Radiation Exposure and Safety Considerations in Interventional Radiology: Comparison of a Twin Robotic X-ray System to a Conventional Angiography System
Abstract
:1. Introduction
2. Materials and Methods
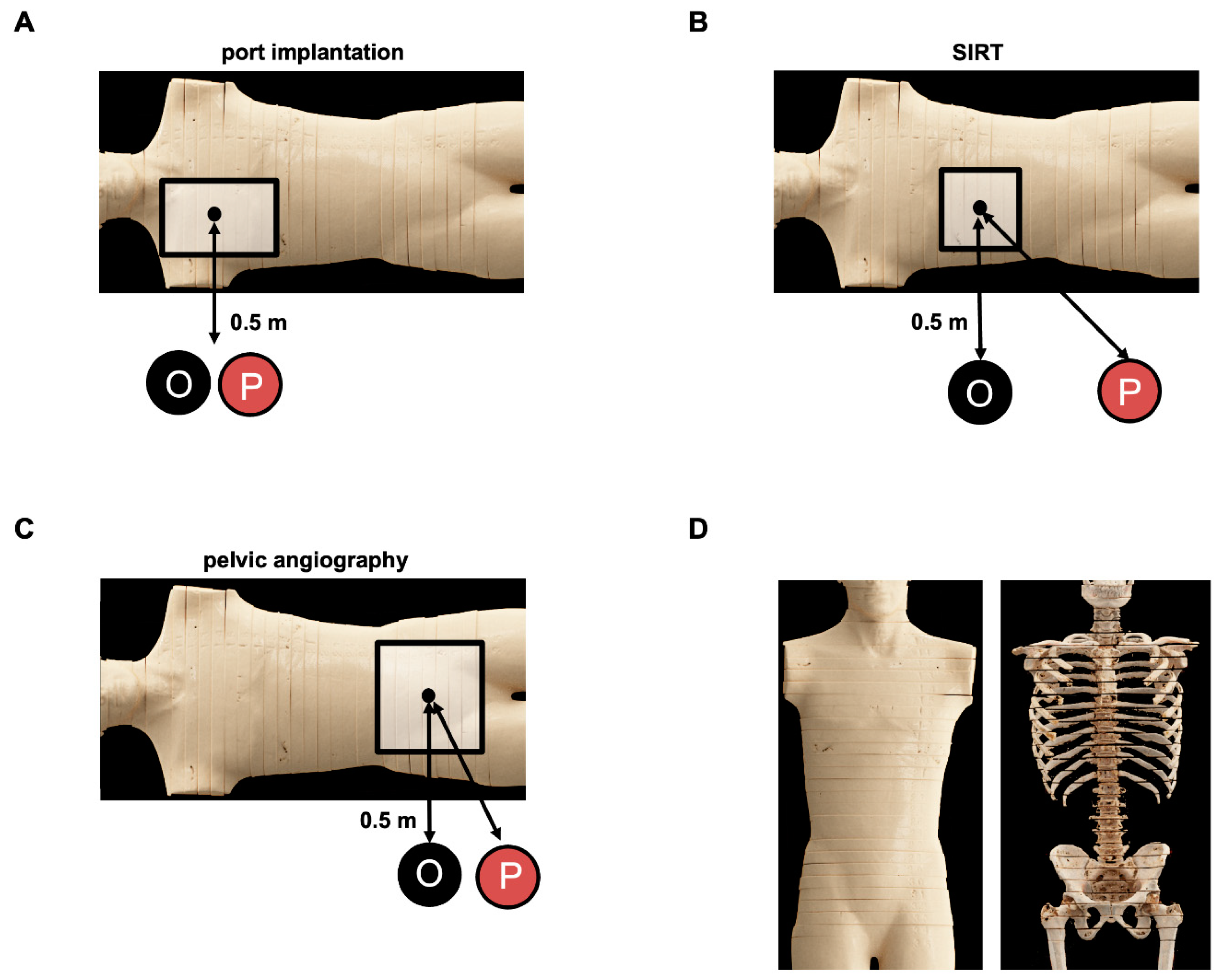
2.1. Phantom Study
2.2. Angiography Systems
2.3. Experimental Dose Exposure Measurements for Patients
2.4. Experimental Dose Exposure Measurements for Procedural Staff
2.5. Statistical Analysis
3. Results
3.1. Patient Radiation Exposure in Relation to the Anatomical Localization and Procedure
3.2. Radiation Exposure during Ported Venous Catheter Placements
3.3. Radiation Exposure during the SIRT Procedure
3.4. Radiation Exposure during Invasive Angiography of the Pelvis
3.5. Radiation Exposure for the Performing Proceduralist during SIRT and Invasive Angiography of the Pelvis Using the Multitom Rax System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koenig, T.R.; Wolff, D.; Mettler, F.A.; Wagner, L.K. Skin injuries from fluoroscopically guided procedures: Part 1, characteristics of radiation injury. Am. J. Roentgenol. 2001, 177, 3–11. [Google Scholar] [CrossRef]
- Koenig, T.R.; Mettler, F.A.; Wagner, L.K. Skin injuries from fluoroscopically guided procedures: Part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. Am. J. Roentgenol. 2001, 177, 13–20. [Google Scholar] [CrossRef]
- Rehani, M.; Ciraj-Bjelac, O.; Vañó, E.; Miller, D.; Walsh, S.; Giordano, B.; Persliden, J. ICRP Publication 117. Radiological protection in fluoroscopically guided procedures performed outside the imaging department. Ann. ICRP 2010, 40, 1–102. [Google Scholar] [CrossRef] [PubMed]
- Bryk, S.G.; Censullo, M.L.; Wagner, L.K.; Rossman, L.L.; Cohen, A.M. Endovascular and interventional procedures in obese patients: A review of procedural technique modifications and radiation management. J. Vasc. Interv. Radiol. 2006, 17, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Cole, W.C.; Haase, G.M. Radiation protection in humans: Extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br. J. Radiol. 2004, 77, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hirsch, J.A.; Rehani, M.M.; Ganguli, S.; Yang, K.; Liu, B. Radiation Effective Dose Above 100 mSv From Fluoroscopically Guided Intervention: Frequency and Patient Medical Condition. Am. J. Roentgenol. 2020, 215, 433–440. [Google Scholar] [CrossRef]
- Bartal, G.; Vano, E.; Paulo, G.; Miller, D.L. Management of patient and staff radiation dose in interventional radiology: Current concepts. Cardiovasc. Interv. Radiol. 2014, 37, 289–298. [Google Scholar] [CrossRef]
- Plastaras, C.; Appasamy, M.; Sayeed, Y.; McLaughlin, C.; Charles, J.; Joshi, A.; Macron, D.; Pukenas, B. Fluoroscopy procedure and equipment changes to reduce staff radiation exposure in the interventional spine suite. Pain Physician 2013, 6, E731–E738. [Google Scholar] [CrossRef]
