Recent Advances in Carbon-Based Single-Atom Catalysts for Electrochemical Oxygen Reduction to Hydrogen Peroxide in Acidic Media
Abstract
:1. Introduction
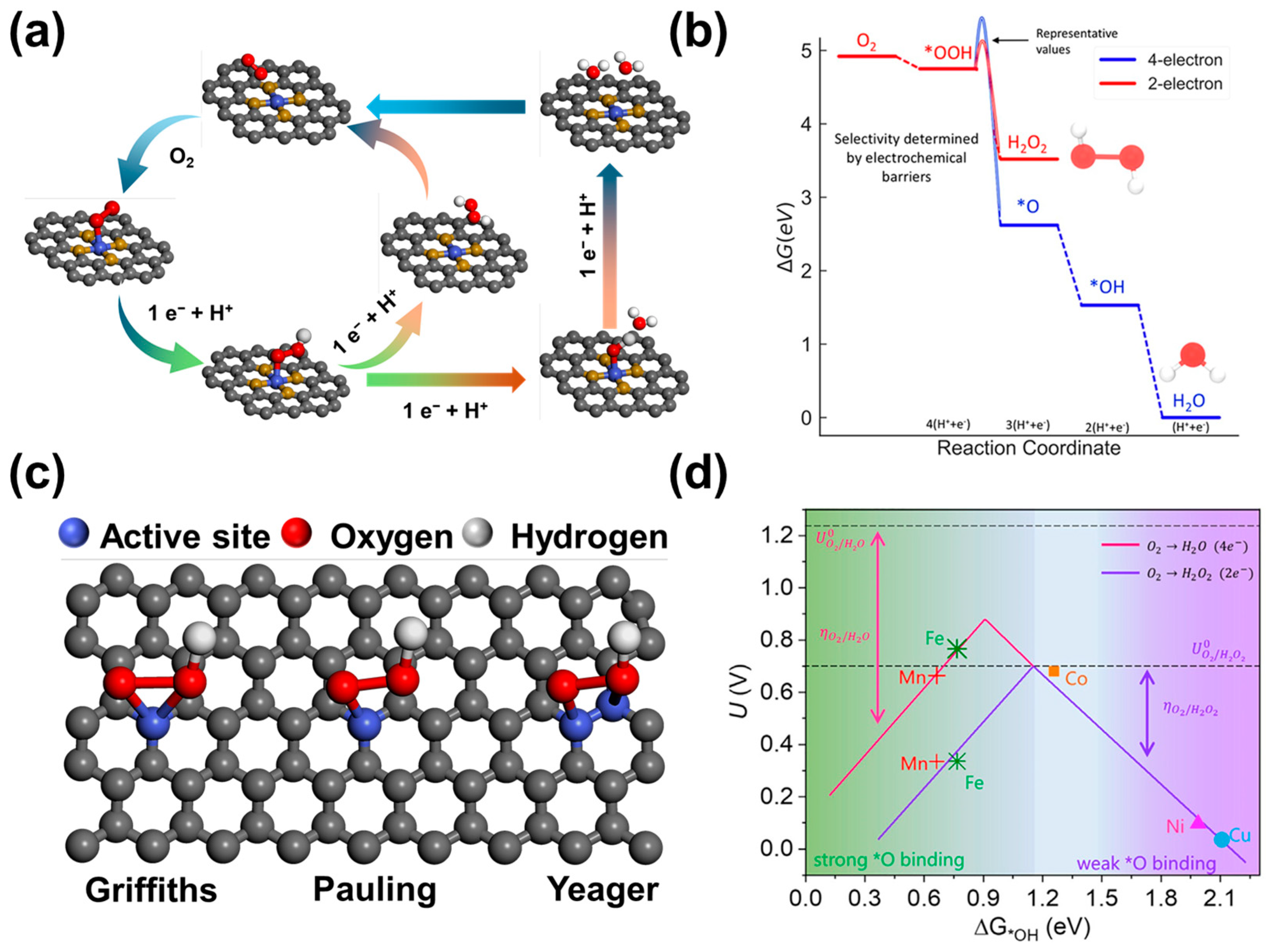
2. Reaction Mechanism of the ORR to H2O2
3. Carbon-Based Single Atom Catalysts
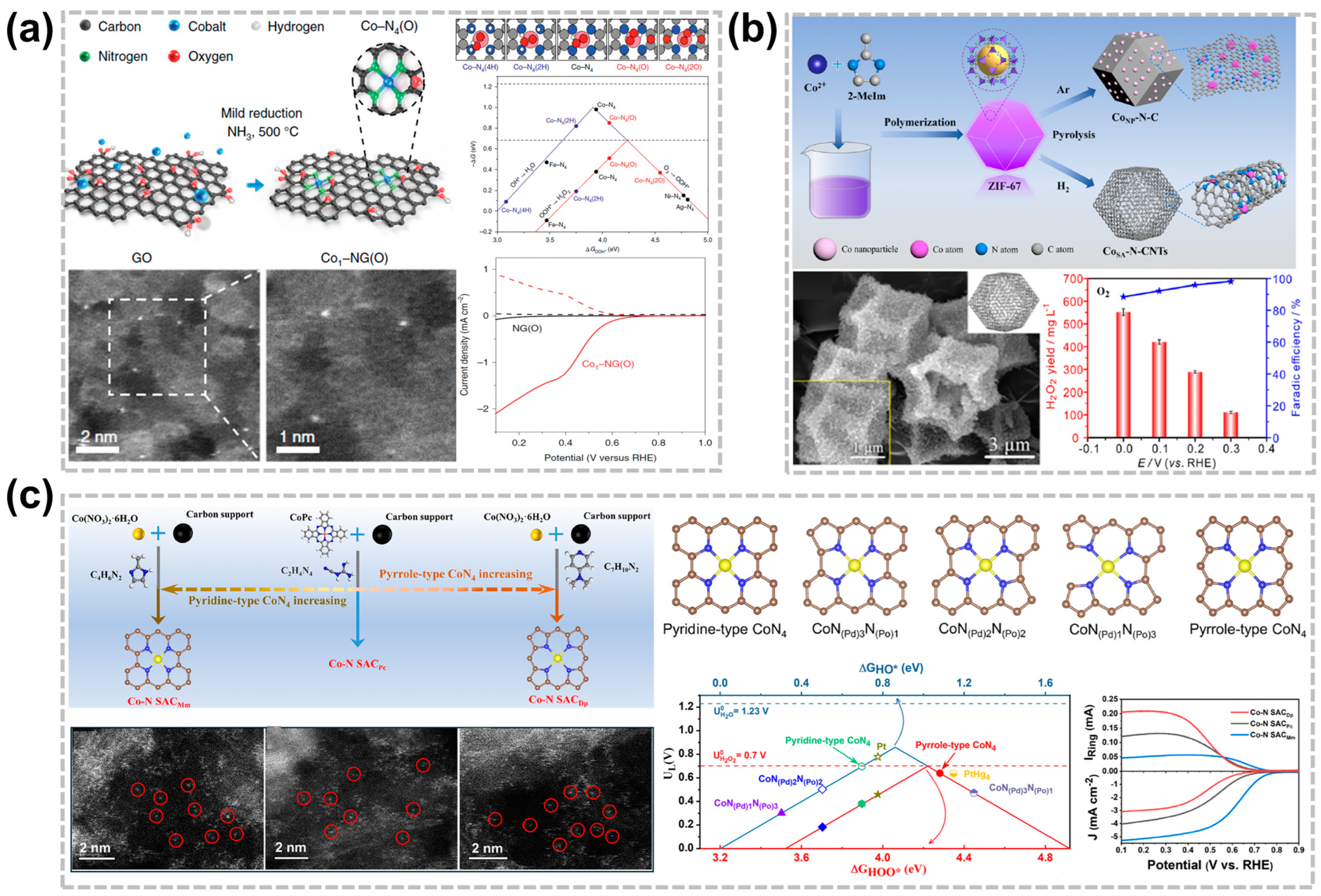
3.1. Metal Active Center Regulation Strategy
3.2. Effect of Nitrogen Coordination
3.3. Effect of Heteroatom Coordination
3.4. Effect of Surface Functional Groups or Substituent Groups
4. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Wang, Y.; Waterhouse, G.I.N.; Shang, L.; Zhang, T. Electrocatalytic Oxygen Reduction to Hydrogen Peroxide: From Homogeneous to Heterogeneous Electrocatalysis. Adv. Energy Mater. 2020, 11, 2003323. [Google Scholar] [CrossRef]
- Chen, S.; Luo, T.; Li, X.; Chen, K.; Fu, J.; Liu, K.; Cai, C.; Wang, Q.; Li, H.; Chen, Y.; et al. Identification of the Highly Active Co−N4 Coordination Motif for Selective Oxygen Reduction to Hydrogen Peroxide. J. Am. Chem. Soc. 2022, 144, 14505–14516. [Google Scholar] [CrossRef] [PubMed]
- Siahrostami, S.; Villegas, S.J.; Bagherzadeh Mostaghimi, A.H.; Back, S.; Farimani, A.B.; Wang, H.; Persson, K.A.; Montoya, J. A Review on Challenges and Successes in Atomic-Scale Design of Catalysts for Electrochemical Synthesis of Hydrogen Peroxide. ACS Catal. 2020, 10, 7495–7511. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Xia, C.; Wang, H.-F.; Tang, C. Recent advances in electrocatalytic oxygen reduction for on-site hydrogen peroxide synthesis in acidic media. J. Energy Chem. 2022, 67, 432–450. [Google Scholar] [CrossRef]
- Noll, N.; Krause, A.-M.; Beuerle, F.; Würthner, F. Enzyme-like water preorganization in a synthetic molecular cleft for homogeneous water oxidation catalysis. Nat. Catal. 2022, 5, 867–877. [Google Scholar] [CrossRef]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Huang, Y.-X.; Jin, R.-C.; Huang, B.-C. Single atom catalysts for use in the selective production of hydrogen peroxide via two-electron oxygen reduction reaction: Mechanism, activity, and structure optimization. Appl. Catal. B Environ. 2023, 337, 122987. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, L.; Gu, F.; Wu, J.; Gao, J.; Zhang, Y.-C.; Zhu, X.