Research Progress of Heavy-Metal-Free Quantum Dot Light-Emitting Diodes
Abstract
:1. Introduction
2. The Importance of Heavy-Metal-Free QDs
2.1. Hazards with Heavy-Metal-Containing QDs
2.1.1. Environmental Pollution
2.1.2. Human Health Risks
2.2. Main Representatives of Heavy-Metal-Free QDs
3. The Synthesis Methods of Heavy-Metal-Free Nanostructures
4. Overview of the Development in Heavy-Metal-Free QLEDs
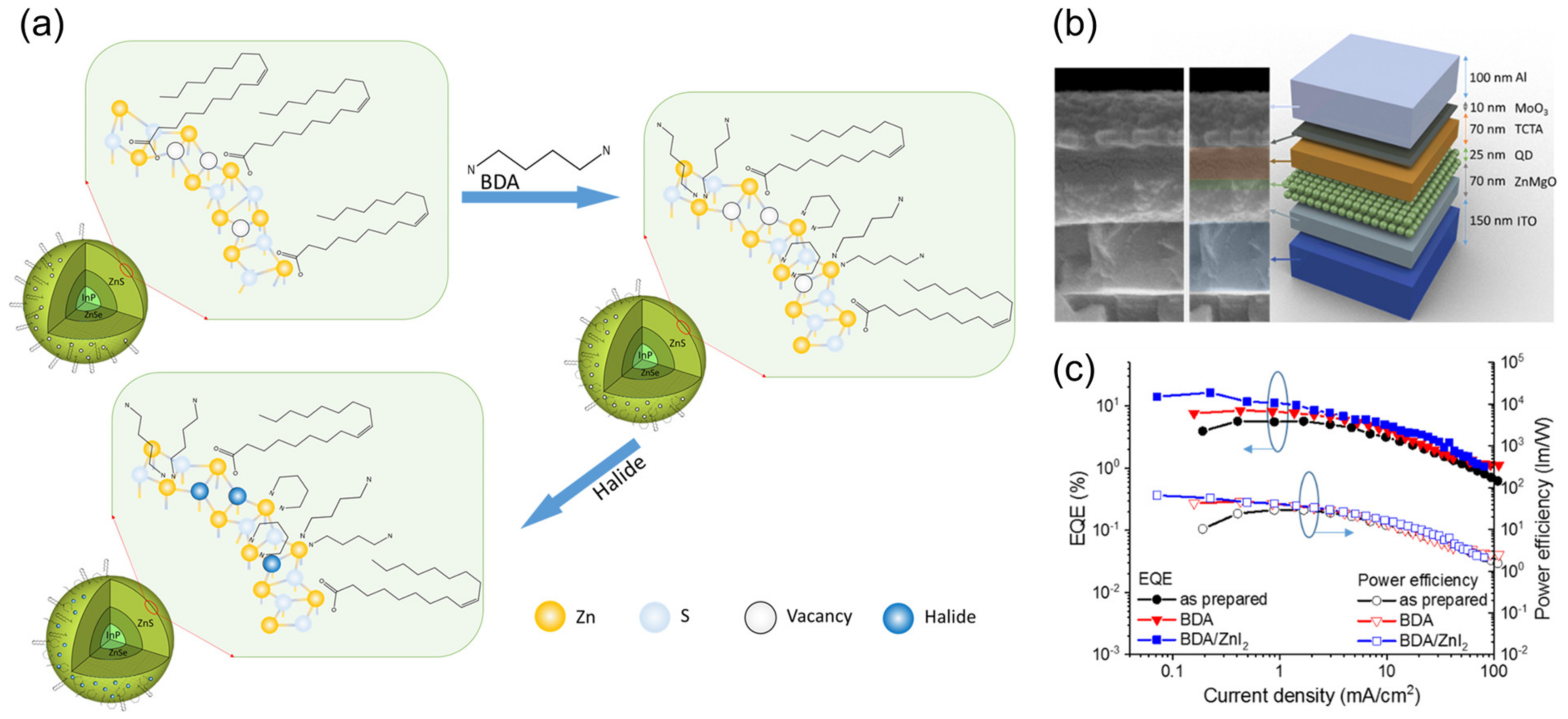
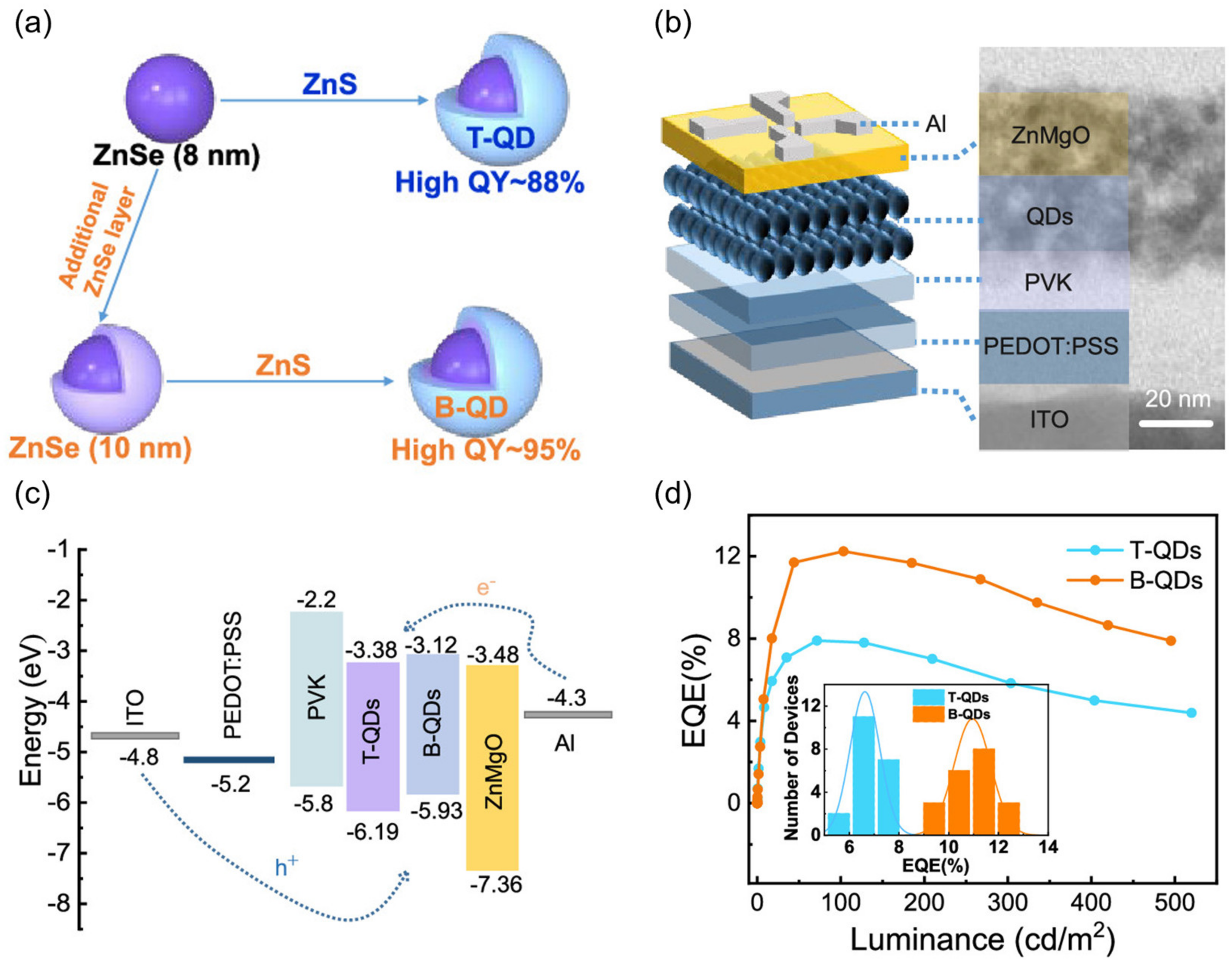
4.1. QD-Emitting Layer
4.2. Hole Transport Layer
4.3. Electron Transport Layer
| QDs | LED Structure 1 | EQE (%) | Turn-On Voltage (V) | Max. lum (cd/m2) | Peak CE 2 (cd/A) | Lifetime | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| InP/ZnSe/ZnS | ZnO/QDs/CBP/HAT-CN/Al | 6.6 | 2 | 1600 | 13.6 | 2018 | [84] | |
| InP/GaP/ZnS//ZnS | PEDOT:PSS/TFB/QDs/ZnO/Al | 6.3 | 2.98 | 2938 | 13.7 | 2019 | [85] | |
| InP/ZnSe/ZnS | PEDOT:PSS/TFB/QDs/ZnMgO/Al | 21.4 | 1.8 | 100,000 | 4300 h 3 | 2019 | [16] | |
| InP/ZnSe/ZnS | PEDOT:PSS/TFB/QDs/ZnMgO/Al | 22.2 | 1.87 | 110,000 | >32,000 h 4 | 2022 | [89] | |
| InP/ZnSe/ZnS | ZnMgO/QDs/TCTA/MoO3/Al | 16.3 | 2.2 | 12,646.3 | 57.5 | 2021 | [90] | |
| ZnSe/ZnS | PEDOT:PSS/PVK/QDs/ZnMgO/Al | 12.2 | 4.1 | 1055 | 1.7 | 237 h 5 | 2021 | [91] |
| ZnTeSe/ZnSe/ZnS | PEDOT:PSS/TFB/QD-Cl/ZnMgO/Al | 20.2 | 88,900 | 15,850 h 5 | 2020 | [7] | ||
| InP/ZnSe/ZnS | ZnMgO/QDs/DBTA/PCBBiF/HATCN/Al | 21.8 | 3.5 | 23,300 | 23.4 | 72,848 h 5 | 2020 | [94] |
| ZnSe/ZnS | PEDOT:PSS/TFB/C8-BTBT/QDs/ZnO:PVP/Ag | 7.23 | 7.73 | 2023 | [99] | |||
| InP | ZnMgO/QDs/TCTA/MoO3/Al | ~6.4 | 2.32 | 13,000 | 6.38 | 2021 | [103] | |
| InP/ZnSe/ZnS | NiZnO/QDs/DBTA/PCBBiF/HATCN/Al | 10.6 | 10.4 | 164,048 h 5 | 2023 | [104] | ||
| InP/ZnS | PEDOT:PSS/TFB/QDs/LZO@MgO/Al | 9.7 | 2.1 | 22,200 | 13.8 | 2023 | [105] | |
| InP | PEDOT:PSS/TFB/QDs/ZnO/Al | 5.42 | 2.2 | 1692 | 21.22 | 25 h 6 | 2021 | [106] |
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mashford, B.S.; Stevenson, M.; Popovic, Z.; Hamilton, C.; Zhou, Z.; Breen, C.; Steckel, J.; Bulovic, V.; Bawendi, M.; Coe-Sullivan, S.; et al. High-Efficiency Quantum-Dot Light-Emitting Devices with Enhanced Charge Injection. Nat. Photonics 2013, 7, 407–412. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.-H.; Ding, T.; Wang, N.; Chen, G.; Dang, C.; Demir, H.V.; Sun, X.W. High-Efficiency All-Inorganic Full-Colour Quantum Dot Light-Emitting Diodes. Nano Energy 2018, 46, 229–233. [Google Scholar] [CrossRef]
- Shen, H.; Gao, Q.; Zhang, Y.; Lin, Y.; Lin, Q.; Li, Z.; Chen, L.; Zeng, Z.; Li, X.; Jia, Y.; et al. Visible Quantum Dot Light-Emitting Diodes with Simultaneous High Brightness and Efficiency. Nat. Photonics 2019, 13, 192–197. [Google Scholar] [CrossRef]
- Sayevich, V.; Robinson, Z.L.; Kim, Y.; Kozlov, O.V.; Jung, H.; Nakotte, T.; Park, Y.S.; Klimov, V.I. Highly Versatile Near-Infrared Emitters Based on an Atomically Defined HgS Interlayer Embedded into A CdSe/CdS Quantum Dot. Nat. Nanotechnol. 2021, 16, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Gaponik, N.; Borchert, H.; Rogach, A.L.; Haase, M.; Weller, H. Etching of Colloidal InP Nanocrystals with Fluorides: Photochemical Nature of The Process Resulting in High Photoluminescence Efficiency. J. Phys. Chem. B 2002, 106, 12659–12663. [Google Scholar] [CrossRef]
- Li, L.S.; Pradhan, N.; Wang, Y.; Peng, X. High Quality ZnSe and ZnS Nanocrystals Formed by Activating Zinc Carboxylate Precursors. Nano Lett. 2004, 4, 2261–2264. [Google Scholar] [CrossRef]