- Power, S.; Mirza, M.; Thakorlal, A.; Ganai, B.; Gavagan, L.D.; Given, M.F.; Lee, M.J. Efficacy of a radiation absorbing shield in reducing dose to the interventionalist during peripheral endovascular procedures: A single centre pilot study. Cardiovasc. Interv. Radiol. 2015, 38, 573–578. [Google Scholar] [CrossRef]
- van Rooijen, B.D.; de Haan, M.W.; Das, M.; Arnoldussen, C.W.K.P.; de Graaf, R.; van Zwam, W.H.; Backes, W.H.; Jeukens, C.R.L.P.N. Efficacy of radiation safety glasses in interventional radiology. Cardiovasc. Interv. Radiol. 2014, 37, 1149–1155. [Google Scholar] [CrossRef]
- Bartal, G.; Vano, E.; Paulo, G. Get Protected! Recommendations for Staff in IR. Cardiovasc. Interv. Radiol. 2021, 44, 871–876. [Google Scholar] [CrossRef]
- Stecker, M.S.; Balter, S.; Towbin, R.B.; Miller, D.L.; Vañó, E.; Bartal, G.; Angle, J.F.; Chao, C.P.; Cohen, A.M.; Dixon, R.G.; et al. Guidelines for patient radiation dose management. J. Vasc. Interv. Radiol. 2009, 20 (Suppl. S7), S263–S273. [Google Scholar] [CrossRef]
- Elbakri, I.A. Estimation of dose-area product-to-effective dose conversion factors for neonatal radiography using PCXMC. Radiat. Prot. Dosim. 2014, 158, 43–50. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Aventín, D.L.; Gil, I.; González, D.M.L.; Pujol, R.M. Chronic scalp ulceration as a late complication of fluoroscopically guided cerebral aneurysm embolization. Dermatology 2012, 224, 198–203. [Google Scholar] [CrossRef]
- Balter, S.; Miller, D.L. Patient skin reactions from interventional fluoroscopy procedures. Am. J. Roentgenol. 2014, 202, W335–W342. [Google Scholar] [CrossRef]
- Dauer, L.T.; Miller, D.L.; Schueler, B.; Silberzweig, J.; Balter, S.; Bartal, G.; Chambers, C.; Collins, J.D.; Damilakis, J.; Dixon, R.G.; et al. Occupational radiation protection of pregnant or potentially pregnant workers in IR: A joint guideline of the Society of Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 2015, 26, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Vañó, E.; Bartal, G.; Balter, S.; Dixon, R.; Padovani, R.; Schueler, B.; Cardella, J.F.; de Baère, T. Occupational radiation protection in interventional radiology: A joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc. Interv. Radiol. 2010, 33, 230–239. [Google Scholar] [CrossRef]






| Characteristics | FGI | DSA | ||
|---|---|---|---|---|
| Port Implantation | Artis Zeego | Multitom Rax | Artis Zeego | Multitom Rax |
| Tube voltage (kV) | 67.7 | 73 | 70 | 67.5 |
| Cu (mm) | 0.2 | 0.2 | 0 | 0 |
| Frames/second | 7.5 | 10 | final, single image | final, single image |
| Acquisition time for experimental dose exposure measurements for patients (minutes) | 1:16 | 1:16 | single image | single image |