-D. Recent advances in single-atom catalysts for acidic electrochemical oxygen reduction to hydrogen peroxide. Nano Energy 2023, 116, 108798. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Norskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Choi, C.H.; Kwon, H.C.; Yook, S.; Shin, H.; Kim, H.; Choi, M. Hydrogen Peroxide Synthesis via Enhanced Two-Electron Oxygen Reduction Pathway on Carbon-Coated Pt Surface. J. Phys. Chem. C 2015, 119, 11160–11170. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, B.; Chen, P.; Zhao, T.; Zhao, S. Palladium-based single atom catalysts for high-performance electrochemical production of hydrogen peroxide. Chem. Eng. J. 2022, 428, 131112. [Google Scholar] [CrossRef]
- He, H.; Liu, S.; Liu, Y.; Zhou, L.; Wen, H.; Shen, R.; Zhang, H.; Guo, X.; Jiang, J.; Li, B. Review and perspectives on carbon-based electrocatalysts for the production of H2O2 via two-electron oxygen reduction. Green Chem. 2023, 25, 9501–9542. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, W.; Guo, K.; Xiong, M.; Zhang, J.; Lu, X. A Pentagonal Defect-Rich Metal-Free Carbon Electrocatalyst for Boosting Acidic O2 Reduction to H2O2 Production. J. Am. Chem. Soc. 2023, 145, 11589–11598. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Hu, Y.; Liu, Y.; Chen, S. Intrinsic Carbon Defects for the Electrosynthesis of H2O2. J. Phys. Chem. Lett. 2022, 13, 8914–8920. [Google Scholar] [CrossRef]
- Tian, Q.; Jing, L.; Du, H.; Yin, Y.; Cheng, X.; Xu, J.; Chen, J.; Liu, Z.; Wan, J.; Liu, J.; et al. Mesoporous carbon spheres with programmable interiors as efficient nanoreactors for H2O2 electrosynthesis. Nat. Commun. 2024, 15, 983. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Zhao, X.; Yang, J.; Li, N.; Gong, W.; Liu, Y.; Burguete-Lopez, A.; Li, Y.; Niu, X.; Fratalocchi, A. Enhanced Selectivity in the Electroproduction of H2O2 via F/S Dual-Doping in Metal-Free Nanofibers. Adv. Mater. 2023, 35, 2208533. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, X.; Xia, C.; Wu, Z.Y.; Zhu, P.; Kim, J.Y.T.; Bai, X.; Gao, G.; Hu, Y.; Zhong, J.; et al. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 2021, 12, 4225. [Google Scholar] [CrossRef]