- Kim, T.; Kim, K.H.; Kim, S.; Choi, S.M.; Jang, H.; Seo, H.K.; Lee, H.; Chung, D.Y.; Jang, E. Efficient and Stable Blue Quantum Dot Light-Emitting Diode. Nature 2020, 586, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Rutherford, M.; Peng, X. Formation of High-Quality I-III-VI Semiconductor Nanocrystals by Tuning Relative Reactivity of Cationic Precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef]
- Li, L.; Daou, T.J.; Texier, I.; Chi, T.T.K.; Liem, N.Q.; Reiss, P. Highly Luminescent CuInS2/ZnS Core/Shell Nanocrystals: Cadmium-Free Quantum Dots for In Vivo Imaging. Chem. Mater. 2009, 21, 2422–2429. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; An, X.; Wang, Z.; Yang, X.; Yu, M.; Zhang, R.; Sun, Z.; Wang, Q. Controlled Synthesis of Ag2Te@Ag2S Core-Shell Quantum Dots with Enhanced and Tunable Fluorescence in the Second Near-Infrared Window. Small 2020, 16, 2001003. [Google Scholar] [CrossRef]
- Xiong, Q.; Yang, J.; Ding, H.; Du, J.; Tang, X.; Shi, T.; Liu, Z.; Wu, D.; Lin, H.; Leng, Y. Low-Threshold Amplification of Spontaneous Emission from AgInS2 Quantum Dots. J. Mater. Chem. C 2020, 8, 8515–8520. [Google Scholar] [CrossRef]
- Pradhan, N.; Peng, X. Efficient and Color-Tunable Mn-Doped ZnSe Nanocrystal Emitters: Control of Optical Performance via Greener Synthetic Chemistry. J. Am. Chem. Soc. 2007, 129, 3339–3347. [Google Scholar] [CrossRef]
- Xie, R.; Peng, X. Synthesis of Cu-Doped InP Nanocrystals (d-dots) with ZnSe Diffusion Barrier as Efficient and Color-Tunable NIR Emitters. J. Am. Chem. Soc. 2009, 131, 10645–10651. [Google Scholar] [CrossRef]
- Zhang, W.; Lou, Q.; Ji, W.; Zhao, J.; Zhong, X. Color-Tunable Highly Bright Photoluminescence of Cadmium-Free Cu-Doped Zn–In–S Nanocrystals and Electroluminescence. Chem. Mater. 2014, 26, 1204–1212. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, J.; Zhao, J.; Yang, Z.; Shang, M.; Li, C.; Yang, W.; Fang, X. Robust and Stable Ratiometric Temperature Sensor Based on Zn-In-S Quantum Dots with Intrinsic Dual-Dopant Ion Emissions. Adv. Funct. Mater. 2016, 26, 7224–7233. [Google Scholar] [CrossRef]
- Won, Y.H.; Cho, O.; Kim, T.; Chung, D.Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly Efficient and Stable InP/ZnSe/ZnS Quantum Dot Light-Emitting Diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Manshian, B.B.; Aubert, T.; Himmelreich, U.; Demeester, J.; De Smedt, S.C.; Hens, Z.; Braeckmans, K. Cytotoxicity of Cadmium-Free Quantum Dots and Their Use in Cell Bioimaging. Chem. Res. Toxicol. 2014, 27, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, R.; Liu, J.; Zhang, B.; Wang, Y.; Liu, X.; Law, W.C.; Liu, L.; Ye, L.; Yong, K.T. Cytotoxicity Assessment of Functionalized CdSe, CdTe and InP Quantum Dots in Two Human Cancer Cell Models. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qiu, H.; Liu, Y.; Huang, C.; Sheng, J.; Cui, J.; Su, W.; Xiao, Q. Systematical Investigation of In Vitro Interaction of InP/ZnS Quantum Dots with Human Serum Albumin by Multi-spectroscopic Approach. Colloids Surf. B Biointerfaces 2016, 148, 165–172. [Google Scholar] [CrossRef]
- Horstmann, C.; Kim, K. Comparing Transcriptome Profiles of Saccharomyces Cerevisiae Cells Exposed to Cadmium Selenide/Zinc Sulfide and Indium Phosphide/Zinc Sulfide. Genes 2021, 12, 428. [Google Scholar] [CrossRef]
- Kupper, H. Lead Toxicity in Plants. Met. Ions. Life Sci. 2017, 17, 491–500. [Google Scholar]