| Acquisition time for experimental dose exposure measurements for height profiles (minutes) | 00:10 | 00:10 | 00:10 | 00:10 |
| SIRT | ||||
| Tube voltage (kV) | 67.7 | 73 | 68 | 67.5 |
| Cu (mm) | 0.2 | 0.2 | 0.6/0.3 | 0 |
| Frames/second | 7.5 | 10 | 2 | 2 |
| Acquisition time for experimental dose exposure measurement for patients (minutes) | 13: 42 | 13: 42 | 00:01 | 00:01 |
| Acquisition time for experimental dose exposure measurements for height profiles (minutes) | 00:10 | 00:10 | 00:10 | 00:10 |
| diagnostic pelvic angiography | ||||
| Tube voltage (kV) | 65 | 73 | 66.4 | 67.5 |
| Cu (mm) | 0.2 | 0.2 | 0.3 | 0 |
| Frames/second | 7.5 | 10 | 2 | 2 |
| Acquisition time for experimental dose exposure measurement for patients (minutes) | 4:30 | 4:30 | 01:00 | 01:00 |
| Acquisition time for experimental dose exposure measurements for height profiles (minutes) | 00:10 | 00:10 | 00:10 | 00:10 |
| Location | Artis Zeego Means +/− SD (mGy) | Multitom Rax Means +/− SD (mGy) | p Value | Cohen’s D |
|---|---|---|---|---|
| port implantation (FGI 1 min 16 s, DSA single image) | ||||
| thyroid gland | 0.5 ± 0.1 | 1.8 ± 0.8 | <0.001 | −2.311 |
| esophagus | 0.9 ± 0.4 | 0.8 ± 0.5 | 0.979 | 0.012 |
| spinal cord | 0.9 ± 0.3 | 0.6 ± 0.2 | 0.02 | 1.116 |
| heart | 0.5 ± 0.1 | 1.0 ± 0.2 | <0.001 | −3.030 |
| skin (entry dose) | 3.3 ± 0.7 | 4.0 ± 1.4 | 0.101 | −0.631 |
| skin (exit dose) | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.473 | −0.284 |
| depth–dose profile | 1.9 ± 1.0 | 2.2 ± 1.8 | 0.412 | −0.199 |
| angiography for SIRT (FGI 13 min 42 s, DSA 1 s) | ||||
| gallbladder | 14.6 ± 1.6 | 12.8 ± 3.5 | 0.459 | 0.669 |
| spinal cord | 24 ± 18.3 | 2.1 ± 0.5 | 0.107 | 1.695 |
| heart | 7.1 ± 1.0 | 43.1 ± 12.3 | <0.001 | −3.950 |
| skin (entry dose) | 71.2 ± 38 | 43.5 ± 27.1 | 0.004 | 0.867 |
| skin (exit dose) | 3.8 ± 1.2 | 2.2 ± 1.0 | <0.001 | 1.441 |
| depth–dose profile | 33.6 ± 28.7 | 22.1 ± 23.1 | 0.117 | 0.438 |
| diagnostic pelvic angiography (FGI 4 min 30 s, DSA 60 s) | ||||
| prostate | 13.1 ± 5.7 | 49.8 ± 14.4 | <0.001 | −3.353 |
| uterus | 13.9 ± 4.3 | 48.9 ± 14.6 | <0.001 | −3.256 |
| ovaries | 10.3 ± 2.8 | 47.5 ± 11 | <0.001 | −4.527 |
| urinary bladder | 5.6 ± 1.5 | 96 ± 27.8 | 0.005 | −4.600 |
| rectum | 20.2 ± 8.8 | 26.9 ± 12.4 | 0.01 | −0.624 |
| skin (entry dose) | 42 ± 25.9 | 216.6 ± 172.6 | <0.001 | −1.552 |
| skin (exit dose) | 1.5 ± 0.7 | 4.2 ± 1.5 | <0.001 | −2.195 |
| depth–dose profile | 18 ± 18.2 | 120.5 ± 144.1 | 0.002 | −0.987 |
| SIRT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | (1) No X-ray Protection | (2) Ceiling-Suspended Screen | (3) Ceiling-Suspended Screen + X-ray Protective Drapes | p Value (1) vs. (2) | % of Initial Dose (1) vs. (2) | p Value (1) vs. (3) | % of Initial Dose (1) vs. (3) | p Value (2) vs. (3) | % of Initial Dose (2) vs. (3) |