- Jia, N.; Yang, T.; Shi, S.; Chen, X.; An, Z.; Chen, Y.; Yin, S.; Chen, P. N,F-Codoped Carbon Nanocages: An Efficient Electrocatalyst for Hydrogen Peroxide Electroproduction in Alkaline and Acidic Solutions. ACS Sustain. Chem. Eng. 2020, 8, 2883–2891. [Google Scholar] [CrossRef]
- Cao, P.; Quan, X.; Nie, X.; Zhao, K.; Liu, Y.; Chen, S.; Yu, H.; Chen, J.G. Metal single-site catalyst design for electrocatalytic production of hydrogen peroxide at industrial-relevant currents. Nat. Commun. 2023, 14, 172. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, Q.; Mao, X.; Cheng, C.; Zhang, L.; Wang, L.; Liu, T.-F.; Li, Y.; Li, Y. Theory-guided design of hydrogen-bonded cobaltoporphyrin frameworks for highly selective electrochemical H2O2 production in acid. Nat. Commun. 2022, 13, 2721. [Google Scholar] [CrossRef]
- Yan, L.; Wang, C.; Wang, Y.; Wang, Y.; Wang, Z.; Zheng, L.; Lu, Y.; Wang, R.; Chen, G. Optimizing the binding of the *OOH intermediate via axially coordinated Co-N5 motif for efficient electrocatalytic H2O2 production. Appl. Catal. B 2023, 338, 123078. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; She, F.; Chen, J.; Liu, F.; Qu, J.; Cairney, J.M.; Wu, C.; Liu, K.; Yang, W.; et al. Heterogeneous molecular Co–N–C catalysts for efficient electrochemical H2O2 synthesis. Energy Environ. Sci. 2023, 16, 446–459. [Google Scholar] [CrossRef]
- Du, J.; Han, G.; Zhang, W.; Li, L.; Yan, Y.; Shi, Y.; Zhang, X.; Geng, L.; Wang, Z.; Xiong, Y.; et al. CoIn dual-atom catalyst for hydrogen peroxide production via oxygen reduction reaction in acid. Nat. Commun. 2023, 14, 4766. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, C.; Zhang, J.; Huang, X.; Song, M.; Li, J.; He, F.; Yang, H.; Zhang, J.; Wang, D. Tuning the atomic configuration of Co-N-C electrocatalyst enables highly-selective H2O2 production in acidic media. Appl. Catal. B 2022, 310, 121312. [Google Scholar] [CrossRef]
- Bi, X.; Jiang, Y.; Chen, R.; Du, Y.; Zheng, Y.; Yang, R.; Wang, R.; Wang, J.; Wang, X.; Chen, Z. Rechargeable Zinc–Air versus Lithium–Air Battery: From Fundamental Promises Toward Technological Potentials. Adv. Energy Mater. 2024, 14, 2302388. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Wang, X.-X.; Guan, D.-H.; Miao, C.-L.; Kong, D.-C.; Zheng, L.-J.; Xu, J.-J. Metal–Organic Framework-Based Mixed Conductors Achieve Highly Stable Photo-assisted Solid-State Lithium–Oxygen Batteries. J. Am. Chem. Soc. 2023, 145, 5718–5729. [Google Scholar] [CrossRef]
