Serum Plasminogen Activator Inhibitor-1, α 1-Acid Glycoprotein, C-Reactive Protein, and Platelet Factor 4 Levels—Promising Molecules That Can Complete the “Puzzle” of the Biochemical Milieu in Severe Burns: Preliminary Results of a Cohort Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Included in This Research
2.2. Inclusion and Exclusion Criteria for the Children with a Burn (Study Group)
2.2.1. Inclusion Criteria: Children with a Burn (Study Group)
- -
- Age below 18 years old;
- -
- Thermal burns involving at least 25% TBSA;
- -
- Patients admitted to the burn unit in less than 48 h from the moment of burn infliction;
- -
- The patient’s parents or legal guardian read, understood, and signed the informed consent that states their agreement for the enrolment of their child/children into the present study;
- -
- Patient agreement to be part of this study.
2.2.2. Exclusion Criteria: Children with a Burn (Study Group)
- -
- Pre-existing autoimmune health condition.
- -
- Local or systemic infection at the moment of admission into the burn unit;
- -
- Pre-existing oncologic condition;
- -
- Patients who have been receiving hormonal treatment;
- -
- Patients who have been receiving oncologic treatment;
- -
- Patients who have been receiving immunosuppressive therapy;
- -
- Refusal of the parents or legal guardians to enroll the patient into the present study;
- -
- Refusal of the patient to be included in this study.
2.3. Inclusion and Exclusion Criteria for the Control Group
2.3.1. Inclusion Criteria: Control Group
- -
- Age below 18 years old;
- -
- The individual agrees to be included in the control group;
- -
- The patient’s parents or legal guardians read, understood, and signed the informed consent that states their agreement for the enrolment of their child/children into the present study.
