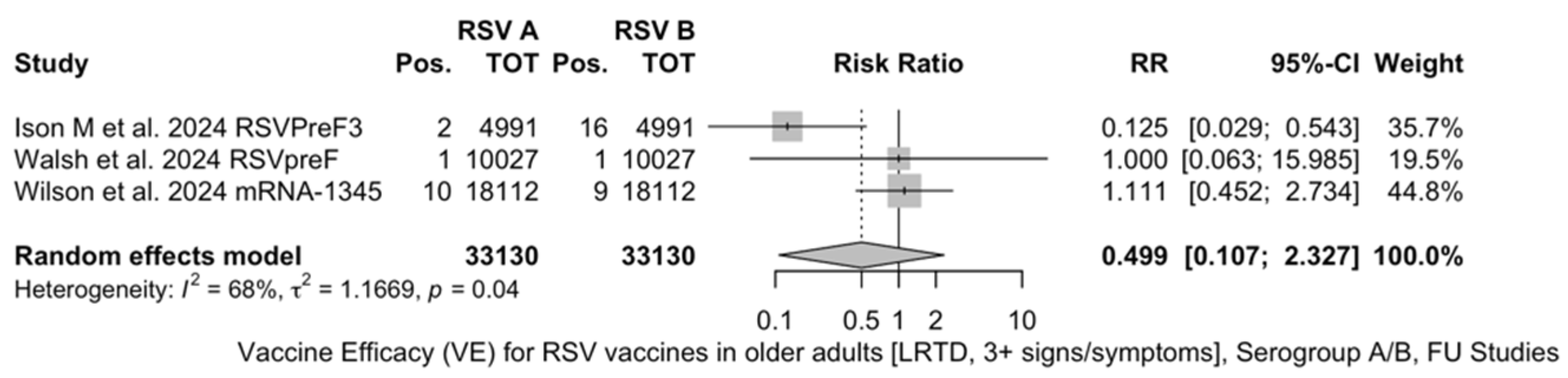
| LRTD (3+ symptoms) | [48,61,85] | 26/33,130 (0.08%) | 82/38,068 (0.22%) | 50.25% (22.62; 68.02) | 0.0% (0.0; 89.6) | 0.000 (0.000; 2.270) | 0.57 (0.750) |
| Outcome | Studies | Total Samples | RSV A (/Total, %) | RSV B (/Total, %) | RR (95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| I2 (95% CI) | τ2 (95% CI) | Q (p-Value) | ||||||
| 1st season, RSV A vs. RSV B | ||||||||
| ARI | [51,52,83] | 35,694 | 32 (0.09%) | 38 (0.11%) | 0.849 (0.529; 1.363) | 0.0% (0.0; 89.6) | 0.000 (0.000; 2.523) | 1.56 (0.458) |
| LRTD (3+ symptoms) | [51,52,81] | 47,253 | 4 (0.01%) | 8 (0.2%) | 0.505 (0.150; 1.706) | 0.0% (0.0; 89.6) | 0.000 (0.000; 7.469) | 0.31 (0.856) |
| Follow-up studies, RSV A vs. RSV B | ||||||||
| LRTD (3+ symptoms) | [48,51,52] | 33,130 | 13 (0.4%) | 26 (0.8%) | 0.499 (0.107; 2.327) | 0.0% (0.0; 89.6) | 1.167 (0.000; 59.266) | 6.29 (0.043) |
| Outcome | Studies | Subjects Aged ≥70/75 Years (/Total, %) | Subjects Aged <70/75 Years (/Total, %) | RR (95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 (95% CI) | τ2 (95% CI) | Q (p-Value) | |||||
| First Season | |||||||
| ARI | [51,52,82] | 18/13,627 (0.13%) | 43/23,951 (0.18%) | 0.800 (0.459; 1.392) | 0.0% (0.0; 89.6) | 0.000 (0.000; 6.721) | 1.40 (0.496) |
| LRTD (2 symptoms) | [51,52,82] | 6/13,627 (0.04%) | 24/23,951 (0.10%) | 0.522 (0.211; 1.291) | 0.0% (0.0; 89.6) | 0.000 (0.000; 14.509) | 0.84 (0.657) |
| LRTD (3+ symptoms) | [51,52,81,82] | 5/19,130 (0.03%) | 13/30,914 (0.04%) | 0.819 (0.304; 2.207) | 0.0% (0.0; 84.7) | 0.000 (0.000; 7.504) | 1.30 (0.729) |
| Outcome | Studies |
Follow-Up (/Total, %) |
Primary Season (/Total, %) | RR (95% CI) | Heterogeneity | Outcome | Studies |
|---|---|---|---|---|---|---|---|
| I2 (95% CI) | τ2 (95% CI) | Q (p-Value) | |||||