| FGI (means +/− SD (μSV)) | |||||||||
| eye level | 2.853 ± 0.070 | 0.032 ± 0.004 | 0.020 ± 0.001 | <0.001 | 1.1 | <0.001 | 0.1 | 1 | 62.9 |
| front of phantom | 3.544 ± 0.134 | 0.131 ± 0.001 | 0.058 ± 0.002 | <0.001 | 3.7 | <0.001 | 1.6 | 0.881 | 44.5 |
| table level | 1.46 ± 0.054 | 0.168 ± 0.010 | 0.084 ± 0.005 | <0.001 | 11.5 | <0.001 | 5.7 | 0.051 | 49.7 |
| DSA (means +/− SD (μSV)) | |||||||||
| eye level | 41.837 ± 0.720 | 0.238 ± 0.016 | 0.160 ± 0.008 | <0.001 | 0.6 | <0.001 | 0.4 | 1 | 67 |
| front of phantom | 61.03 ± 1.866 | 1.675 ± 0.053 | 0.621 ± 0.022 | <0.001 | 2.7 | <0.001 | 1.0 | 0.829 | 37.1 |
| table level | 20.83 ± 0.693 | 1.748 ± 0.027 | 0.895 ± 0.020 | <0.001 | 8.4 | <0.001 | 4.2 | 0.121 | 51.2 |
| diagnostic pelvic angiography | |||||||||
| Location | (1) no x-ray protection | (2) ceiling-suspended screen | N/A | p value (1) vs. (2) | % of initial dose (1) vs. (2) | ||||
| FGI (means +/− SD (μSV)) | |||||||||
| eye level | 4.911 ± 0.091 | 0.42 ± 0.008 | N/A | <0.001 | 8.6 | ||||
| front of phantom | 1.893 ± 0.004 | 0.489 ± 0.016 | N/A | <0.001 | 25.8 | ||||
| table level | 1.352 ± 0.017 | 0.689 ± 0.024 | N/A | <0.001 | 51 | ||||
| DSA (means +/− SD (μSV)) | |||||||||
| eye level | 84.82 ± 1.663 | 6.295 ± 0.119 | N/A | <0.001 | 7.4 | ||||
| front of phantom | 30.06 ± 1.125 | 6.935 ± 0.239 | N/A | <0.001 | 23.1 | ||||
| table level | 23.26 ± 6.178 | 9.2 ± 0.499 | N/A | <0.017 | 39.6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruff, C.; Partovi, S.; Strobel, I.; Kaleth, S.; Herz, K.; Nikolaou, K.; Levitin, A.; Kirksey, L.; Syha, R.; Artzner, C.; et al. Radiation Exposure and Safety Considerations in Interventional Radiology: Comparison of a Twin Robotic X-ray System to a Conventional Angiography System. J. Clin. Med. 2024, 13, 2732. https://doi.org/10.3390/jcm13102732
Ruff C, Partovi S, Strobel I, Kaleth S, Herz K, Nikolaou K, Levitin A, Kirksey L, Syha R, Artzner C, et al. Radiation Exposure and Safety Considerations in Interventional Radiology: Comparison of a Twin Robotic X-ray System to a Conventional Angiography System. Journal of Clinical Medicine. 2024; 13(10):2732. https://doi.org/10.3390/jcm13102732
Chicago/Turabian StyleRuff, Christer, Sasan Partovi, Isabella Strobel, Stella Kaleth, Klaus Herz, Konstantin Nikolaou, Abraham Levitin, Levester Kirksey, Roland Syha, Christoph Artzner, and et al. 2024. "Radiation Exposure and Safety Considerations in Interventional Radiology: Comparison of a Twin Robotic X-ray System to a Conventional Angiography System" Journal of Clinical Medicine 13, no. 10: 2732. https://doi.org/10.3390/jcm13102732