2.3.2. Exclusion Criteria: Control Group
- -
- Inflammatory systemic condition.
- -
- Autoimmune health condition;
- -
- Local or systemic infection;
- -
- Oncologic condition;
- -
- Individual under hormonal treatment, or oncologic treatment, or immunosuppressive therapy;
- -
- Individuals with oral health conditions (teeth, gums, mucosa);
- -
- Refusal of the parents or legal guardians to enroll the patient in the present study;
- -
- Refusal of the individual to be part of this study.
2.4. Sample Collection
2.4.1. Sample Collection in the Study Group
- -
- Forty-eight hours after the burn trauma (T1);
- -
- Ten days after the burn trauma (T2);
- -
- Twenty-one days after the burn trauma (T3).
2.4.2. Sample Collection in the Control Group
2.5. Sample Preservation
2.6. Sample Analyzing and Data Collecting Using Multiplex Technique
2.7. Data Analysis
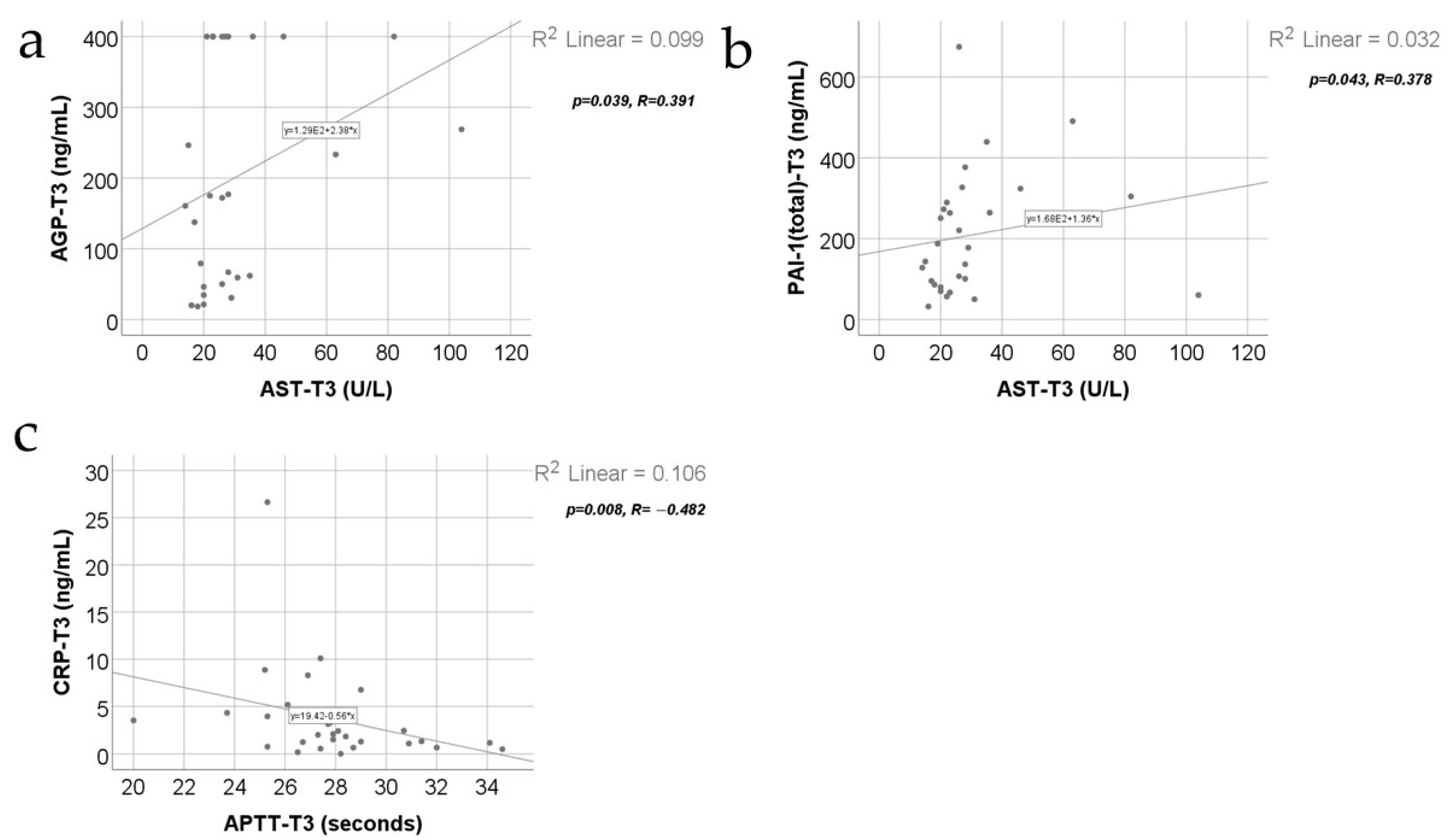
3. Results
- -
- Fibrinogen and triiodothyronine concentrations were significantly lower at T3 than at T2 (p = 0.015/p = 0.025) or T1 (p < 0.001/p = 0.004);
- -
- White blood cells (WBCs) were significantly increased at T2 compared to at T1 (p < 0.001) or T3 (p = 0.007);
- -
- Platelets (PLTs) and thyroxine were significantly increased at T2 (p < 0.001/p < 0.001) or T3 (p < 0.001/p < 0.001) compared to at T1;
- -
- TSH values were significantly higher at T3 compared to at T1 (p = 0.004).
4. Discussion
- The fact that it was not a multi-center study.
- The limited number of patients enrolled in this study because this study was conducted during the COVID-19 pandemic and immediately after the pandemic.
- The patients were studied only during the first 21 days post-burn as the post-discharge follow-up was very difficult to conduct: many patients would not come for scheduled check-ups.