- Yan, L.; Li, P.; Zhu, Q.; Kumar, A.; Sun, K.; Tian, S.; Sun, X. Atomically precise electrocatalysts for oxygen reduction reaction. Chem 2023, 9, 280–342. [Google Scholar] [CrossRef]
- Chen, J.-W.; Zhang, Z.; Yan, H.-M.; Xia, G.-J.; Cao, H.; Wang, Y.-G. Pseudo-adsorption and long-range redox coupling during oxygen reduction reaction on single atom electrocatalyst. Nat. Commun. 2022, 13, 1734. [Google Scholar] [CrossRef]
- Guo, X.; Lin, S.; Gu, J.; Zhang, S.; Chen, Z.; Huang, S. Simultaneously Achieving High Activity and Selectivity toward Two-Electron O2 Electroreduction: The Power of Single-Atom Catalysts. ACS Catal. 2019, 9, 11042–11054. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, W.; Gong, S.; Zheng, G.; Tian, Z.; Wen, Y.; Peng, L.; Zhang, L.; Lu, Z.; Chen, L. Atomically dispersed Lewis acid sites boost 2-electron oxygen reduction activity of carbon-based catalysts. Nat. Commun. 2020, 11, 5478. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Liu, B. Coordination Engineering of Single-Atom Catalysts for the Oxygen Reduction Reaction: A Review. Adv. Energy Mater. 2021, 11, 2002473. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Chen, H.; Wang, Y.; Song, S. Recent advances in electrochemical 2e oxygen reduction reaction for on-site hydrogen peroxide production and beyond. Chem. Commun. 2020, 56, 12109–12121. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, D.; Xu, L.; Li, M.; Chen, H.; Wu, Z.; Zhang, S. Strategies for Sustainable Production of Hydrogen Peroxide via Oxygen Reduction Reaction: From Catalyst Design to Device Setup. Nano-Micro Lett. 2023, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Zhong, R.; Hu, S.; Wu, H.; Zhou, Y.; Liu, Z.-Q. Strategies and challenges on selective electrochemical hydrogen peroxide production: Catalyst and reaction medium design. Chem. Catal. 2022, 2, 1919–1960. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Ma, L.; Duan, T.; Du, Y.; Wu, J.; Zou, J.-J.; Gao, J.; Zhu, X.-D.; Zhang, Y.-C. Recent Progress of Transition Metal Selenides for Electrochemical Oxygen Reduction to Hydrogen Peroxide: From Catalyst Design to Electrolyzers Application. Small 2024, e2309448. [Google Scholar] [CrossRef]