- Li, Y.; Chen, L.; Liang, S.; Zhou, H.; Liu, Y.R.; Zhong, H.; Yang, Z. Looping Mercury Cycle in Global Environmental–Economic System Modeling. Environ. Sci. Technol. 2022, 56, 2861–2879. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.D.; Jacob, D.J.; Corbitt, E.S.; Mao, J.; Yang, X.; Talbot, R.; Slemr, F. Global Atmospheric Model for Mercury Including Oxidation by Bromine Atoms. Atmos. Chem. Phys. 2010, 10, 12037–12057. [Google Scholar] [CrossRef]
- Selin, N.E. Global Biogeochemical Cycling of Mercury: A Review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Meng, B.; Feng, X.; Qiu, G.; Liang, P.; Li, P.; Chen, C.; Shang, L. The Process of Methylmercury Accumulation in Rice (Oryza sativa L.). Environ. Sci. Technol. 2011, 45, 2711–2717. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, X.; Qiu, G.; Anderson, C.W.; Yao, H. Prediction of Methyl Mercury Uptake by Rice Plants (Oryza sativa L.) Using the Diffusive Gradient in Thin Films Technique. Environ. Sci. Technol. 2012, 46, 11013–11020. [Google Scholar] [CrossRef] [PubMed]
- Miklavcic, A.; Mazej, D.; Jacimovic, R.; Dizdarevio, T.; Horvat, M. Mercury in Food Items from the Idrija Mercury Mine Area. Environ. Res. 2013, 125, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Díaz-Fernández, L.; Urango-Cárdenas, I.; Araméndiz-Tatis, H.; Vergara-Flórez, V.; Bravo, A.G.; Díez, S. Transfer and Bioaccumulation of Mercury from Soil in Cowpea in Gold Mining Sites. Chemosphere 2020, 250, 126142. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Carmichael, N.G.; Backhouse, B.L.; Winder, C.; Lewis, P.D. Teratogenicity, Toxicity and Perinatal Effects of Cadmium. Hum. Toxicol. 1982, 1, 159–186. [Google Scholar] [CrossRef]
- Sugawara, N.; Sugawara, C. Effect of Cadmium, in Vivo Andin Vitro, on Intestinal Brush Border ALPase and ATPase. Bull. Environ. Contam. Toxicol. 1975, 14, 653–656. [Google Scholar] [CrossRef]
- Galeone, A.; Vecchio, G.; Malvindi, M.A.; Brunetti, V.; Cingolani, R.; Pompa, P.P. In Vivo Assessment of CdSe-ZnS Quantum Dots: Coating Dependent Bioaccumulation and Genotoxicity. Nanoscale 2012, 4, 6401–6407. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; He, M.; Chen, B.; Wu, Q.; Zhang, Z.; Pang, D.; Zhu, Y.; Hu, B. Cellular Uptake, Elimination and Toxicity of CdSe/ZnS Quantum Dots in HepG2 Cells. Biomaterials 2013, 34, 9545–9558. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, F.; Wei, H.; Yang, B. The effects of Composition and Surface Chemistry on the Toxicity of Quantum Dots. J. Mater. Chem. B 2013, 1, 6485–6494. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Maysinger, D.; Jain, M.; Roder, B.; Hackbarth, S.; Winnik, F.M. Long-Term Exposure to CdTe Quantum Dots Causes Functional Impairments in Live Cells. Langmuir 2007, 23, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.T.; Law, W.C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity Assessment of Quantum Dots: From Cellular to Primate Studies. Chem. Soc. Rev. 2013, 42, 1236–1250. [Google Scholar] [CrossRef]
- Buffard, A.; Dreyfuss, S.; Nadal, B.; Heuclin, H.; Xu, X.Z.; Patriarche, G.; Mezailles, N.; Dubertret, B. Mechanistic Insight and Optimization of InP Nanocrystals Synthesized with Aminophosphines. Chem. Mater. 2016, 28, 5925–5934. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Dai, X.; Yao, Z.; Lv, L.; Jin, Y.; Peng, X. Stoichiometry-Controlled InP-Based Quantum Dots: Synthesis, Photoluminescence, and Electroluminescence. J. Am. Chem. Soc. 2019, 141, 6448–6452. [Google Scholar] [CrossRef]