- The differences of median age of the study group and of the control group.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herndon, D.N. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet 2016, 388, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.; Vlig, M.; Boekema, B.K.; Stoop, M.M.; Pijpe, A.; Van Zuijlen, P.P.; De Jong, E.; van Cranenbroek, B.; Joosten, I.; Koenen, H.J. Persistent systemic inflammation in patients with severe burn injury is accompanied by influx of immature neutrophils and shifts in T cell subsets and cytokine profiles. Front. Immunol. 2021, 11, 621222. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G. Postburn hypermetabolism: Past, present, and future. J. Burn Care Res. 2016, 37, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Lu, G.-Z.; Zhao, H.-S.; Liu, L.-J.; Jin, J.; Wu, Y.-F.; Wu, J.; Zhao, F.-L.; Liu, N.; Liu, W.-M. Clinical features and mortality-related factors of extensive burns among young adults: The Kunshan disaster experience. Ann. Transl. Med. 2020, 8, 1053. [Google Scholar] [CrossRef]
- Kelter, B.; Holavanahalli, R.; Suman, O.; Ryan, C.; Schneider, J. Recognizing the long-term sequelae of burns as a chronic medical condition. Burns 2020, 46, 493–496. [Google Scholar] [CrossRef]
- Gruys, E.; Toussaint, M.; Niewold, T.; Koopmans, S. Acute phase reaction and acute phase proteins. J. Zhejiang Univ.-Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, C.; Wolf, S.D.; Bode, J.G. Acute-phase protein synthesis: A key feature of innate immune functions of the liver. Biol. Chem. 2021, 402, 1129–1145. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Finnerty, C.C.; Kulp, G.A.; Kraft, R.; Herndon, D.N. Can we use C-reactive protein levels to predict severe infection or sepsis in severely burned patients? Int. J. Burn. Trauma 2013, 3, 137. [Google Scholar]
- Song, J.; Ozhathil, D.K.; El Ayadi, A.; Golovko, G.; Wolf, S.E. C-reactive protein elevation is associated with increased morbidity and mortality in elderly burned patients. Burns 2023, 49, 806–812. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Dewez, J.E.; Nijman, R.G.; Fitchett, E.J.; Li, E.C.; Luu, Q.F.; Lynch, R.; Emonts, M.; de Groot, R.; van der Flier, M.; Philipsen, R. Adoption of C-reactive protein rapid tests for the management of acute childhood infections in hospitals in the Netherlands and England: A comparative health systems analysis. BMC Health Serv. Res. 2024, 24, 351. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Kontakiotis, T.; Lazaridis, L.; Tsotsolis, N.; Koumis, J.; Kyriazis, G.; Bitzani, M. Inflammatory markers in patients with severe burn injury: What is the best indicator of sepsis? Burns 2007, 33, 189–194. [Google Scholar] [CrossRef]
- Khera, A.; McGuire, D.K.; Murphy, S.A.; Stanek, H.G.; Das, S.R.; Vongpatanasin, W.; Wians, F.H.; Grundy, S.M.; de Lemos, J.A. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 2005, 46, 464–469. [Google Scholar] [CrossRef]
- Ball, R.L.; Keyloun, J.W.; Brummel-Ziedins, K.; Orfeo, T.; Palmieri, T.L.; Johnson, L.S.; Moffatt, L.T.; Pusateri, A.E.; Shupp, J.W. Burn-induced coagulopathies: A comprehensive review. Shock 2020, 54, 154. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef] [PubMed]
- Glas, G.; Levi, M.; Schultz, M. Coagulopathy and its management in patients with severe burns. J. Thromb. Haemost. 2016, 14, 865–874. [Google Scholar] [CrossRef]