- Gao, J.; Yang, H.b.; Huang, X.; Hung, S.-F.; Cai, W.; Jia, C.; Miao, S.; Chen, H.M.; Yang, X.; Huang, Y.; et al. Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst. Chem 2020, 6, 658–674. [Google Scholar] [CrossRef]
- Sun, Y.; Silvioli, L.; Sahraie, N.R.; Ju, W.; Li, J.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.; et al. Activity-Selectivity Trends in the Electrochemical Production of Hydrogen Peroxide over Single-Site Metal-Nitrogen-Carbon Catalysts. J. Am. Chem. Soc. 2019, 141, 12372–12381. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Cui, J.; Wu, J.; Vajtai, R.; Sun, D.; Ajayan, P.M. Improving the Catalytic Activity of Carbon-Supported Single Atom Catalysts by Polynary Metal or Heteroatom Doping. Small 2020, 16, 1906782. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-H.; Miao, Z.; Wang, Y.-X.; Wang, J.-Z.; Chou, S.-L. Atomic-Local Environments of Single-Atom Catalysts: Synthesis, Electronic Structure, and Activity. Adv. Energy Mater. 2019, 9, 1900722. [Google Scholar] [CrossRef]
- Lee, B.-H.; Shin, H.; Rasouli, A.S.; Choubisa, H.; Ou, P.; Dorakhan, R.; Grigioni, I.; Lee, G.; Shirzadi, E.; Miao, R.K.; et al. Supramolecular tuning of supported metal phthalocyanine catalysts for hydrogen peroxide electrosynthesis. Nat. Catal. 2023, 6, 234–243. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, D.E.; Lee, S.Y.; Park, C.B.; Kim, C.K. Cobalt nanoparticles-encapsulated holey nitrogen-doped carbon nanotubes for stable and efficient oxygen reduction and evolution reactions in rechargeable Zn-air batteries. Appl. Catal. B 2023, 325, 122386. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Xu, M.-W.; Bao, S.-J.; Tan, K.; Chai, H.; Cai, C.-J.; Ji, C.-C.; Zhang, Q. Facile preparation of nitrogen-doped reduced graphene oxide as a metal-free catalyst for oxygen reduction reaction. J. Mater. Sci. Technol. 2013, 48, 8101–8107. [Google Scholar] [CrossRef]
- Maouche, C.; Zhou, Y.; Li, B.; Cheng, C.; Wu, Y.; Li, J.; Gao, S.; Yang, J. Thermal treated three-dimensional N-doped graphene as efficient metal free-catalyst for oxygen reduction reaction. J. Electroanal. Chem. 2019, 853, 113536. [Google Scholar] [CrossRef]