- Shan, X.; Li, B.; Ji, B. Synthesis of Wurtzite in and Ga Phosphide Quantum Dots Through Cation Exchange Reactions. Chem. Mater. 2021, 33, 5223–5232. [Google Scholar] [CrossRef]
- Pradhan, N.; Goorskey, D.; Thessing, J.; Peng, X. An Alternative of CdSe Nanocrystal Emitters: Pure and Tunable Impurity Emissions in ZnSe Nanocrystals. J. Am. Chem. Soc. 2005, 127, 17586–17587. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Hong, A.; Kim, J.H.; Yang, H.; Lee, K.; Jang, H.S. Highly Bright Yellow-Green-Emitting CuInS2 Colloidal Quantum Dots with Core/Shell/Shell Architecture for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 6764–6771. [Google Scholar] [CrossRef]
- Du, Y.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q. Near-Infrared Photoluminescent Ag2S Quantum Dots from a Single Source Precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef]
- Yang, H.; Li, R.; Zhang, Y.; Yu, M.; Wang, Z.; Liu, X.; You, W.; Tu, D.; Sun, Z.; Zhang, R.; et al. Colloidal Alloyed Quantum Dots with Enhanced Photoluminescence Quantum Yield in the NIR-II Window. J. Am. Chem. Soc. 2021, 143, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ma, R.; Zhang, W.; Hua, J.; Meng, X.; Zhong, X.; Zhang, J.; Zhao, J.; Li, H. Dual Emissive Manganese and Copper Co-Doped Zn-In-S Quantum Dots as a Single Color-Converter for High Color Rendering White-Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 8659–8666. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Yoon, H.C.; Yoo, H.; Oh, J.H.; Yang, H.; Do, Y.R. Highly Efficient Green Zn-Ag-In-S/Zn-In-S/ZnS QDs by a Strong Exothermic Reaction for Down-Converted Green and Tripackage White LEDs. Adv. Funct. Mater. 2017, 27, 1602638. [Google Scholar] [CrossRef]
- Harris, D.K.; Bawendi, M.G. Improved Precursor Chemistry for the Synthesis of III-V Quantum Dots. J. Am. Chem. Soc. 2012, 134, 20211–20213. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, J.; Leppert, V.J.; Lam, S.-T.; Gibson, G.A.; Nauka, K.; Yang, C.C.; Zhou, Z.-L. Rapid Oxidation of InP Nanoparticles in Air. Solid State Commun. 2007, 141, 624–627. [Google Scholar] [CrossRef]
- Chen, B.; Li, D.; Wang, F. InP Quantum Dots: Synthesis and Lighting Applications. Small 2020, 16, 2002454. [Google Scholar] [CrossRef]
- Fang, X.; Zhai, T.; Gautam, U.K.; Li, L.; Wu, L.; Bando, Y.; Golberg, D. ZnS Nanostructures: From Synthesis to Applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Reiss, P.; Protiere, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Van der Stam, W.; Berends, A.C.; De Mello Donega, C. Prospects of Colloidal Copper Chalcogenide Nanocrystals. ChemPhysChem 2016, 17, 559–581. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibanez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound Copper Chalcogenide Nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef]
- Knowles, K.E.; Hartstein, K.H.; Kilburn, T.B.; Marchioro, A.; Nelson, H.D.; Whitham, P.J.; Gamelin, D.R. Luminescent Colloidal Semiconductor Nanocrystals Containing Copper: Synthesis, Photophysics, and Applications. Chem. Rev. 2016, 116, 10820–10851. [Google Scholar] [CrossRef] [PubMed]
- Regulacio, M.D.; Han, M.Y. Multinary I-III-VI2 and I2-II-IV-VI4 Semiconductor Nanostructures for Photocatalytic Applications. Acc. Chem. Res. 2016, 49, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Cai, W. Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Oner, I.; Varlikli, C.; Ozsoy, C.; Zafer, C. Efficiency Enhancement in a Single Emission Layer Yellow Organic Light Emitting Device: Contribution of CIS/ZnS Quantum Dot. Thin Solid Film. 2015, 589, 153–160. [Google Scholar] [CrossRef]