- Condron, M.; Rowell, S.; Dewey, E.; Anderson, T.; Lealiiee, L.; Farrell, D.; Hinson, H. The procoagulant molecule plasminogen activator inhibitor-1 is associated with injury severity and shock in patients with and without traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.C.; Chang, L.-Y.E.; Lemaire, S.M.; Burris, A.; Purdue, G.F.; Hunt, J.L.; Arnoldo, B.D.; Horton, J.W. Epistatic interactions are critical to gene-association studies: PAI-1 and risk for mortality after burn injury. J. Burn Care Res. 2008, 29, 168–175. [Google Scholar] [CrossRef]
- Woźnica-Niesobska, E.; Leśnik, P.; Janc, J.; Zalewska, M.; Łysenko, L. The Role of Plasminogen Activator Inhibitor 1 in Predicting Sepsis-Associated Liver Dysfunction: An Observational Study. Int. J. Environ. Res. Public Health 2023, 20, 4846. [Google Scholar] [CrossRef]
- Levine, J.A.; Oleaga, C.; Eren, M.; Amaral, A.P.; Shang, M.; Lux, E.; Khan, S.S.; Shah, S.J.; Omura, Y.; Pamir, N. Role of PAI-1 in hepatic steatosis and dyslipidemia. Sci. Rep. 2021, 11, 430. [Google Scholar] [CrossRef]
- Rivas, G.; Hummer-Bair, B.; Bezinover, D.; Kadry, Z.; Stine, J. Plasminogen activator inhibitor is significantly elevated in liver transplant recipients with decompensated NASH cirrhosis. BMJ Open Gastroenterol. 2021, 8, e000683. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, R.; Kaji, K.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Kitade, M.; Takaya, H.; Aihara, Y.; Douhara, A.; Asada, K. Novel oral plasminogen activator inhibitor-1 inhibitor TM5275 attenuates hepatic fibrosis under metabolic syndrome via suppression of activated hepatic stellate cells in rats. Mol. Med. Rep. 2020, 22, 2948–2956. [Google Scholar] [CrossRef]
- Huang, Z.; Ung, T. Effect of alpha-1-acid glycoprotein binding on pharmacokinetics and pharmacodynamics. Curr. Drug Metab. 2013, 14, 226–238. [Google Scholar] [PubMed]
- Abbas, M.; Watson, D.; Shah, S.I.; Anwar, M.S.; Alossaimi, M.A.; Ming, L. Determination of α1-Acid Glycoprotein (AGP) concentration by HPLC in patients following local infiltration analgesia for primary total hip arthroplasty and its relation with ropivacaine (total and unbound). Front. Pharmacol. 2023, 14, 1145962. [Google Scholar] [CrossRef] [PubMed]
- Čaval, T.; Lin, Y.-H.; Varkila, M.; Reiding, K.R.; Bonten, M.J.; Cremer, O.L.; Franc, V.; Heck, A.J. Glycoproteoform profiles of individual patients’ plasma alpha-1-antichymotrypsin are unique and extensively remodeled following a septic episode. Front. Immunol. 2021, 11, 608466. [Google Scholar] [CrossRef]
- Mundt, F.; Albrechtsen, N.J.W.; Mann, S.P.; Treit, P.; Ghodgaonkar-Steger, M.; O’Flaherty, M.; Raijmakers, R.; Vizcaíno, J.A.; Heck, A.J.; Mann, M. Foresight in clinical proteomics: Current status, ethical considerations, and future perspectives. Open Res. Eur. 2023, 3, 59. [Google Scholar] [CrossRef]
- Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: Highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Shipley, M.J.; Bell, J.A.; Canonico, M.; Elbaz, A.; Kivimäki, M. Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality. CMAJ 2017, 189, E384–E390. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Fujimura, R.; Hiramoto, Y.; Murata, R.; Nishida, K.; Bi, J.; Imafuku, T.; Komori, H.; Maeda, H.; Mukunoki, A. An acute phase protein α1-acid glycoprotein mitigates AKI and its progression to CKD through its anti-inflammatory action. Sci. Rep. 2021, 11, 7953. [Google Scholar] [CrossRef]