- Jung, E.; Shin, H.; Lee, B.H.; Efremov, V.; Lee, S.; Lee, H.S.; Kim, J.; Hooch Antink, W.; Park, S.; Lee, K.S.; et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Lei, Z.; Yu, L.; Wu, W.; Wang, Z.; Cheng, N. Electronic modulation optimizes OH* intermediate adsorption on Co-Nx-C sites via coupling CoNi alloy in hollow carbon nanopolyhedron toward efficient reversible oxygen electrocatalysis. Appl. Catal. B 2022, 304, 121006. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition metal-based bimetallic MOFs and MOF-derived catalysts for electrochemical oxygen evolution reaction. Energy Environ. Sci. 2021, 14, 1897–1927. [Google Scholar] [CrossRef]
- Chen, M.X.; Zhu, M.; Zuo, M.; Chu, S.Q.; Zhang, J.; Wu, Y.; Liang, H.W.; Feng, X. Identification of Catalytic Sites for Oxygen Reduction in Metal/Nitrogen-Doped Carbons with Encapsulated Metal Nanoparticles. Angew 2020, 132, 1644–1650. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; He, F.; Song, M.; Huang, X.; Shen, T.; Li, J.; Zhang, C.; Zhang, J.; Wang, D. Highly dispersed Co atoms anchored in porous nitrogen-doped carbon for acidic H2O2 electrosynthesis. Chem. Eng. J 2022, 438, 135619. [Google Scholar] [CrossRef]
- Hu, X.; Chen, S.; Chen, L.; Tian, Y.; Yao, S.; Lu, Z.; Zhang, X.; Zhou, Z. What is the Real Origin of the Activity of Fe-N-C Electrocatalysts in the O2 Reduction Reaction? Critical Roles of Coordinating Pyrrolic N and Axially Adsorbing Species. J. Am. Chem. Soc. 2022, 144, 18144–18152. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, L.; Li, H.; Li, L.; Jiao, Y.; Zheng, Y.; Xu, H.; Davey, K.; Qiao, S.Z. Tailoring Acidic Oxygen Reduction Selectivity on Single-Atom Catalysts via Modification of First and Second Coordination Spheres. J. Am. Chem. Soc. 2021, 143, 7819–7827. [Google Scholar] [CrossRef]
- Yuan, J.; Yin, H.; Ge, X.; Pan, R.; Huang, C.; Chen, D.; Hu, L.; Xie, H. Superior Efficiency Hydrogen Peroxide Production in Acidic Media through Epoxy Group Adjacent to Co-O/C Active Centers on Carbon Black. Chem. Eng. J 2023, 465, 142691. [Google Scholar] [CrossRef]
- Zhou, W.; Xie, L.; Gao, J.; Nazari, R.; Zhao, H.; Meng, X.; Sun, F.; Zhao, G.; Ma, J. Selective H2O2 electrosynthesis by O-doped and transition-metal-O-doped carbon cathodes via O2 electroreduction: A critical review. Chem. Eng. J 2021, 410, 128368. [Google Scholar] [CrossRef]