- Guo, W.; Sun, X.; Jacobson, O.; Yan, X.; Min, K.; Srivatsan, A.; Niu, G.; Kiesewetter, D.O.; Chang, J.; Chen, X. Intrinsically Radioactive [64Cu]CuInS/ZnS Quantum Dots for PET and Optical Imaging: Improved Radiochemical Stability and Controllable Cerenkov Luminescence. ACS Nano 2015, 9, 488–495. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, Z. Controllable Growth of Semiconductor Heterostructures Mediated by Bifunctional Ag2S Nanocrystals as Catalyst or Source-Host. J. Am. Chem. Soc. 2011, 133, 148–157. [Google Scholar] [CrossRef]
- Pourahmad, A. Ag2S Nanoparticle Encapsulated in Mesoporous Material Nanoparticles and Its Application for Photocatalytic Degradation of Dye in Aqueous Solution. Superlattices Microstruct. 2012, 52, 276–287. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.; Mao, S.; Wang, Y.; Shen, W.; Wang, W.; Huang, L.; Li, H.; Tang, J. Recent Progress in Synthetic Methods and Applications in Solar Cells of Ag2S Quantum Dots. Mater. Res. Bull. 2018, 106, 113–123. [Google Scholar] [CrossRef]
- Lu, C.; Chen, G.; Yu, B.; Cong, H. Recent Advances of Low Biological Toxicity Ag2S QDs for Biomedical Application. Adv. Eng. Mater. 2018, 20, 1700940. [Google Scholar] [CrossRef]
- Ding, C.; Huang, Y.; Shen, Z.; Chen, X. Synthesis and Bioapplications of Ag2S Quantum Dots with Near-Infrared Fluorescence. Adv. Mater. 2021, 33, 2007768. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhao, Z.; Wang, C.; Rao, H.; Zhao, B.; Liu, Z.; Bian, Z.; Huang, C. Lead-Free Tin-Based Perovskite Solar Cells: Strategies Toward High Performance. Solar RRL 2019, 3, 1900213. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G. Hybrid Germanium Iodide Perovskite Semiconductors: Active lone Pairs, Structural Distortions, Direct and Indirect Energy Gaps, and Strong Nonlinear Optical Properties. J. Am. Chem. Soc. 2015, 137, 6804–6819. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-Liquid-Solid Mechanism of Single Crystal Growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Trentler, T.J.; Hickman, K.M.; Goel, S.C.; Viano, A.M.; Gibbons, P.C.; Buhro, W.E. Solution-Liquid-Solid Growth of Crystalline III-V Semiconductors: An Analogy to Vapor-Liquid-Solid Growth. Science 1995, 270, 1791–1794. [Google Scholar] [CrossRef]
- Wang, F.; Buhro, W.E. Determination of the Rod–Wire Transition Length in Colloidal Indium Phosphide Quantum Rods. J. Am. Chem. Soc. 2007, 129, 14381–14387. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-Assembly of ZnO: From Nanodots to Nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191. [Google Scholar] [CrossRef]
- Yu, J.H.; Joo, J.; Park, H.M.; Baik, S.-I.; Kim, Y.W.; Kim, S.C.; Hyeon, T. Synthesis of Quantum-Sized Cubic ZnS Nanorods by the Oriented Attachment Mechanism. J. Am. Chem. Soc. 2005, 127, 5662–5670. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Ning, Y.; Li, M.; Zhang, H.; Yang, B. “One-pot” Synthesis and Shape Control of ZnSe Semiconductor Nanocrystals in Liquid Paraffin. J. Mater. Chem. 2010, 20, 4451–4458. [Google Scholar] [CrossRef]