- Hsiao, S.-Y.; Lai, Y.-R.; Kung, C.-T.; Tsai, N.-W.; Su, C.-M.; Huang, C.-C.; Wang, H.-C.; Cheng, B.-C.; Su, Y.-J.; Lin, W.-C. α-1-acid glycoprotein concentration as an outcome predictor in adult patients with sepsis. BioMed Res. Int. 2019, 2019, 3174896. [Google Scholar] [CrossRef]
- Williams, J.P.; Weiser, M.R.; Pechet, T.T.; Kobzik, L.; Moore, F.D., Jr.; Hechtman, H.B. α1-Acid glycoprotein reduces local and remote injuries after intestinal ischemia in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 1997, 273, G1031–G1035. [Google Scholar] [CrossRef] [PubMed]
- Spiller, F.; Carlos, D.; Souto, F.O.; De Freitas, A.; Soares, F.S.; Vieira, S.M.; Paula, F.J.; Alves-Filho, J.C.; Cunha, F.Q. α1-Acid glycoprotein decreases neutrophil migration and increases susceptibility to sepsis in diabetic mice. Diabetes 2012, 61, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Lecchi, C. The immune functions of α1 acid glycoprotein. Curr. Protein Pept. Sci. 2019, 20, 505–524. [Google Scholar] [CrossRef]
- Maier, M.; Geiger, E.V.; Henrich, D.; Bendt, C.; Wutzler, S.; Lehnert, M.; Marzi, I. Platelet factor 4 is highly upregulated in dendritic cells after severe trauma. Mol. Med. 2009, 15, 384–391. [Google Scholar] [CrossRef]
- Jian, J.; Pang, Y.; Yan, H.H.; Min, Y.; Achyut, B.R.; Hollander, M.C.; Lin, P.C.; Liang, X.; Yang, L. Platelet factor 4 is produced by subsets of myeloid cells in premetastatic lung and inhibits tumor metastasis. Oncotarget 2017, 8, 27725. [Google Scholar] [CrossRef]
- Maier, M.; Wutzler, S.; Bauer, M.; Trendafilov, P.; Henrich, D.; Marzi, I. Altered gene expression patterns in dendritic cells after severe trauma: Implications for systemic inflammation and organ injury. Shock 2008, 30, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Greene, M.I.; Zhu, Z.; Zhang, H. Structural features and PF4 functions that occur in heparin-induced thrombocytopenia (HIT) complicated by COVID-19. Antibodies 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Warkentin, T.E. Platelet factor 4 triggers thrombo-inflammation by bridging innate and adaptive immunity. Int. J. Lab. Hematol. 2023, 45, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Roy, A.K.; Saha, D. Assessment of histopathological changes in the thyroid gland of fatal burn patients: A cross-sectional study. Burn. Open 2022, 6, 164–167. [Google Scholar] [CrossRef]
- Batista, A.S.; Zane, L.L.; Smith, L.M. Burn-induced myxedema crisis. Clin. Pract. Cases Emerg. Med. 2017, 1, 98. [Google Scholar] [CrossRef]
- Korytskyi, V. Peculiarities of structural reorganization of the thyroid gland vessels in dynamics after experimental thermal trauma. Rep. Vinnytsia Natl. Med. Univ. 2018, 22, 610–615. [Google Scholar] [CrossRef]
- Sen, S.; Palmieri, T.; Greenhalgh, D. Thyroid storm in a pediatric high voltage electrical burn injury. Burn. Open 2018, 2, 76–78. [Google Scholar] [CrossRef]