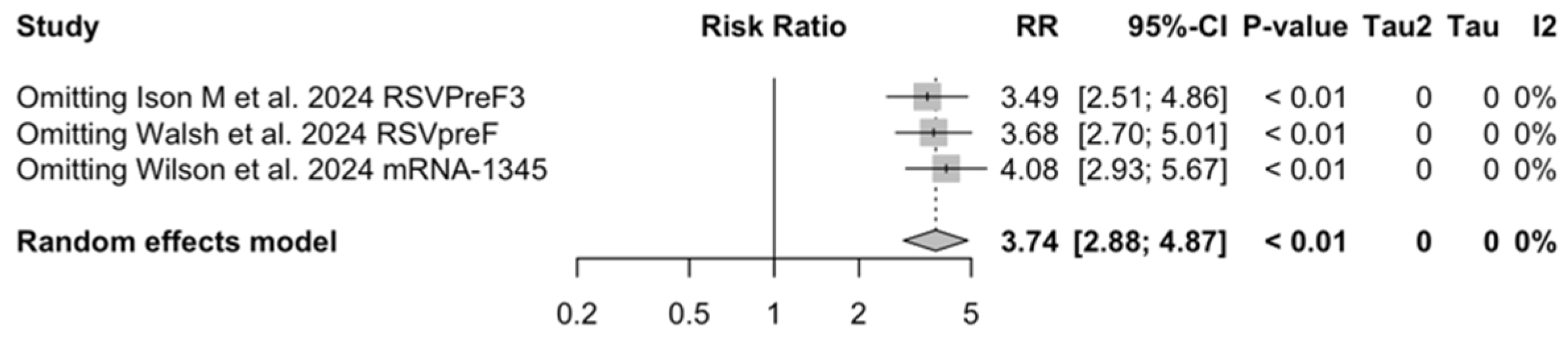
| ARI | [48,61,85] | 190/33,130 (0.57%) | 80/47,256 (0.17%) | 3.740 (2.875; 4.866) | 0.0% (0.0; 89.6) | 0.000 (0.000; 0.731) | 0.79 (0.675) |
| LRTD (3+ symptoms) | [48,61,85] | 42/33,130 (0.13%) | 17/47,256 (0.04%) | 4.326 (2.415; 7.748) | 0.0% (0.0; 89.6) | 0.000 (0.000; 6.872) | 0.65 (0.721) |
| Settings | Pathogen | t | df | p-Value | Bias (SE) |
|---|---|---|---|---|---|
| First season | ARI | 1.46 | 3 | 0.241 | 4.819 (3.303) |
| LRTD 2 symptoms | 2.09 | 2 | 0.171 | 36.073 (17.219) | |
| LRTD 3+ symptoms | −1.70 | 2 | 0.232 | −0.494 (0.291) | |
| Follow-up | ARI | 1.66 | 1 | 0.345 | 5.566 (3.348) |
| LRTD 3+ symptoms | −2.76 | 1 | 0.222 | −1.774 (0.644) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Cascio, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Giuri, P.G.; Gori, D.; Manzoni, P. Efficacy of Respiratory Syncytial Virus Vaccination to Prevent Lower Respiratory Tract Illness in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines 2024, 12, 500. https://doi.org/10.3390/vaccines12050500
Riccò M, Cascio A, Corrado S, Bottazzoli M, Marchesi F, Gili R, Giuri PG, Gori D, Manzoni P. Efficacy of Respiratory Syncytial Virus Vaccination to Prevent Lower Respiratory Tract Illness in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines. 2024; 12(5):500. https://doi.org/10.3390/vaccines12050500
Chicago/Turabian StyleRiccò, Matteo, Antonio Cascio, Silvia Corrado, Marco Bottazzoli, Federico Marchesi, Renata Gili, Pasquale Gianluca Giuri, Davide Gori, and Paolo Manzoni. 2024. "Efficacy of Respiratory Syncytial Virus Vaccination to Prevent Lower Respiratory Tract Illness in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Vaccines 12, no. 5: 500. https://doi.org/10.3390/vaccines12050500