- Goldberger, J.; He, R.; Zhang, Y.; Lee, S.; Yan, H.; Choi, H.-J.; Yang, P. Single-Crystal Gallium Nitride Nanotubes. Nature 2003, 422, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fuge, G.M.; Fox, N.A.; Riley, D.J.; Ashfold, M.N.R. Synthesis of Aligned Arrays of Ultrathin ZnO Nanotubes on a Si Wafer Coated with a Thin ZnO Film. Adv. Mater. 2005, 17, 2477–2481. [Google Scholar] [CrossRef]
- Shuai, X.M.; Shen, W.Z. A Facile Chemical Conversion Synthesis of ZnO/ZnS Core/Shell Nanorods and Diverse Metal Sulfide Nanotubes. J. Phys. Chem. C 2011, 115, 6415–6422. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, M.; Chen, D.; Chen, W.; Wang, F.; Anwar, S.J.; Saunders, M.; Rowles, M.R.; Liu, L.; Liu, S.; et al. Why Do Colloidal Wurtzite Semiconductor Nanoplatelets Have an Atomically Uniform Thickness of Eight Monolayers? J. Phys. Chem. Lett. 2019, 10, 3465–3471. [Google Scholar] [CrossRef] [PubMed]
- Van der Stam, W.; Akkerman, Q.A.; Ke, X.; Van Huis, M.A.; Bals, S.; De Mello Donega, C. Solution-Processable Ultrathin Size- and Shape-Controlled Colloidal Cu2–xS Nanosheets. Chem. Mater. 2015, 27, 283–291. [Google Scholar] [CrossRef]
- Van der Stam, W.; Rabouw, F.T.; Geuchies, J.J.; Berends, A.C.; Hinterding, S.O.M.; Geitenbeek, R.G.; Van der Lit, J.; Prévost, S.; Petukhov, A.V.; De Mello Donega, C. In Situ Probing of Stack-Templated Growth of Ultrathin Cu2–xS Nanosheets. Chem. Mater. 2016, 28, 6381–6389. [Google Scholar] [CrossRef]
- Kim, H.-M.; Cho, S.; Kim, J.; Shin, H.; Jang, J. Li and Mg Co-Doped Zinc Oxide Electron Transporting Layer for Highly Efficient Quantum Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 24028–24036. [Google Scholar] [CrossRef] [PubMed]
- Chestnoy, N.; Hull, R.; Brus, L.E. Higher Excited Electronic States in Cluster of ZnSe, CdSe, and ZnS: Spin-Orbit, Vibronic, and Relaxation Phenomena. J. Chem. Phys. 1986, 85, 2237. [Google Scholar] [CrossRef]
- Wang, Y.; Suna, A.; Mahler, W.; Kasowski, R. PbS in Polymers. From Molecules to Bulk Solids. J. Chem. Phys. 1987, 87, 7315–7322. [Google Scholar] [CrossRef]
- Steigerwald, M.L.; Alivisatos, A.P.; Gibson, J.M.; Harris, T.D.; Kortan, A.R.; Muller, A.J.; Thayer, A.M.; Duncan, T.M.; Douglass, D.C.; Brus, L.E. Surface Derivatization and Isolation of Semiconductor Cluster Molecules. J. Am. Chem. Soc. 1988, 110, 3046–3050. [Google Scholar] [CrossRef]
- Battaglia, D.; Peng, X. Formation of High Quality InP and InAs Nanocrystals in a Noncoordinating Solvent. Nano Lett. 2002, 2, 1027–1030. [Google Scholar] [CrossRef]
- Lim, J.; Bae, W.K.; Lee, D.; Nam, M.K.; Jung, J.; Lee, C.; Char, K.; Lee, S. InP@ZnSeS, Core@Composition Gradient Shell Quantum Dots with Enhanced Stability. Chem. Mater. 2011, 23, 4459–4463. [Google Scholar] [CrossRef]
- Cao, F.; Wang, S.; Wang, F.; Wu, Q.; Zhao, D.; Yang, X. A Layer-by-Layer Growth Strategy for Large-Size InP/ZnSe/ZnS Core–Shell Quantum Dots Enabling High-Efficiency Light-Emitting Diodes. Chem. Mater. 2018, 30, 8002–8007. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, N.; Zeng, Z.; Lin, Q.; Zhang, F.; Tang, A.; Jia, Y.; Li, L.S.; Shen, H.; Teng, F.; et al. High-Efficiency Green InP Quantum Dot-Based Electroluminescent Device Comprising Thick-Shell Quantum Dots. Adv. Opt. Mater. 2019, 7, 1801602. [Google Scholar] [CrossRef]
- Hahm, D.; Chang, J.H.; Jeong, B.G.; Park, P.; Kim, J.; Lee, S.; Choi, J.; Kim, W.D.; Rhee, S.; Lim, J.; et al. Design Principle for Bright, Robust, and Color-Pure InP/ZnSexS1–x/ZnS Heterostructures. Chem. Mater. 2019, 31, 3476–3484. [Google Scholar] [CrossRef]