- Sofianos, C.; Redant, D.P.; Muganza, R.A.; Moore, R.L.; Ferrar, D.S. Thyroid crisis in a patient with burn injury. J. Burn Care Res. 2017, 38, e776–e780. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Selzman, C.H.; Cothren, C.; Sorensen, A.C.; Raeburn, C.D.; Harken, A.H. Diagnostic implications of C-reactive protein. Arch. Surg. 2003, 138, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Daniles, J.C.; Larson, D.L.; Abston, S.; Ritzmann, S.E. SERUM PROTEIN PROFILES IN THERMAL BURNS II. Protease Inhibitors, Complement Factors, and C-Reactive Protein. J. Trauma Acute Care Surg. 1974, 14, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Putra, O.; Saputro, I.; Nurrahman, N.; Herawati, E.; Dewi, L. Effects of empirical antibiotic administration on the level of C-Reactive protein and inflammatory markers in severe burn patients. Ann. Burn. Fire Disasters 2020, 33, 20. [Google Scholar]
- Ruiz-Castilla, M.; Roca, O.; Masclans, J.R.; Barret, J.P. Recent advances in biomarkers in severe burns. Shock Inj. Inflamm. Sepsis Lab. Clin. Approaches 2016, 45, 117–125. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef]
- Aoki, K.; Aikawa, N.; Sekine, K.; Yamazaki, M.; Mimura, T.; Urano, T.; Takada, A. Elevation of plasma free PAI-1 levels as an integrated endothelial response to severe burns. Burns 2001, 27, 569–575. [Google Scholar] [CrossRef]
- Galganski, L.A.; Greenhalgh, D.G.; Sen, S.; Palmieri, T.L. Randomized comparison of packed red blood cell-to-fresh frozen plasma transfusion ratio of 4: 1 vs 1: 1 during acute massive burn excision. J. Burn Care Res. 2017, 38, 194–201. [Google Scholar] [CrossRef]
- Lavrendieva, A.; Parlapani, A.; Thomareis, O.; Soulountsi, V.; Bitzani, M. Early coagulation alterations in intensive care unit burn patients. Crit. Care 2007, 11 (Suppl. S2), P365. [Google Scholar] [CrossRef]
- Sillen, M.; Miyata, T.; Vaughan, D.E.; Strelkov, S.V.; Declerck, P.J. Structural insight into the two-step mechanism of PAI-1 inhibition by small molecule TM5484. Int. J. Mol. Sci. 2021, 22, 1482. [Google Scholar] [CrossRef]
- Barbier, J.M.; Viana, M.V.; Pantet, O.; Alberio, L.; Berger, M.M. Blood coagulation alterations over the first 10 days after severe burn injury. Burn. Open 2022, 6, 10–18. [Google Scholar] [CrossRef]
- Xia, Z.-F.; Coolbaugh, M.I.; He, F.; Herndon, D.N.; Papaconstantinou, J. The effects of burn injury on the acute phase response. J. Trauma Acute Care Surg. 1992, 32, 245–251. [Google Scholar] [CrossRef]
- Gottschlich, M.; Baumer, T.; Jenkins, M.; Khoury, J.; Warden, G. The prognostic value of nutritional and inflammatory indices in patients with burns. J. Burn Care Rehabil. 1992, 13, 105–113. [Google Scholar] [PubMed]
- Zimmermann-Belsing, T.; Rasmussen, Å.; Feldt-Rasmussen, U.; Bøg-Hansen, T. The influence of alpha1-acid glycoprotein (orosomucoid) and its glycoforms on the function of human thyrocytes and CHO cells transfected with the human TSH receptor. Mol. Cell. Endocrinol. 2002, 188, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W.; Landgrafe, G.; Fotiadou, E.H. TSH and thyrotropic agonists: Key actors in thyroid homeostasis. J. Thyroid Res. 2012, 2012, 351864. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Pereira, M.; Mendes, R.A. Systemic Inflammatory Disorders, Immunosuppressive Treatment and Increase Risk of Head and Neck Cancers—A Narrative Review of Potential Physiopathological and Biological Mechanisms. Cells 2023, 12, 2192. [Google Scholar] [CrossRef]