- Yang, L.; Sun, G.; Fu, H.; Zhang, L. Isolation of cobalt single atoms on hollow B, N co-doped defective carbon nanotubes for hydrogen peroxide production and tandem reagent-free electro-Fenton oxidation. Chem. Eng. J 2023, 472, 145052. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Bedford, N.M.; Han, Z.; Thomsen, L.; Smith, S.; Amal, R.; Lu, X. Direct insights into the role of epoxy groups on cobalt sites for acidic H2O2 production. Nat. Commun. 2020, 11, 4181. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Lai, W.-H.; Xiao, F.; Lyu, Y.; Liao, C.; Shao, M. Approaching a high-rate and sustainable production of hydrogen peroxide: Oxygen reduction on Co–N–C single-atom electrocatalysts in simulated seawater. Energy Environ. Sci. 2021, 14, 5444–5456. [Google Scholar] [CrossRef]
- Sun, L.; Jin, X.; Su, T.; Fisher, A.C.; Wang, X. Conjugated Nickel Phthalocyanine Derivatives for Heterogeneous Electrocatalytic H2O2. Adv. Mater. 2023, 36, e2306336. [Google Scholar] [CrossRef]
- Dey, S.; Mondal, B.; Chatterjee, S.; Rana, A.; Amanullah, S.; Dey, A. Molecular electrocatalysts for the oxygen reduction reaction. Nat. Rev. Chem. 2017, 1, 0098. [Google Scholar] [CrossRef]
- Kumar, R.; Sankar, M. Synthesis, Spectral, and Electrochemical Studies of Electronically Tunable β-Substituted Porphyrins with Mixed Substituent Pattern. Inorg. Chem. 2014, 53, 12706–12719. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Pan, R.; Zou, M.; Ge, X.; Shi, C.; Yuan, J.; Huang, C.; Xie, H. Recent Advances in Carbon-Based Single-Atom Catalysts for Electrochemical Oxygen Reduction to Hydrogen Peroxide in Acidic Media. Nanomaterials 2024, 14, 835. https://doi.org/10.3390/nano14100835
Yin H, Pan R, Zou M, Ge X, Shi C, Yuan J, Huang C, Xie H. Recent Advances in Carbon-Based Single-Atom Catalysts for Electrochemical Oxygen Reduction to Hydrogen Peroxide in Acidic Media. Nanomaterials. 2024; 14(10):835. https://doi.org/10.3390/nano14100835
Chicago/Turabian StyleYin, Hao, Ronglan Pan, Manman Zou, Xin Ge, Changxuan Shi, Jili Yuan, Caijuan Huang, and Haibo Xie. 2024. "Recent Advances in Carbon-Based Single-Atom Catalysts for Electrochemical Oxygen Reduction to Hydrogen Peroxide in Acidic Media" Nanomaterials 14, no. 10: 835. https://doi.org/10.3390/nano14100835