- Li, C.-H.A.; Zhou, Z.; Vashishtha, P.; Halpert, J.E. The Future Is Blue (LEDs): Why Chemistry Is the Key to Perovskite Displays. Chem. Mater. 2019, 31, 6003–6032. [Google Scholar] [CrossRef]
- Wei, Z.; Xing, J. The Rise of Perovskite Light-Emitting Diodes. J. Phys. Chem. Lett. 2019, 10, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, W.; Bian, Y.; Ahn, T.K.; Shen, H.; Ji, B. ZnF2-Assisted Synthesis of Highly Luminescent InP/ZnSe/ZnS Quantum Dots for Efficient and Stable Electroluminescence. Nano Lett. 2022, 22, 4067–4073. [Google Scholar] [CrossRef]
- Chao, W.-C.; Chiang, T.-H.; Liu, Y.-C.; Huang, Z.-X.; Liao, C.-C.; Chu, C.-H.; Wang, C.-H.; Tseng, H.-W.; Hung, W.-Y.; Chou, P.-T. High Efficiency Green InP Quantum Dot Light-Emitting Diodes by Balancing Electron and Hole Mobility. Commun. Mater. 2021, 2, 96. [Google Scholar] [CrossRef]
- Gao, M.; Yang, H.; Shen, H.; Zeng, Z.; Fan, F.; Tang, B.; Min, J.; Zhang, Y.; Hua, Q.; Li, L.S.; et al. Bulk-like ZnSe Quantum Dots Enabling Efficient Ultranarrow Blue Light-Emitting Diodes. Nano Lett. 2021, 21, 7252–7260. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Chang, J.H.; Hahm, D.; Jeong, B.G.; Kim, J.; Lee, H.; Lim, J.; Hwang, E.; Kwak, J.; Bae, W.K. Tailoring the Electronic Landscape of Quantum Dot Light-Emitting Diodes for High Brightness and Stable Operation. ACS Nano 2020, 14, 17496–17504. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Hahm, D.; Kim, K.; Bae, W.K.; Lee, C.; Kwak, J. Highly Efficient and Bright Inverted Top-Emitting InP Quantum Dot Light-Emitting Diodes Introducing a Hole-Suppressing Interlayer. Small 2019, 15, 1905162. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.E.; Shin, D.H.; Lampande, R.; Jung, Y.H.; Mude, N.N.; Park, J.H.; Kwon, J.H. Good Charge Balanced Inverted Red InP/ZnSe/ZnS-Quantum Dot Light-Emitting Diode with New High Mobility and Deep HOMO Level Hole Transport Layer. ACS Energy Lett. 2020, 5, 3868–3875. [Google Scholar] [CrossRef]
- Zhao, J.; Bardecker, J.A.; Munro, A.M.; Liu, M.S.; Niu, Y.; Ding, I.-K.; Luo, J.; Chen, B.; Jen, A.K.-Y.; Ginger, D.S. Efficient CdSe/CdS Quantum Dot Light-Emitting Diodes Using a Thermally Polymerized Hole Transport Layer. Nano Lett. 2006, 6, 463–467. [Google Scholar] [CrossRef]
- Jing, P.; Zheng, J.; Zeng, Q.; Zhang, Y.; Liu, X.; Liu, X.; Kong, X.; Zhao, J. Shell-Dependent Electroluminescence from Colloidal CdSe Quantum Dots in Multilayer Light-Emitting Diodes. J. Appl. Phys. 2009, 105, 044313. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Ban, M.; Huang, Q.; Sun, T.; Zhang, Q.; Song, T.; Sun, B. Crosslinked Conjugated Polymers as Hole Transport Layers in High-Performance Quantum Dot Light-Emitting Diodes. Nanoscale Horiz. 2017, 2, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-Y.; Lee, S.-H.; Song, S.-W.; Yoon, S.-Y.; Jo, J.-H.; Jo, D.-Y.; Kim, H.-M.; Lee, B.-J.; Kim, H.-S.; Yang, H. More Than 9% Efficient ZnSeTe Quantum Dot-Based Blue Electroluminescent Devices. ACS Energy Lett. 2020, 5, 1568–1576. [Google Scholar] [CrossRef]
- Luo, Z.; Yu, Y.; Yang, K.; Lin, L.; Lin, J.; Guo, T.; Hu, H.; Li, F. High-Performance Cadmium-Free Blue Quantum Dot Light-Emitting Devices with Stepwise Double Hole-Transport Layers. Adv. Electron. Mater. 2023, 9, 2200970. [Google Scholar] [CrossRef]
- Caruge, J.-M.; Halpert, J.E.; Bulović, V.; Bawendi, M.G. NiO as an Inorganic Hole-Transporting Layer in Quantum-Dot Light-Emitting Devices. Nano Lett. 2006, 6, 2991–2994. [Google Scholar] [CrossRef]