- Kowalska, M.A.; Rauova, L.; Poncz, M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb. Res. 2010, 125, 292–296. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Sun, M.-X.; Hua, M.-Q.; Zhang, H.-X.; Mu, G.-Y.; Zhou, S.; Wang, Z.; Xiang, Q.; Cui, Y.-M. New perspectives on the induction and acceleration of immune-associated thrombosis by PF4 and VWF. Front. Immunol. 2023, 14, 1098665. [Google Scholar] [CrossRef]
- Müller, K.A.; Chatterjee, M.; Rath, D.; Geisler, T. Platelets, inflammation and anti-inflammatory effects of antiplatelet drugs in ACS and CAD. Thromb. Haemost. 2015, 114, 498–518. [Google Scholar] [CrossRef]
- Horowitz, A.; Smith, L.; Encabo, K.; Tenggara, I.; Couthouis, J.; Gross, J.; Chan, J.; Luke, A.; Ventura, P.; Sucharov, J. Platelet factors attenuate inflammation and rescue cognition in ageing. Nature 2023, 620, 1071–1079. [Google Scholar]
- Phalane, K.G.; Kriel, M.; Loxton, A.G.; Menezes, A.; Stanley, K.; Van der Spuy, G.D.; Walzl, G.; Chegou, N.N. Differential expression of host biomarkers in saliva and serum samples from individuals with suspected pulmonary tuberculosis. Mediat. Inflamm. 2013, 2013, 981984. [Google Scholar] [CrossRef]
- Thombs, B.D.; Singh, V.A.; Milner, S.M. Children under 4 years are at greater risk of mortality following acute burn injury: Evidence from a national sample of 12,902 pediatric admissions. Shock 2006, 26, 348–352. [Google Scholar] [CrossRef] [PubMed]









| Group/Parameter | Study Group | Control Group | p |
|---|---|---|---|
| Number of patients (Nr., %) | 32 (60.4%) | 21 (39.6%) | - |
| Gender—male (Nr., %) | 22 (68.8%) | 9 (42.9%) | 0.089 * |
| Age (Median (IQR)) | 3 (2–10) | 14 (12–16) | <0.001 ** |
| Hospitalization period (Median (IQR)) | 35 (25–56) | - | - |
| % Burned body surface (Median (IQR)) | 35 (27–45) | - | - |
| Time from event to hospitalization (Median (IQR)) | 8 (4–9.5) | - | - |
| Burn injury mechanism (Nr., %) | |||
| Hot liquid | 15 (46.9%) | - | - |
| Flame | 13 (40.6%) | - | - |
| Electric arc | 4 (12.5%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badoiu, S.C.; Enescu, D.M.; Tatar, R.; Stanescu-Spinu, I.-I.; Miricescu, D.; Greabu, M.; Ionel, I.P.; Jinga, V. Serum Plasminogen Activator Inhibitor-1, α 1-Acid Glycoprotein, C-Reactive Protein, and Platelet Factor 4 Levels—Promising Molecules That Can Complete the “Puzzle” of the Biochemical Milieu in Severe Burns: Preliminary Results of a Cohort Prospective Study. J. Clin. Med. 2024, 13, 2794. https://doi.org/10.3390/jcm13102794
Badoiu SC, Enescu DM, Tatar R, Stanescu-Spinu I-I, Miricescu D, Greabu M, Ionel IP, Jinga V. Serum Plasminogen Activator Inhibitor-1, α 1-Acid Glycoprotein, C-Reactive Protein, and Platelet Factor 4 Levels—Promising Molecules That Can Complete the “Puzzle” of the Biochemical Milieu in Severe Burns: Preliminary Results of a Cohort Prospective Study. Journal of Clinical Medicine. 2024; 13(10):2794. https://doi.org/10.3390/jcm13102794
Chicago/Turabian StyleBadoiu, Silviu Constantin, Dan Mircea Enescu, Raluca Tatar, Iulia-Ioana Stanescu-Spinu, Daniela Miricescu, Maria Greabu, Ileana Paula Ionel, and Viorel Jinga. 2024. "Serum Plasminogen Activator Inhibitor-1, α 1-Acid Glycoprotein, C-Reactive Protein, and Platelet Factor 4 Levels—Promising Molecules That Can Complete the “Puzzle” of the Biochemical Milieu in Severe Burns: Preliminary Results of a Cohort Prospective Study" Journal of Clinical Medicine 13, no. 10: 2794. https://doi.org/10.3390/jcm13102794