- Cao, F.; Wu, Q.; Sui, Y.; Wang, S.; Dou, Y.; Hua, W.; Kong, L.; Wang, L.; Zhang, J.; Jiang, T.; et al. All-Inorganic Quantum Dot Light-Emitting Diodes with Suppressed Luminance Quenching Enabled by Chloride Passivated Tungsten Phosphate Hole Transport Layers. Small 2021, 17, 2100030. [Google Scholar] [CrossRef]
- Li, Z.H.; Chen, F.; Wang, L.; Shen, H.B.; Guo, L.J.; Kuang, Y.M.; Wang, H.Z.; Li, N.; Li, L.S. Synthesis and Evaluation of Ideal Core/Shell Quantum Dots with Precisely Controlled Shell Growth: Nonblinking, Single Photoluminescence Decay Channel, and Suppressed FRET. Chem. Mater. 2018, 30, 3668–3676. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Mai, C.; Mu, L.; Li, M.; Wang, J.; Xu, W.; Peng, J. Effects of ZnMgO Electron Transport Layer on the Performance of InP-Based Inverted Quantum Dot Light-Emitting Diodes. Nanomaterials 2021, 11, 1246. [Google Scholar] [CrossRef]
- Mude, N.N.; Yang, H.I.; Thuy, T.T.; Kwon, J.H. Performance enhancement by sol-gel processed Ni-doped ZnO layer in InP-based quantum dot light-emitting diodes. Org. Electron. 2023, 112, 106696. [Google Scholar] [CrossRef]
- Ning, M.J.; Cao, S.; Li, Q.Y.; Luo, H.; Du, Z.T.; Wang, Y.J.; Zhao, J.L. Improving Performance of InP-Based Quantum Dot Light-Emitting Diodes by Controlling Defect States of the ZnO Electron Transport Layer. J. Phys. Chem. C 2023, 127, 824–830. [Google Scholar] [CrossRef]
- Guo, S.H.; Wu, Q.Q.; Wang, L.; Cao, F.; Dou, Y.J.; Wang, Y.M.; Sun, Z.J.; Zhang, C.X.; Yang, X.Y. Boosting Efficiency of InP Quantum Dots-Based Light-Emitting Diodes by an In-Doped ZnO Electron Transport Layer. IEEE Electron Device Lett. 2021, 42, 1806–1809. [Google Scholar] [CrossRef]






| Material | Wavelength (nm) | FWHM (nm) | Method | Applications | References |
|---|---|---|---|---|---|
| InP QDs | 490–610 | 64 | HI | Visible light emission | [38] |
| Cu-doped InP QDs | 630–1100 | HI and SILAR | Visible and NIR light emission | [13] | |
| InP/ZnSe/ZnS QDs | 630 | 35 | HI | Blue, green, and red LEDs | [16] |
| InP/ZnSe/ZnS QDs | 618 | 42 | HI | Red LEDs | [39] |
| InP/ZnSeS and InGaP QDs | 674–754 | 38–50 | CE | Visible emission | [40] |
| ZnS and ZnSe QDs | 10–12 (ZnS) 14 (ZnSe) | HI | UV and blue light emission | [6] | |
| Cu- or Mn-doped ZnSe QDs | 470–550 (Cu) 575–595 (Mn) | HI | Visible light emission | [41] | |
| ZnTeSe/ZnSe/ZnS QDs | 457 | 36 | HI | Blue LEDs | [7] |
| CuInS2/ZnS core/shell QDs | 500–950 | HI | Visible light emission | [8] | |
| CuInS2/ZnS/ZnS core/shell/shell QDs | 559 | 101.9 | HU | White LEDs | [42] |
| Ag2S QDs | 1058 | 21 | HU | NIR light emission | [43] |
| AgAuSe QDs | 820–1170 | 90 | HI and CE | NIR light emission | [44] |
| Cu-doped ZnInS QDs | 450–810 | HU | Red LEDs | [14] | |
| Cu, Mn co-doped ZnInS/ZnS QDs | 190 | HU | White LEDs | [45] | |
| ZnAgInS/ZnInS/ZnS core/shell/shell QDs | 501 | >89 | HI | White LEDs | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Lai, S.; Zhang, Y.; Zhang, X. Research Progress of Heavy-Metal-Free Quantum Dot Light-Emitting Diodes. Nanomaterials 2024, 14, 832. https://doi.org/10.3390/nano14100832
Xu R, Lai S, Zhang Y, Zhang X. Research Progress of Heavy-Metal-Free Quantum Dot Light-Emitting Diodes. Nanomaterials. 2024; 14(10):832. https://doi.org/10.3390/nano14100832
Chicago/Turabian StyleXu, Ruiqiang, Shi Lai, Youwei Zhang, and Xiaoli Zhang. 2024. "Research Progress of Heavy-Metal-Free Quantum Dot Light-Emitting Diodes" Nanomaterials 14, no. 10: 832. https://doi.org/10.3390/nano14100832






